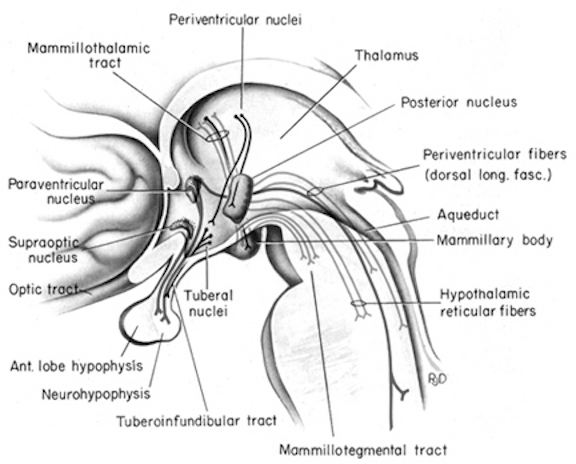
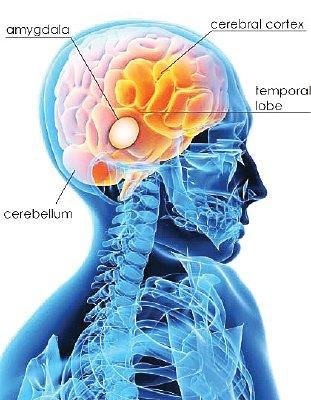
Rhawn Gabriel Joseph, Ph.D.
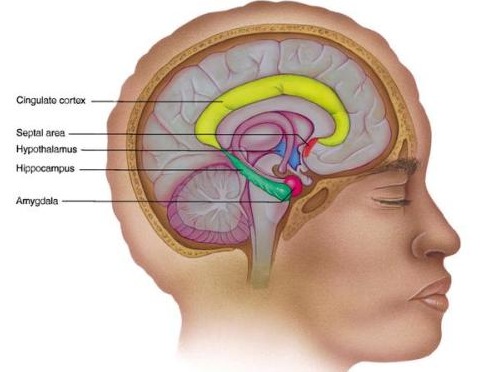
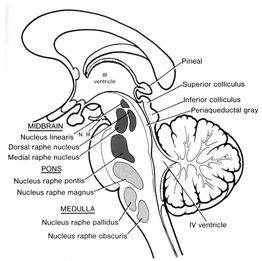
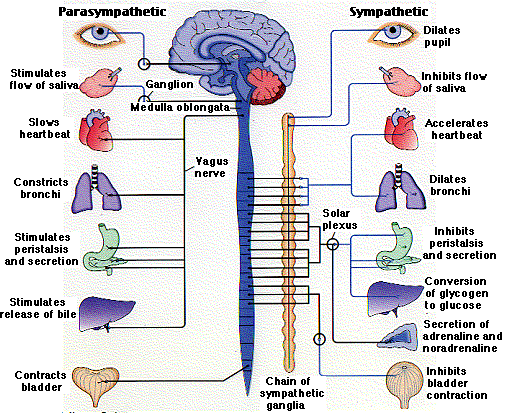
Human language, and the original impetus to vocalize, springs forth from roots buried within the depths of the ancient limbic lobes which is buried in the body of the brain. These vocalizing structures include the hypothalamus, amygdala, cingulate gyrus (Joseph, 1982, 2012a, 1993, 2014, 1999e; Jurgens, 2011, 2012, 2014; MacLean, 2011; Ploog, 2012; Robinson, 1967, 1972) as well as brainstem structures such as the periaqueductal grey.
It is these ancient vocal-emotional centers which explain why non-human animals also vocalize to convey feeling and emotion. Thus, although non-humans and infants generally do not have the capacity to meaningfully communicate in grammatical word sequences, they still vocalize, and these vocalizations are often limbic and emotional in origin (Hauser, 1997; Joseph, 1982, 2012a; Jurgens, 2011; Jurgens and Muller-Preuss, 1977; MacLean, 2011; Meyer et al., 1973; Ploog, 2012; Robinson, 1967, 1972).

We know that language is evoked from the limbic system and not from the neocortical surface of the brain, since electrode stimulation of the neocortex does not provoke vocalization. Although the neocortex can segment, grammatically organized, and sequence the sounds of language, the source of speech belongs to the old limbic brain. Indeed, emotional cries and warning calls have been produced via stimulation of wide areas of the limbic system (Jurgens, 2011, 2012; Jurgens et al. 1982; Jurgens and Muller-Preuss, 1977; MacLean, 2011; Ploog, 2012; Robinson, 1967, 1972) including the human amygdala (Halgren, 2012; Heith, Smith and Halgren, 1988) and the anterior cingulate gyrus (Meyer et al., 1973) which also becomes activated when speaking (Frith and Dolan, 2015; Passingham, 2015; Paulesu et al., 2015; Peterson et al., 1988). And these and other limbic structures often become activated in response to certain emotional sounds. In fact, the limbic system is more vocal than any other part of the brain (Jurgens, 2011; Robinson, 1967).


Characteristically, limbic vocalizations are evoked in situations involving sexual arousal, terror, anger, rage and extreme fear and fright and are similarly expressed by a wide range of species. They are also expressed by infants such as when separated from the primary caretaker when young. Some of these limbic vocalizations begin to be emitted soon after birth.





In fact, because these vocalizations are mediated by the limbic system, human infants and apes and monkeys reared in isolation or (in the case of non-human primates) with surgically muted mothers and thus with little or no "language" experience or training, are able to produce complex and appropriate emotional calls and cries which accurately indicate fear or distress. They are also expressed by children born blind and deaf.
In the case of non-human primates, they will react appropriately to these vocalizations the first time they are exposed to them (Herzog and Hopf, 1984; Winter et al., 1973). For example, squirrel monkeys reared in isolation respond appropriately with fear and anxiety in response to warning "yapping" calls (signifying the presence of a predator) the very first time they are heard (Herzog and Hopf, 1984). They will also produce an appropriate "yapping" cry when they are first exposed to a potential predator.
The first vocalizations of human infants (McGraw, 1969; Milner, 1967; Piaget, 1952; Spitz and Wolf, 1946; Sroufe, 1996), including anencephalics (Lemire et al., 1978; Monnier, 1956) and those born deaf and blind (Eibl-Eisbesfeldt, 1995) are similarly emotional in origin and limbically mediated (Joseph, 1982, 2012a, 1999b,e).
Initially these sounds consist of grunts and sounds indicative of displeasure, and weeks then months later will include pleasure, fear and the separation cry. These vocalizations can also be produced by direct stimulation of the hypothalamus, amygdala, or anterior cingulate gyrus (Jurgens, 2011, 2012, 2014; MacLean, 2011; Meyer et al., 1973; Ploog, 2012; Robinson, 1967, 1972).
The human amygdala (Halgren, 2012; Heith et al., 1988) and anterior cingulate (Frith and Dolan, 2015; Passingham, 2015; Paulesu et al., 2015; Peterson et al., 1988) become activated when hearing or producing emotional words and sounds, whereas the medial hypothalamus (MacLean, 2011; Robinson, 1967, 1972) and the midbrain periaqueductal grey (Casey et al., 2014; Coghill et al., 2014; Zhang et al., 2014) become active when experiencing or vocally expressing negative mood states. Hence the term: "limbic language" (Joseph, 1982; Jurgens, 2011).

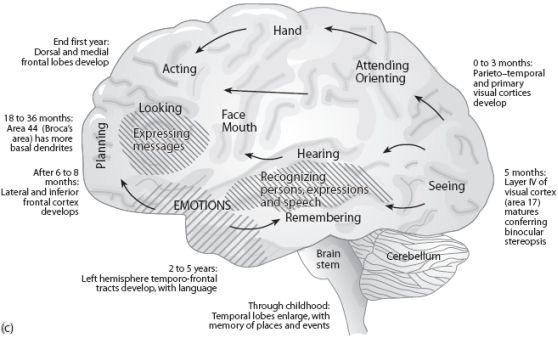
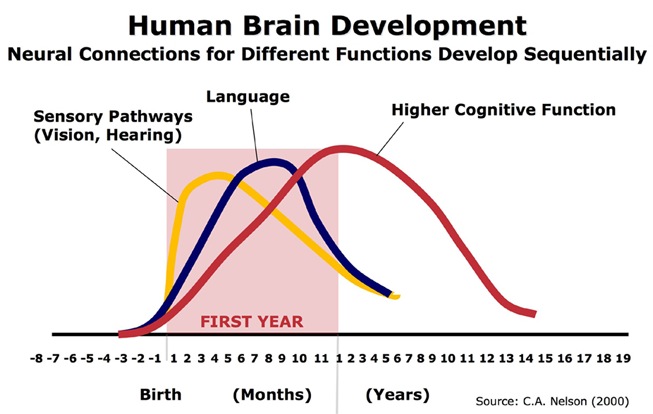
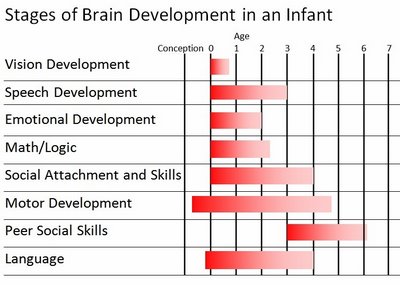
The normal pattern of maturational development, as is evident behaviorally and as based on myelination and metabolic activity, is that the brainstem and cerebellum begin to develop in advance of the forebrain, which in turn matures in a upward, rostral and paramedial to lateral arc, i.e. diencephalon (medial hypothalamus), limbic system (amygdala), striatum, cingulate, neocortex (Barkovich et al., 1988; Brody et al., 1987; Gibson, 1991; Harbord, et al., 2011; Holland, et al., 1986; Lee et al., 1986).
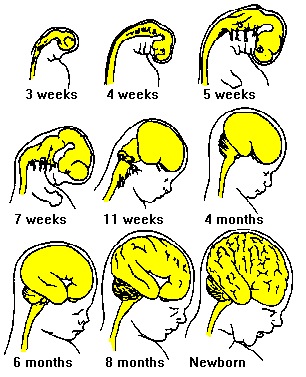
Because the maturation of the forebrain and neocortex are exceedingly prolonged (Benes, 2014; Blinkov and Glezer, 1968; Brody, Kinney, Kloman, and Gilles, 1987; Conel, 1939, 1941; Debakan, 2000; Flechsig, 1901; Holcomb et al., 2012; Huttenlocher, 2011; Paus et al., 1999; Pfefferbaum, et al., 2014; Reiss, et al., 1996; Yakovlev and Lecours, 1967), and as the limbic system matures in advance of the neocortical speech areas, infants (and their mothers) are dependent on limbic language and the limbic system in order to communicate their needs and to discern the social-emotional intentions of others. This is why infants will react appropriately even if the speaker speaks a "foreign language" (Fernald, 2015).
Since these emotional sounds are limbically produced and comprehended, and because these structures are common to all humans and mammals, these sounds are stereotypically vocalized and comprehended cross-culturally, and by infants and different animal species (Beier and Zautra, 1972; Fernald, 2012; Fernald et al., 1989; Hauser, 2015; Joseph, 1988a, 2015; Kramer, 2014; Nakazima, 1975). Hence, the famous aside: "I don't know what they are saying, but I sure don't like the sound of it!"
For example, be it human, primate, or social mammal, certain sounds arouse or convey fear, sadness, caution or alarm (e.g. thunder, growling, low tones), or conversely, pleasure, gaiety, or peaceful conditions (e.g., soft or higher rolling, and smoother pitched tones and melodies). It is because certain sounds can signify and arouse specific and universal mood states that movie and television programs are often accompanied by "mood" music.
As noted, despite neocortical immaturity, and although lacking denotative, grammatical language skills, infants are quite adept at distinguishing between different emotional vocalizations so as to determine the mood state and intentions of others (Fernald, 2015; Haviland and Lelwica, 1987).

For example, it has been demonstrated that preverbal infants between the ages of 10 weeks and 5 months are capable of appropriately discerning, discriminating and responding to social-emotional vocalizations conveying approval, disproval, happiness, and anger (Fernald, 2015; Haviland and Lelwica, 1987). Moreover, 5 month old (American) infants are able to make these discriminations even in the absence of words and vocabulary, and in response to nonsense English, as well as to German and Italian vocalizations (Fernald, 2015). However, infants even younger are capable of producing these same limbic vocalizations (Joseph, 1982), and the same is true of those born deaf and blind, which again indicates that these abilities are innate.
OVERVIEW: LIMBIC LANGUAGE DEVELOPMENT
Be it infant H. sapiens sapiens, or non-human primates and mammals (hereafter referred to as "primates" and "mammals"), different structures within the limbic system produce different (as well as similar) vocalizations. The type of cry or vocalization elicited, in general, depends upon which limbic structure has been activated (Jurgens, 2011; Jurgens and Muller-Preuss, 1977; Robinson, 1967, 1972). This is because different limbic structures, and in fact, different divisions within these nuclei, subserve unique functions, and maintain different anatomical interconnections with various regions of the brain (chapter 13).
For example, portions of the septal nuclei, hippocampus, anterior cingulate, medial amygdala, and medial hypothalamus have been repeatedly shown to generate negative and unpleasant mood states (MacLean, 2011; Olds and Forbes, 1981; Robinson, 1967, 1972). Other limbic tissues, including the lateral hypothalamus, lateral amygdala and portions of the septal nuclei, are associated with pleasurable feelings (chapter 13). Hence, areas associated with pleasurable sensations often give rise, when sufficiently stimulated, to pleasurable calls, whereas those linked to negative mood states, will trigger shrieks and cries of alarm.
However, because these limbic structures mature at somewhat different, albeit overlapping rates (Benes, 2014; Brody et al., 1987; Debakan, 2000; Holcomb et al., 2012; Paus et al., 1999; Pfefferbaum, et al., 2014; Reiss, et al., 1996; Yakovlev and Lecours, 1967) the infant's emotional vocal repertoire also develops in a stereotypical fashion in parallel.
For example, the midbrain periaqueductal gray and hypothalamus are almost fully functional at birth, and as will be detailed, these structures can produce crying, screaming, and grunting sounds--vocalizations characteristic of the human infant. It is the somewhat later to mature amygdala and cingulate gyrus which in turn are responsible for the increasing complexity and range of the infant's vocal-emotional repertoire, including the development of early and late babbling (Joseph 1982, 2012a, 2015).
Initially infant emotional sound production appears to convey generalized meanings, e.g. pleasure, displeasure. These sounds are associated with the hypothalamus and periaqueductal gray. Over the following weeks and months, these vocalizations become modified and more elaborate and produced in specific contexts, and are then increasingly shaped and tied to specific mood states or events and social-emotional phenomenon (Joseph, 1982, 2012a, 1999c; Milner, 1966; Piaget, 1952). That is, the increasing range of sounds produced and perceived become modulated, differentiated, more elaborate and complex, and specifically tied to sadness, joy, affection, sorrow, fear, and so on, rather than just pleasure and displeasure. These increases in vocal complexity in turn are associated with maturational events occurring within the amygdala and anterior cingulate.

Similar developmental alterations and elaborations in limbic vocalizations have been noted among primates. For example, vervet monkeys employ three distinct calls which they differentially produce in the presence of eagles, snakes and leopards (Cheney and Seyfarth, 2011). Experienced and normally reared monkeys respond to these calls by looking up ("eagle"), looking down ("snake"), or climbing up a tree ("Leopard") depending on which call is produced, even when played from a tape recorder (Seyfarth et al. 1980).
However, infants reared in isolation merely respond with generalized alarm when presented with these same calls, and are as likely to look up as down as climb a tree. That is, although they recognize the emotional significance of the call, they are not yet able to differentiate these sounds as signifying particular and specific social-emotional events or situations. These abilities are increasingly acquired over the course of the first few months as the limbic system matures, and in response to specific environmental experiences. In fact, these differential limbic maturational and vocalization events are also associated with and parallel social-emotional developmental behavioral changes which are mediated by these same limbic structures (Joseph, 2012a, 1999c).
LIMBIC LANGUAGE HIERARCHICAL DEVELOPMENT
The brain develops and matures in a caudal to rostral and paramedial to lateral arc such that functions associated with the midbrain periaqueductal gray are soon followed by that of the medial hypothalamus which sits atop and is partly contiguous with the midbrain (detailed in chapters 23 and 24). At birth, the medial hypothalamus appears to be functionally active and/or at a state of semi-functional and anatomical maturity. An advanced state of (neonatal) hypothalamic maturity is indicated by the emergence of associated behaviors and vocalizations (Joseph, 2012a, 2015, 1999c), myelination patterns (e.g., Debakan, 2000; Gibson, 1991; Langworthy, 1937; Yakovlev and Lecours, 1967), and the establishment of its massive fiber interconnections with the midbrain and periaqueductal gray (Gilles et al., 2003; Debakan, 2000; Langworthy, 1937; Yakovlev and Lecours, 1967).
As noted the neonatal and infant neocortex is exceedingly immature and in the case of the neonate, displays almost no functional activity (Blinkov and Glezer, 1968; Chugani, 2014; Conel, 1939, 1941; Debakan, 2000; Flechsig, 1901; Holcomb et al., 2012; Huttenlocher, 2011; Paus et al., 1999; Pfefferbaum, et al., 2014; Reiss, et al., 1996; Yakovlev and Lecours, 1967). Hence, the behavior of the human neonate appears to be mediated by the brainstem and midbrain periaqueductal gray which mediate chewing, sucking, swallowing, phonating, and breathing, and reflexively vocalizes when aroused (Joseph, 1999c; Jurgens, 2012, 2014; Ploog, 2012), as well as the medial hypothalamus which appears to be functionally active at birth.

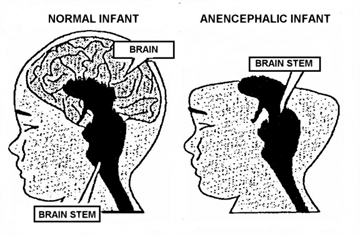
This explains why similar neonatal sounds can be vocalized by anencephalics who are generally devoid of neural tissue anterior to the diencephalon (Emde, Gaensbauer, and Harmon, 1976; Lemire et al., 1978; Monnier, 1956). With complete forebrain destruction, and following depth electrode activation of the brainstem periaqueductal gray (Jurgens, 2014; Larson et al., 2014; Zhang et al., 2014), or with stimulation of the hypothalamus (MacLean, 2011; Robinson, 1967, 1972) grunting, howling and crying can be reflexively triggered, including those similar to those produced by a howling infant or anencephalic.
Brainstem vocalization centers appear to be fully functional at birth, though they also continue to mature and myelinate over the course of the first 3-12 postnatal months (Debakan, 2000; Gilles, 1991; Yakovlev and Lecours, 1967), whereas the neocortex, including the neocortical speech areas can take well over 10 years to reach an advanced stage of maturation.
However, when the hypothalamus is stimulated, not just emotional vocalizations but complex and emotionally congruent behaviors can be elicited including facial displays of rage or pleasure. On the other hand, as with midbrain stimulation, once the stimulation is terminated, the behavior and vocalizations cease. Nevertheless, although the hypothalamus responds in an on/off fashion, so long as it is activated (such as by hunger or pain) and in the case of neonates (because it is so immature) it will continue to react and cry out for long time periods, even in the absence of an obvious external stimulating source.
By contrast, activation of the more highly evolved amygdala and anterior cingulate produces complex and sustained feeling and mood states even after the stimulation is removed (see chapter 13). Moreover, cingulate stimulation can produce emotional sounds that accurately reflect or which have no relation to the individual's mood. These latter structures, however, begin to functionally mature at a later age of development, which is why associated behaviors and emotions appear at later ages as well (Joseph, 1999b,c,).
Specifically, the later maturation of the amygdala is associated with the developing of cooing and other emotional sounds and the onset of "early babbling" (Joseph, 1982, 2012a, 1999b). "Early babbling," although not emotional per se, appears to be a function of the the amygdala's massive fiber pathway to the masticatory centers in the brainstem, which, at this age, are decidedly immature (see Takeuchi et al., 1988). That is, because of its immaturity, it induces rudimentary and repetitive reflexive jaw and lip movements including chewing, sucking, and swallowing--functions that many scientists believe are integral to speech development (Weiss, 1951; for related discussion see Moore and Ruark, 1996).
The much later to develop "late" (canonical) babbling, and other complex emotional vocalizations, are associated with the maturation of the anterior cingulate (Joseph, 2015, 1999b)--a structure which when electrically stimulated produces all manner of complex and repetitive sounds including "dadadada" (Dimmer and Luders, 1995; Penfield and Welch, 1951). The cingulate ("canonical") late babbling stage is followed by jargon babbling and then human speech, beginning with the first words, all of which are associated with increased neocortical maturity and the establishment of neocortical hierarchical control over limbic and brainstem nuclei.
Thus, babbling and limbic language and emotional speech become increasingly complex as the hypothalamus, followed by the amygdala and cingulate gyrus mature--structures which myelinate and functionally develop following the myelination of the brainstem. Moreover, not just speech, but associated limbic behaviors also emerge in parallel. Again, the normal pattern of maturational development is that the brainstem develops in advance of the forebrain, which in turn matures in a caudal to rostral and paramedial to lateral arc, i.e. diencephalon (medial hypothalamus), limbic system (amygdala), striatum, cingulate, neocortex (Barkovich et al., 1988; Brody et al., 1987; Gibson, 1991; Harbord, et al., 2011; Holland, et al., 1986; Lee et al., 1986). Again, however, these neocortical maturational events continue well into late childhood, adolescence and adulthood (e.g., Pfefferebaum, et al., 2014; Jernigan, et al., 1991; Kinney et al., 1988).
With the later maturation of the neocortical speech areas, it appears that limbic language becomes hierarchically and sequentially reorganized, thereby giving rise, in children and adults, to segmented-prosodic, temporal sequential, grammatical, vocabulary-rich human speech (Joseph, 1982, 2012a, 1999a,e; for related discussion see Hallet and Proctor 1996; Herschkowitz, Kagan, and Zilles, 2015).
In this regard, a hierarchy of progressive complexity in emotional vocalization and feeling states could be said to begin with the periaqueductal gray and medial hypothalamus, and then progressively expands so as to incorporate the amygdala followed by the cingulate gyrus and finally the neocortical speech areas which hierarchically mediate and sequence limbic emotional vocalizations thereby producing complex, vocabulary-rich grammatical speech (Joseph, 1999a,e; 2000a).
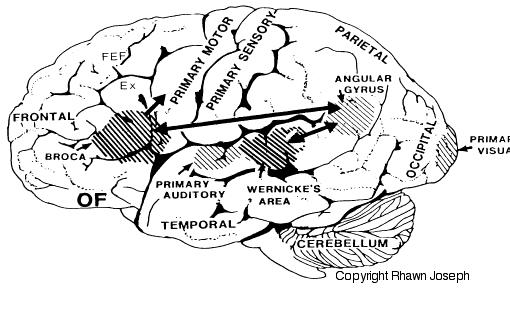
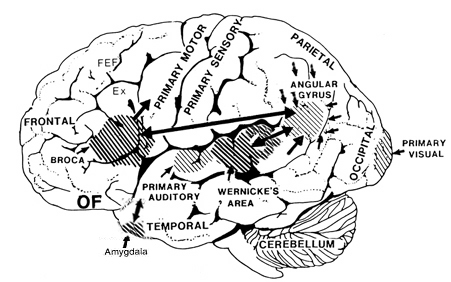
Specifically, with the maturation (and evolution) not only of the neocortical speech areas but the inferior parietal lobule, Broca's and Wernicke's areas become interlocked at the neocortical level thereby giving rise to the language axis, and modern human speech through the hierarchical representation of limbic speech which is punctuated and fractionated into words and temporal sequences (Joseph, 1982, 1999e, 2000a). However, even in the adult, these neocortical tissues remain dependent on the limbic system and the brainstem in order to vocalize and communicate as these tissues are directly connected. If these pathways are destroyed the patient may become mute.
Therefore, because the maturation of the limbic forebrain and neocortex is exceedingly prolonged (Benes, 2014; Blinkov and Glezer, 1968; Brody et al., 1987; Conel, 1939, 1941; Debakan, 2000; Flechsig, 1901; Holcomb et al., 2012; Huttenlocher, 2011; Paus et al., 1999; Pfefferbaum, et al., 2014; Reiss, et al., 1996; Yakovlev and Lecours, 1967) so too is the development and acquisition of human language, the first sounds of which appear to be reflexively uttered by the brainstem periaqueductal gray and the medial hypothalamus.
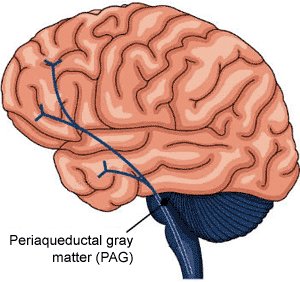
PERIAQUEDUCTAL GRAY AND VOCALIZATION
Activity within the midbrain periaqueductal gray (which receives extensive input from the amygdala, hypothalamus, and other limbic nuclei) can trigger the production of a variety of sounds that are suggestive of exceedingly negative feelings (Larson et al. 2014; Zhang et al. 2014). The periaqueductal gray, in fact, becomes functionally active in response to noxious and painful stimuli as do other pontine-midbrain nuclei as demonstrated through functional imaging (Casey et al., 2014; Coghill et al., 2014). However, if the periaqueductal gray is disconnected from the limbic system and neocortex (such as by a midbrain transection), stimulation of this nuclei will continue to evoke vocalization. Nevertheless, with the exception of facial contortions (produced by the fifth and 7th cranial nerves) and changes in breathing and thus vocalization, stimulation of the isolated periaqueductal gray is not accompanied by complex behavioral displays, and when the stimulation is removed, the vocalizations immediately cease. Moreover, patients with "bulbar palsy" (due to partial brainstem injury and disconnection), report that their vocalizations do not correspond to their actual feelings.
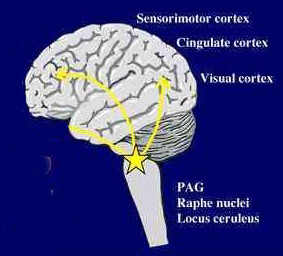
This suggests that periaqueductal gray sound production, that is, at the level of the midbrain, is due to the activation of pre-programmed motor engrams that are stored within the brainstem. Moreover, this suggests that the periaqueductal gray responds reflexively in response to painful stimuli (such as when an individual cries "ouch"), and in reaction to emotional impulses transmitted via the amygdala and hypothalamus -nuclei with which it is intimately interconnected.
However, if denied forebrain input, or if provided abnormal input, although the periaqueductal gray may vocalize, the "emotions" conveyed may not have a corresponding feeling state but instead represents a reflexive motor program involving the vocalization centers. In fact, the coordinated activity of these tissues and activation of these motor programs would enable an individual to laugh, cry, or howl, even if the brain anterior to the midbrain were dead and there was no evidence of consciousness. Hence, similar vocalizations are produced by anencephalics born with only a brainstem (Emde et al., 1976; Lemire et al., 1978; Monnier, 1956).
Specifically, the midbrain periaqueductal gray receives input from throughout the brainstem, spinal cord, as well as the hypothlamus, amygdala, cingulate, and the speech areas in the left and right frontal lobes, and is able to activate and coordinates the laryngeal, oral-facial, and principal and accessory muscles of respiration and inspiration thereby producing a wide range of vocalizations (Jurgens, 2011, 2012, 2014; Larson et al. 2014; Zhang et al. 2014). The periaqueductal gray appears to be the site where particular vocalization motor patterns are stored.
When the periaqueductal gray is activated by impulses received from the limbic system or neocortex, it activates the appropriate motor program and then organizes and coordinates the oral-laryngeal and respiratory muscles so that the appropriate sounds can be produced (Jurgens, 2014; Zhang et al, 2014). In this manner the felt aspects of emotion (as generated within the forebrain) are accompanied by appropriate sound production as released and mediated by the brainstem and cranial nerves (see chapter 17).
THE MEDIAL HYPOTHALAMUS: CRYING AND SCREAMING
By birth the hypothalamus has established massive fiber interconnections with the midbrain and periaqueductal gray (Gilles et al. 2003; Debakan, 2000; Langworthy, 1937; Yakovlev and Lecours, 1967) and is thus capable of reflexively activating this structure. That is, the infantile periaqueductal gray reflexively vocalizes in response to hypothalamic influences and thus may cry in reaction to hunger or thirst. As detailed in chapter 13, the hypothalamus is involved in all aspects of endocrine, hormonal, visceral and autonomic nervous system functioning and contains lipostatic, glucose, and osmoreceptors which are sensitive to the body's fat content and fluctuations in circulating metabolities and water levels. The hypothalamus also becomes exceedingly active when hungry and while eating or simply looking at food (Nakamura and Ono, 1986; Rolls, Burton, and Mora, 1976). Thus, the monitoring of internal homeostasis is a major function of the hypothalamus (see chapter 13). As the remainder of the forebrain is exceedingly immature, the vocalizing behavior and auditory capabilities of the neonate appear to reflect brainstem-periaqueductal and medial hypothalamic influences, such that the neonate will grunt and cry in response to hunger, thirst, pain.
As noted, for the first several weeks of life, the infant displays only two behavioral states suggestive of emotion: Quiescence and displeasure --demonstrated by crying, screaming, or inactivity. Among its many functions the medial hypothalamus is also associated with quiescence, the parasympathetic nervous system, and the experience and expression of extreme distress and negative mood states (Olds and Forbes, 1981) including the production of extremely negative vocalizations (MacLean, 2011; Robinson, 1967, 1972). Depth electrode activation of the medial hypothalamus is so aversive subjects will work to reduce it (Olds and Forbes, 1981).
From the perspective of the maturing brain, for the first several days after birth, the infant is little more than a hypothalamus, a brainstem, and a very immature autonomic nervous system. These structures exert reflexive behavioral control over the infant which responds reflexively to bodily needs.
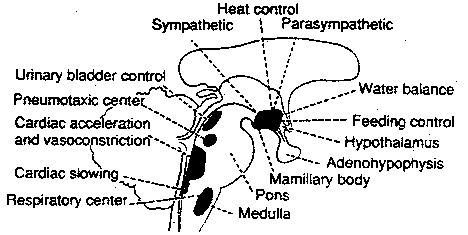
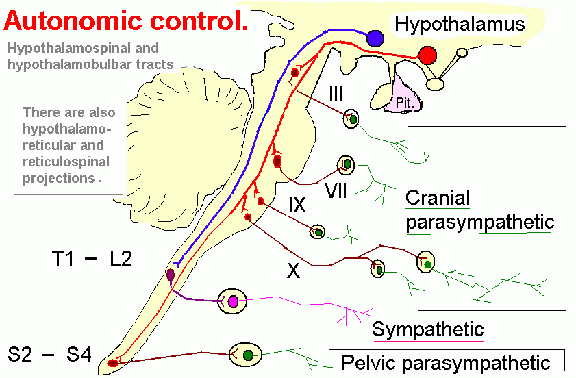
As the remainder of the forebrain is exceedingly immature, quiescence and distress, the two predominate behavioral states demonstrated by the infant (McGraw, 1969; Milner, 1967; Piaget, 1952; Spitz and Wolff, 1946), appear to be associated with medial hypothalamic activity which can act directly on the periaqueductal gray. Thus, for example, when experiencing hunger or thirst, the infantile hypothalamus may activate the brainstem including the periaqueductal gray which reflexively reacts by crying. However, once sated, the medial hypothalamus becomes quiescent.

THE LATERAL HYPOTHALAMUS AND THE PLEASURE PRINCIPLE
The medial hypothalamus begins to mature before the lateral nucleus--a developmental process which may not be complete until late puberty (Yakovlev and Lecours, 1967). However, as the lateral hypothalamus (and other forebrain nuclei) mature, it increasingly exerts its own unique influences and the developing infant will increasingly demonstrate and vocalize feelings of pleasure (Joseph, 2012a; see also Hershkowitz et al. 2015).
Lateral hypothalamic functional maturation, however, also overlaps with that of other forebrain structures, including the amygdala. And, feelings and vocalizations indicative of pleasure have been triggered following excitation of a number of diverse limbic areas including the amygdala, cingulate gyrus, and the medial forebrain bundle (Jurgens, 2011; Jurgens and Muller-Preuss, 1977; Olds and Forbes, 1981; Robinson, 1967, 1972). Following depth-electrode placement, animals will repeatedly engage in self-stimulatory activity to deliver electric impulses to these nuclei. However, the greatest area of concentration of reward sites, and the highest rates of self-stimulatory activity occur in the lateral hypothalamus. According to Olds (1956), animals "would contine to stimulate as rapidly as possible until physical fatigue forced them to slow or to sleep." By contrast, if the lateral region is destroyed the experience of pleasure and emotional responsiveness is almost completely attenuated (Marshall and Teitelbaum, 1974).
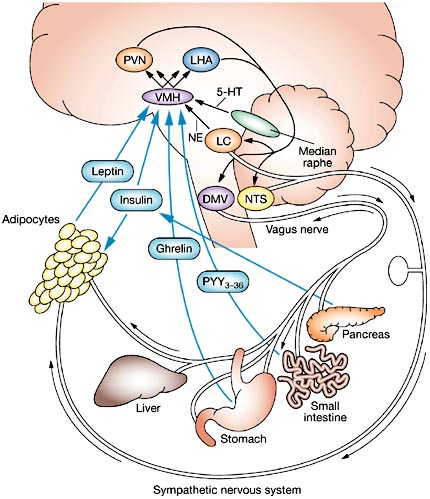
Thus, whereas the medial hypothalamus produces distress and quiescence, the later to mature lateral hypothalamus produces feelings of pleasure, such that by 3 months of age, infants will smile and vocalize with genuine pleasure (Sroufe, 1996). In this regard, it could be said that the hypothalamus mediates the pleasure principle (Joseph, 2012a). In fact, activation of the lateral hypothalamus produces vocalizations suggestive of extreme pleasure, and in humans can even trigger uncontrolled laughter (Davison and Kelman, 1939; Ironside, 1956; Martin, 1950. Hence, as the lateral hypothalamus (and other forebrain structures mature) infants also begin to laugh around 3-4 months of age.
Presumably the hypothalamus activates the periaqueductal gray and brainstem respiratory centers (e.g. Boliek, Hixon, Watson, and Morgan, 1996) which reflexively produces facial expressions and respiration-related vocalizations suggestive of pleasure, including laughter, or conversely, cries of distress. However, by 3 months of age the amygdala is also significantly contributing to the infant's behavior and speech patterns.
Nevertheless, as forebrain maturity continues in a medial to lateral and a caudal to rostral arc, initially the hypothalamus matures at an earlier age than the amygdala (and forebrain), and exerts a more profound influence on neonatal behavior. Over the ensuing weeks, and as the lateral and medial hypothalamus mature, followed by the amygdala/forebrain, the vocal as well as emotional and behavioral repertoire of the infant expands (Joseph, 1982, 2012a), whereas crying begins to wane due to increased cortical inhibitory control (Hershkowitz et al. 2015).
By 3-4 months of age infants will smile with genuine pleasure and produce laughter similar to adult laughter (Sroufe, 1996); a function of increasingy forebrain maturity (e.g. Hallett and Proctor, 1996; Herschkowitz et al., 2015). As the amygdala and the forebrain mature, the infant's ability to vocalize becomes increasingly differentiated and expressive of more complex emotions such as joy, wariness and fear.
THE AMYGDALA, HYPOTHALAMUS, AND PERIAQUEDUCTAL GRAY
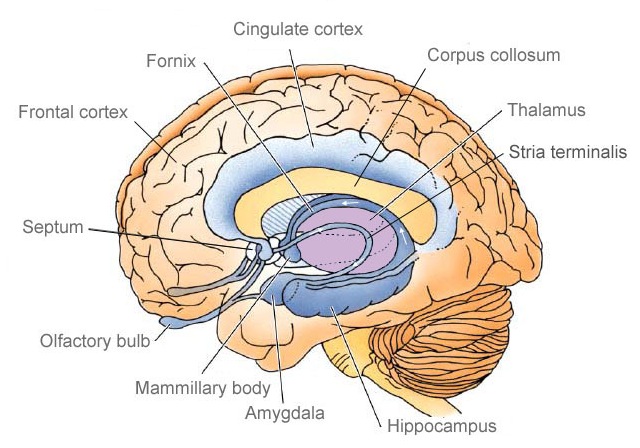
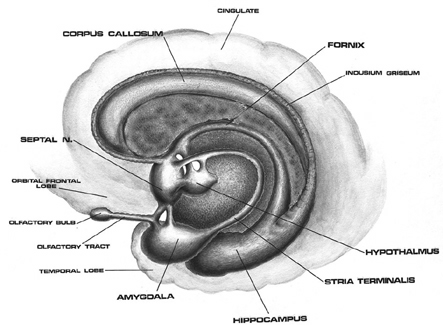
The amygdala responds to and in turn, exerts inhibitory and excitatory influences on the brainstem and hypothalamus and thus emotional behavior, through the amygdalofugal fiber pathway and stria terminalis (Davis et al. 2015; Joseph, 2012a; Rosen and Schulkin, 1998). The stria terminalis and amygdalofugal pathways are bidirectional and interlink the medial and lateral amygdala with the medial and lateral hypothalamus and the periaqueductal gray (Krettek and Price, 1978).
Through these same pathways the amygdala can activate the brainstem including the periaqueductal gray, and is able to modulate and even control rudimentary emotional forces governed by the hypothalamus as well as act at the behest of hypothalamically induced drives. For example, if certain nutritional requirements need to be meet, the hypothalamus signals the amygdala via the stria terminalis. The amygdala then surveys the external environment in search of an appropriate stimulus.
On the other hand, if presented with a potentially threatening or motivationally significant stimulus, the amygdala may stimulate hypothalamic activity (as well as the brainstem and striatum, e.g. Davis et al. 2015; LeDoux, 1996; Rosen and Schulkin, 1998) so that the organism is mobilized to take appropriate action. Hence, when the hypothalamus is activated and driven by the amygdala, instead of responding in an on/off manner which is typical of the hypothalamus, cellular activity continues for an appreciably longer time period (Dreifuss, Murphy, and Gloor, 1968; Rolls 2012).
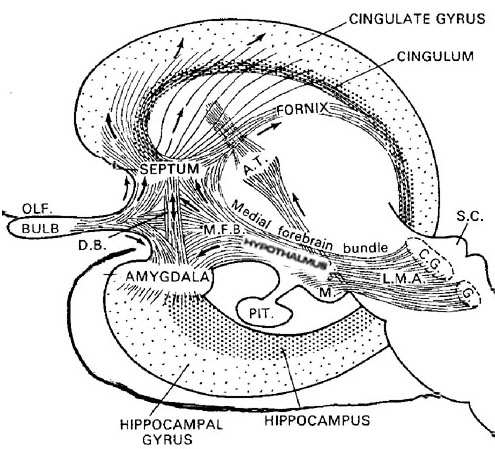
The amygdala also acts independently of the hypothalamus, and can, for example, generate extreme feelings of pleasure and corresponding facial expressions and vocalizations (chapter 13). Indeed, the lateral amygdala receives and contributes fibers to the medial forebrain bundle which in turn has its site of origin in the lateral hypothalamus (Mehler, 1980) and projects to the brainstem. Moreover, the amygdala is rich in cells containing enkephalins, and opiate receptors can be found throughout this nucleus (Atweh and Kuhar, 1977; Uhl, Kuhar, and Snyder, 1978). It is also a major pleasure center which promotes self-stimulatory activity (Olds and Forbes, 1981) and the vocalization of pleasure (e.g. cooing and gooing).
Therefore, as the medial and then the lateral amygdala mature and gains hierarchical control over the hypothalamus and brainstem, the emotional repertoire expands as does the infant's ability to perceive and produce a variety of exceedingly complex social-emotional behaviors and vocalizations, including, as will be detailed later, the development of "early babbling."
THE AMYGDALA AND SOCIAL-EMOTIONAL VOCALIZATION
Although there is much debate as to the infant's emotional capabilities, or lack thereof (e.g. Hershkowitz et al. 2015; Izard, 1991; Sroufe, 1996), for the first several weeks of postnatal life the limbic forebrain is simply too immature to accurately perceive or vocalize emotional feelings other than displeasure. Likewise, although the newborn and week-old infant will lift the corners of its mouth, as if "smiling," these initial "smiles" do not appear to represent true emotions (Spitz, Emde, and Metcalf, 2000; Sroufe, 1996; Wolff, 1963) but are probably brainstem reflexes (Joseph, 1999c). It is only as the lateral hypothalamus, and medial and lateral amygdala (and other forebrain structures) begin to mature that the infant becomes capable of truly "smiling," laughing, and producing complex social-emotional vocalizations (for related discussion see Boliek et al. 1996; Hallet and Proctor, 1996; Herschkowitz et al. 2015; Nobre and Plunkett 2015).
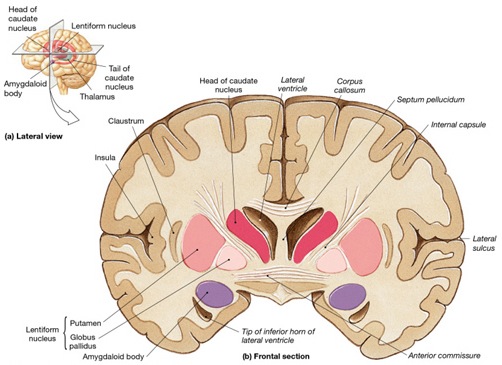
The amygdala is an exceedingly complex structure consisting of a variety of nuclei including the claustrum and the limbic and corpus striatum (Heimer and Alheid, 1991; MacLean, 2011; Mogenson and Yang, 1991). It also maintains massive interconnections with the hypothalamus, cingulate, frontal and parietal lobes, and periaqueductal gray (Amaral, Price, Pitkanen, and Thomas, 2012; Krettek and Price, 1978; Mesulam and Mufson, 1982; O'Keefe and Bouma, 1969). In addition, the amygdala receives direct input from the auditory areas in the temporal lobe via a thick neural pathway, the inferior arcuate fasciculus, and through the claustrum which is a "broken off" segment of the amygdala situated near and is connected with the auditory receiving areas in the temporal lobe. Hence the human amygdala responds to complex auditory-affective stimuli including words and sentences (Halgren, 2012; Heit et al., 1988), and if electrically stimulated, patients report hearing voices which tend to be experienced as emotionally significant (Gloor, Olivier, and Quesney, 1981).
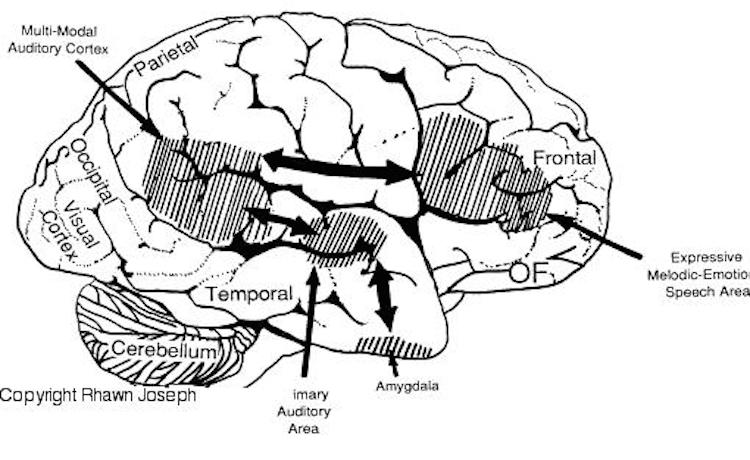
The medial amygdala (in conjunction with the hippocampus) forms a cortical bulbous protrusion in the anterior-medial temporal lobe (the uncus), and over the course of evolution gave rise to sheets of 3-layered allocortex, then 5-layered mesocortex, and finally neocortex and contributed to the formation of the auditory neocortex and Wernicke's area, which are thus, in part, evolutionary extensions of the amygdala. As noted, immediately beneath the insula and approaching the auditory neocortex is a thick band of amygdala-cortex, the claustrum. Over the course of evolution the claustrum apparently split off from the amygdala due to the expansion of the temporal lobe and the passage of additional axons coursing throughout the white matter (Gilles et al., 2003) including the arcuate fasciculus.
Nevertheless, the claustrum maintains rich interconnections with the auditory cortex as well as the amygdala (Gilles et al., 2003) which in turn is linked directly with the neocortical auditory areas. This is evident from dissection of the human brain which reveals that the fibers of the arcuate fasciculus (and claustrum) project to and from the amygdala, leading not only to Wernicke's area, but continuing through the inferior parietal lobule, projecting directly to Broca's area.

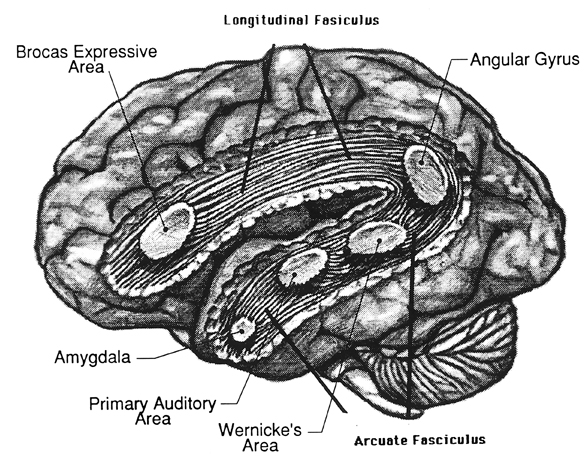

Hence, via these extensive interconnections with the auditory neocortex (and the midbrain inferior-auditory colliculus and medial geniculate of the thalamus) the amygdala is able to receive and analyze auditory input in order to discern and identify stimuli which are emotionally and motivationally significant (Gloor, 2015; Halgren, 2012). When emotional, sexual and motivationally relevant stimuli (e.g. food, sex partner) are detected the amygdala can organize appropriate behavioral and vocal responses and can trigger startle or defensive reactions, as in response to transient sounds, or those typically made by predators, prey, or potential mates (Edeline and Weinberger, 1991; Hitchock and Davis, 1991; Gloor, 1960, 1986, 2015; Hocherhman and Yirmiya, 2011; Rolls, 2012; Ursin and Kaada, 1960). These motor-behavioral reactions, in turn, are mediated by the limbic and corpus striatum, the periaqueductal gray, and lower brainstem, all at the behest of the amygdala.
Moreover, it can vocalize via the neocortical speech areas and pariaqueductal gray. In fact, in conjunction with the anterior cingulate the amygdala is one of the most vocally responsive structures of the brain (Jurgens, 2011, 2012; Robinson, 1967, 1972) and become activated in response to emotional sounds and words (Halgren, 2012; Heit et al., 1988).
In humans, destruction limited to the normal amygdala, the right amygdala in particular, can severely disrupt the ability to sing, convey melodic information or to enunciate properly via vocal inflection, and can result in great changes in pitch and the timbre of speech (Freeman and Williams, 1952, 1963; Joseph, 2012a). With bilateral destruction of the normal (vs the diseased) amygdala, emotional speech production and the capacity to respond appropriately to emotionally significant visual or auditory stimuli is significantly disrupted (Lilly, Cummings, Benson, and Frankel, 2003; LeDoux, 1996; Marlowe, Mancall, Thomas,1975; Scott et al., 2015; Terzian and Ore, 1955).
The amygdala, therefore is primary in regard to the perception and expression of social and emotional nuances and in large part is responsible for the expression and comprehension of not just limbic language, but human speech, the sounds of which are shunted to and from the amygdala via the inferior fasciculus and the claustrum which is a "broken off" segment of the amygdala situated near the primary auditory receiving areas.
As noted (and described briefly below), portions of the auditory neocortex -which extends from the anterior and medial temporal lobe and beyond the insula to include the superior temporal lobe and the inferior parietal lobule- is in part, an evolutionary derivative of the amygdala. In this regard it could be argued that the primary, secondary and auditory association areas including Wernicke's area, have evolved (at least in part) from the amygdala, and in fact remain extensively interconnected with this nuclei via the inferior portions of the arcuate fasciculus as well as the claustrum.
In consequence, when the neocortical auditory areas are injured, the amygdala is sometimes disconnected and can no longer extract or inpart emotional nuances to incoming or outgoing sounds. For example, with right superior temporal lobe lesions, patients may suffer from a receptive auditory affective agnosia, as well as an agnosia for environmental sounds (see chapter 10). They may have difficulty ascertaining the feelings of others or perceiving social emotional nuances. Hence, emotional peception and expression may become grossly disorganized and inappropriate.
By contrast, if the left amygdala is disconnected from the left superior temporal lobe, patients may verbally complain that they consciously feel cut off from their emotions (chapter 10). Moreover, with left temporal lobe dysfunction, speech and thought processes may come to be abnormally invested or devoid of emotion, and patients may be diagnosed as psychotic and/or paranoid.
Hence, although the left and right amygdala are functionally lateralized, with the right amygdala significantly larger than the left, both contribute significantly to the perception and expression of language, and assist in maintaining the functional integrity of the neocortical auditory areas in the right and left temporal lobe. It is through these interconnections that limbic languages comes to be hierarchically organized at the level of the temporal neocortex.
BEHAVIORAL INDICES OF AMYGDALA MATURATION
The amygdala is the most emotional and socially responsive structure of the brain (Davis, Walker, and Lee, 2015; Gloor, 2015; Halgren, 2012; Kling and Brothers 2012; LeDoux 1996; Rolls 2012; Rosen and Schulkin, 1998) and appears responsible for the ability to experience and covey complex emotions including love and guilt, and to form intense emotional (and sexual) attachments (Joseph, 2012a, 1999b).
Nevertheless, at birth and over the course of the ensuing several weeks, the amygdala is so immature that its contributions appear to be rather negligible, which is which is why, for example, infants remain fearless until after 6 months of age. Fear is a primary emotion associated with the amygdala (Chapman, 1960; Davis et al. 2015; Gloor, 2015; Halgren, 2012; Rosen and Schulkin, 1998; Ursin and Kaada, 1960).
However, as based on behavioral-emotional indices, it also appears that neurons within the immature amygdala (and overlying temporal lobe), i.e. those which are responsive to the faces and eyes (Hasselmo, Rolls, and Baylis, 1989; Morris, Frith, Perett, Rowland, Young, Calder, and Colan, 1996; Kawashima et al., 1999; Rolls, 1984), may well be responsible for the newborn's tendency to briefly attend to facelike stimuli (Carpenter, 1974; Goren, Sarty, and Wu, 1975). In fact, in conjunction with the overlying (partly contiguous) temporal lobe, the amygdala contains neurons which selectively respond to smiles, to the eyes, the direction of gaze, and which differentiate between male and female faces and the emotions they convey (Hasselmo et al. 1989; Kawashima, et al., 1999; Morris et al. 1996; Rolls, 1984).
For example, the normal human amygdala will respond to frightened faces by altering and increasing its activity (Morris et al. 1996). Moreover, the left amygdala acts to discriminate the direction of another person's gaze, whereas the right amygdala becomes activated while making eye-to-eye contact (Kawashima, et al., 1999).
Again, however, initially the amygdala is so immature that its influences are nearly negligible. Indeed, the amygdala (uncus) does not reach advanced levels of myelination until around the end of the first postnatal year (Yakovlev and Lecours, 1967). However, as the amygdala and its auditory and facial-featuring detecting neurons develop, and around 2-months of age, the infant increasingly recognizes and orients toward familiar faces, and by 6-months it can discriminate between male and female faces. By 9 months it can easily discriminate between different facial expressions (Caron, Caron and Myers, 1985; Carpenter, 1974; Sroufe, 1996)--functions associated with the temporal lobe and amygdala.
As these structures and their facial-feature-detecting neurons mature, infants increasingly attend to and demonstrate an almost irresistible interest in facial stimuli (Bronson, 1972). If presented with a strange face, although the infant may look away, it will also quickly look back, "and will make eye-to-eye contact as though drawn by a magnet" (Sroufe, 1996, p. 103).
Moreover, these facial-detecting-neurons are richly interconnected with amygdala-temporal lobe neurons concerned with social emotional functioning, including those which trigger smiling, laughter, and even a crying, sobbing, and a fear response (e.g., Chen and Forster, 1973; Offen et al., 1976; Sethi and Rao, 1976). Therefore, activation of these tissues can also trigger eye-to-eye facial contact, smiling, social behavior, as well as a variety of social-emotional vocalizations.
The localization of these functions to the amygdala is exceedingly adaptive, as the face as well as the voice serve as major sources of information. When simultaneously employed these dual input/output channels promote social communication and thus language, and provide amygdala neurons and neural pathways with growth promoting stimulation (Joseph, 1999b). Face-to-face interaction provokes and reinforces the tendency to vocalize, and provides added meaning to what is heard and said, and also contributes to the formation of emotional attachments and the seeking of social contact and stimulation.
Therefore, as the amygdala increasingly attends to the human face, emotional vocalizations become more complex, and, around 3-4 months of age (in conjunction with increased forebrain control), smiling and crying become less reflexive, cooing increases in frequency, and the ability to produce a "social" smile becomes more pronounced (e.g. Hershkowitz et al. 2015). The infant becomes increasingly social, and coos and smiles during greeting, and when making face-to-face and eye-to-eye contact, especially with their mothers (Ainsworth, 1973; D'Odorico, 1984; Sroufe, 1996; Wolff, 1969).
Likewise, between the ages of 3 weeks to 4 months infants become increasingly capable of appropriately discerning, discriminating and responding to social-emotional vocalizations conveying approval, disproval, happiness, and anger (Fernald, 2015; Haviland and Lelwica, 1987). Infants are able to make these discriminations based merely on the perception of emotional prosody, in the absence of words and vocabulary, and in response to non-sense English, as well as to German and Italian vocalizations (Fernald, 2015).
THE AMYGDALA, FEAR, AND ATTACHMENT
As noted, medial forebrain structures begin to mature before lateral nuclei. Thus the medial hypothalamus begins to mature before the lateral nuclei, and the medial amygdala before the lateral amygdala. Thus, whereas the medial amygdala becomes increasingly well myelinated around 3-4 months (Yakovlev and Lecours, 1967), the functional maturation of the lateral amygdala is much more prolonged. It is the lateral amygdala which mediates the fear response (Chapman, 1960; Davis et al. 2015; Gloor, 2015; Halgren, 2012; Rosen and Schulkin, 1998; Ursin and Kaada, 1960); an emotion which, in conjunction with the development of "working memory" (Hershkowitz et al., 2015) and the maturation of the hippocampus, septal nuclei and cingulate, exerts significant influences on the development of attachment and fear of strangers (Joseph, 2012, 1998b, 1999b).
As the amygdala does not approach advanced levels of myelination until around 8-12 months of age, the infant remains basically fearless for the first 8 months of postnatal development. This is exceedingly adaptive. If fear were to emerge at an earlier age, the infant might withdraw from those who normally provide it with loving social-emotional stimulation. It is only as the medial and lateral amygdala reach an advanced stage of myelination and development that the fear response and related vocalizations emerge (Joseph, 2012a), that is, around the 8-12 months (Emde et al. 1976; Sroufe and Waters, 1976). Infants, therefore, become increasingly wary and fearful of strangers--emotions evoked by the amygdala. Therefore, if strangers approach, the child may look away or Cover their eyes. Or the child will try to get away.


Fear is the most common emotional reaction elicited from amygdala stimulation in human and non-humans (Chapman, 1960; Davis et al. 2015; Gloor, 2012, 2015; Halgren, 2012; Rosen and Schulkin, 1998; Ursin and Kaada, 1960). Likewise, the human amygdala becomes activated when experiencing fear (Halgren, 2012; Rauch et al., 1996). Abnormal activity in the amygdala or the overlying temporal lobe can in fact evoke overwhelming, terrifying feelings of death-like "nightmarish" fear (Herman and Chambria, 1980; Strauss, Risser, and Jones, 1982). Hence, the fear response and the expression of fearful vocalizations, which appears around 8-12 months of age, are obvious indications of, and are correlated with, the later stages of amygdala maturation, a structure which also significantly contributes to the formation of specific social-emotional attachments (Joseph, 2012a, 1999b). Indeed, because the infant experiences fear, the fear response directly contributes to the formation of specific attachments and the avoidance of those who are unfamiliar.


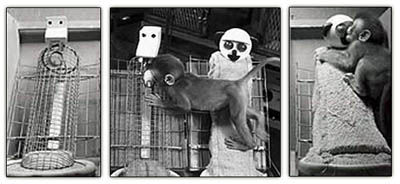
Although the amygdala is associated with the fear response, this structure also promotes social contact seeking. Therefore, as noted, destruction of the amygdala abolishes social behavior, and animals and humans will actively avoid social contact. In the infant, however, social contact seeking is basically indiscriminate due to the immaturity of this structure and the paucity of counterbalancing influences normally exerted by the septal nucleus and cingulate gyrus on the amygdala and these tendendencies (Joseph, 2012a, 1999b). Thus, as the amygdala matures (and since the septal nuclei and cingulate do not begin to mature until later in development), until the infant is 6-7 months of age it will smile at the approach of anyone, even complete strangers. The child will also vigorously protest any form of separation from strangers (e.g. if they leave the room). This stage corresponds to the amygdaloid maturational period where septal and cingulate influences are still less well developed.
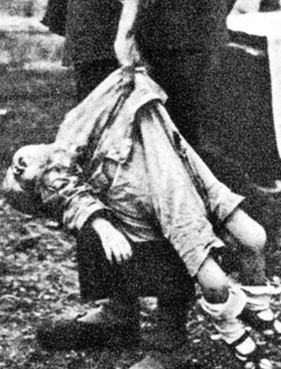
In fact, among humans, so pervasive is this need for physical interaction and social stimulation that when grossly reduced or denied, the result is often death. For example, in several well known studies of children raised in foundling homes during the early 1900's when the need for contact was not well recongized, morbidity rates for children less than 1 year of age was over 70%. Of 10,272 children admitted to the Dublin Foundling home during a single 25 year period, only 45 survived (Langmeier and Matejcek, 1975).
SEPTAL SOCIAL BEHAVIOR
As detailed in chapter 22, the septal nuclei does not begin to differentiate or mature until receiving axonal projections from the amygdala and extended amygdala (Brown, 2003; Humphrey, 1967), and does not begin to reach advanced stages of development until around after 3 years of age (Yakovlev and Lecours, 1967).
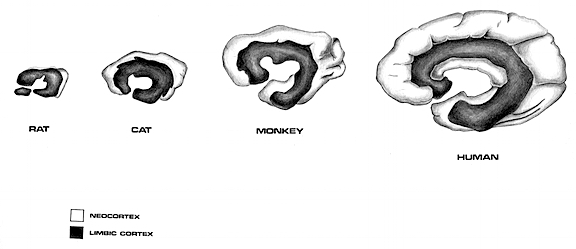
Phylogenetically the septal nuclei appears to be a derivative of the hypothalamus and hippocampus, and contributed to the evolution of the medial portions of the hemispheres (Sanides 2014) including portions of the cingulate gyrus. It also increases in relative size and complexity as we ascend the ancestral tree, attaining its greatest degree of development in humans.

As detailed in chapter 14, the septal nuclei is implicated in memory functioning and arousal, and in this regard contributes to the formation of specific attachments through memory functioning and the modulation of emotional arousal. Moreover, like the amygdala and cingulate, the septal nuclei also produces emotional vocalizations.
The septal nuclei is able to exert facilitatory or inhibitory influences on medial vs lateral hypothalamic arousal (Mogenson, 1976) and maintains a counterbalancing relationship with the amygdala particularly in regard to influences exerted on the hypothalamus (Joseph, 2012a). In addition, the amygdala acts to either facilitate or inhibit septal functioning whereas septal influences on the amygdala are largely inhibitory.
For example, whereas the lateral amygdala may activate the lateral hypothalamus, the septal nuclei may activated the medial hypothalamus (Kolb and Whishaw, 1977; Mogenson, 1976; Petsche et al. 1962, 1965) and can counter lateral hypothalamic self-stimulatory activity (Mogenson, 1976). As noted, the medial hypothalamus (and the septal nuclei) are associated with unpleasant mood states. In fact, electrophysiological alterations in septal activity which correspond to subjective feelings of aversion have been reported in humans (Heath, 1976).
Electrical stimulation of the septal nuclei counters and inhibits aggressive behavior (Rubenstein and Delgado, 1963) and suppresses the expression of rage reactions following hypothalamic stimulation (Siegel and Edinger, 1976). If the septal nucleus is destroyed, these counterbalancing influences are removed such that initially there results dramatic increases in aggressive behavior, including rage (Ahmad and Harvey, 1968; Blanchard and Blanchard, 1968; Brady and Nauta, 1953; King, 1958). Bilateral lesions in fact give rise to explosive emotional reactivity to tactile, visual, or auditory stimulation which can take the form of attack or flight. However, if the amygdala is subsequently lesioned, the septal rage and emotional reactivity are completely attenuated (King and Meyer, 1958). Hence, septal lesions appear to result in a loss of modulatory and inhibitory restraint which are normally exerted, in part, on the amgydala as well as the hypothalamus (McClary, 1961, 1966; Poplawsky, 1987).
Because of the loss of this inhibitory restraint, the amygdala begins to promote indiscriminant socializing and, as is the case with a five month old infant, will display an extreme need for social and physical contact (Joseph, 2012a). That is, in contrast to amygdaloid lesions which produce a severe social-emotional agnosia and social avoidance and withdrawal, septal lesions produce a dramatic and persistent increase in social cohesiveness and contact seeking (Jonason and Enloe, 1971; Jonason et al., 1973; McClary, 1961, 1966; Meyer et al. 1978).
With complete bilateral destruction of the septal nuclei in animals, the drive for social contact appears to be irresistable such that persistent attempts to make physical contact occurs--due in part, presumably, to the disinhibitory release of the amygdala. Septally lesioned animals will in fact seek contact with other species, including animals that might kill and eat them. If a group of septally lesioned animals are placed together, extreme huddling results. So intense is this need for contact comfort following septal lesions, that if other animals are not available they will seek out blocks of wood, old rags, bare wire frames or walls and then attempt to cuddle. Similar behaviors are demonstrated by human and non-human infant primates who are denied sufficient emotional and maternal stimulation (Joseph, 1999b,c).
Among human infant, and most mammals, the desire for maternal and physical-emotional contact is a normal aspect of development.



However, in some cases, similar behaviors can be triggered by amygdala as well as anterior cingulate hyperactivation (see chapter 25). It is probably the abnormal interactions of these nuclei that account for stalking behaviors and the formation of delusional attachments to actresses, sports stars, coworkers, and so on.
LIMBIC LOVE, HATE AND RELATIONSHIPS
The limbic system makes it possible to experience and communicate social-emotional nuances via multiple modalities, such as is reflected in the evolution and development of emotional speech, including the ability to laugh, cry, and to express sympathy and compassion, or the desire to form or maintain an emotional attachment. It is the evolution and development of these limbic nuclei (i.e. the amygdala, septal nuclei, cingulate gyrus) and their differential rates of maturation, which in fact enable humans and other higher mammals to form long lasting emotional and loving attachments, including the need and desire for contact comfort during infancy and early childhood.

Unfortunately, when the limbic system has been activated in this manner, feelings of rage may soon be manifested as acts of murder. For example, the frontal lobes and the rest of the brain may be overwhelmed by these limbic upheavals and the person may act on his or her limbic attachment needs, either in an extremely dependent and despairing fashion ("Don't leave me or I'll kill myself") or violent and enraged manner ("Don't leave or I'll kill you"). Loss of love, such as occurs when a relationship ends, seconded only by jealousy and money are prime elicitors of such murderous feelings and are due to the high involvement of the limbic system in all affairs of the heart.
Thus, if a person who has met another individuals primary needs for love, affection and physical intimacy were to leave, or want to end the affair, the limbic system of the male or female, being "abandoned," may respond in an infantile fashion; i.e. with desperation, frustration, anger, rage or depression and dispair -similar to the emotional stages demonstrated by children who are progressively deprived of mothering.

For example, children who are temporarily separated from their mothers and placed in a hospital, childrens home, or what not, will pass through three stages of emotional turmoil, the first of which is characterized by a protest period where they frequently cry and scream for their mommy, and display signs of rage. This is followed by a stage of despair in which the child ceases to cry, loses interest in his environment and withdraws, and this too can persist for months. In the final stage he ceases to show interest in others, loses his appetite, and fails to respond to the affection offered by others and becomes quite passive and unresponsive. He may sit or lay for long periods with a frozen expression on his face, staring for hours at nothing. If the separation continues he deteriorates further and becomes physically ill and may die (Spitz 1945). In general, males are more severely affected than females (Bowlby, 1940, 1960; Spitz 1945).
Those who were only temporarily removed, once they were returned home would desperately cling to their mother, follow them everywhere, and became extremely fearful when left alone even for short time periods. Those who were deprived of maternal contact for 6 months or more instead behaved in a withdrawn, depressed manner and showed no interest in and were unable to reestablish their normal attachment to their mother. According to J. Bowlby (1940, 1960), children who suffer long term or repeated separations during the first three to five years of life are usually permanently affected.
However, when this same boy (or girl) grows to be an adult, instead of scrying, screaming, and raging helplessly when abandoned or neglected, he may plead as well as threaten, stalk, beat or even kill his former spouse or girlfriend and perhaps even his own children. Or, if its his job he's lost, he may threaten, attack, or kill his boss and coworkers.
Fortunately, it is a small percentage of the population who act on these limbic impulses. Nevertheless, even among those humans who maintain high levels of frontal inhibitory control in regard to all matters of the heart, the amygdala, cingulate, septal nuclei, hypothalamus, inferior temporal lobe, make possible not only intense feelings of emotion for a mate or lover, but correspondingly (at least in some people), an occasional "irrational" urge to throw them in front of a train (metaphorically speaking of course).
Indeed, because it provides the neurological foundation for emotion, attachment, jealously and desire, the limbic system enables human beings not only to coo words of love and sorrow but to experience all the joys, lusts, warmth, thrills, romance, passions, sexual excitement, and "craziness" of "true love."
Summary
In summary, during the amygdaloid maturational phase of early infant development, there is indiscriminant approach and contact seeking, which, if thwarted, may lead to emotional contact-seeking behaviors directed at inanimate objects. During the septal-cingulate stage (see below), indiscriminant social contact seeking is inhibited whereas specific attachments are narrowed, strengthened, reinforced and maintained due to the influences of these structures and the generation of fear response. However, if sufficient maternal emotional stimulation is not provided or is denied for long time periods, the infant will become enraged and then increasingly depressed.
The differential rates of amygdala and septal-cingulate development are crucial in promoting survival and social interaction with significant others. If these structures matured at an earlier age and if the infant experienced fear or wariness, social contact seeking might be prevented or avoided, and the result, might be severe social emotional and limbic system abnormalities (Joseph, 1999b,c) and even death.
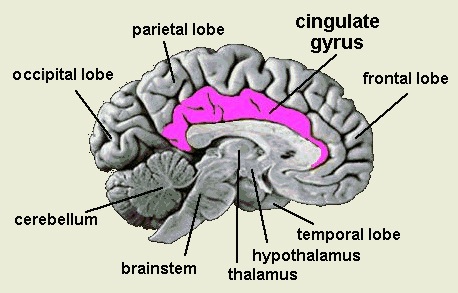
THE CINGULATE GYRUS
The five layered cingulate gyrus sits atop the corpus callosum and can be broadly divided into two segments: the anterior cingulate (areas 24, 25, and 33) which is concerned with vocalizing and emotional and motoric functioning involving the hands, and regulating autonomic and endocrine activities; and the posterior (area 23) cingulate which is involved in visual-spatial and tactile analysis as well as motor output and memory.
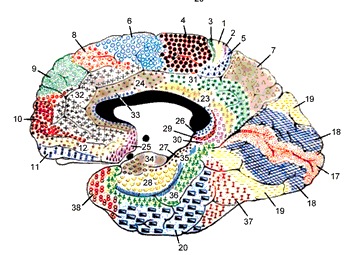

THE POSTERIOR CINGULATE
The posterior cingulate gyrus is richly interconnected with the superior parietal lobe (area 7) the parahippocampal (inferior) temporal and superior temporal lobe (area 22), frontal lobe, caudate, putamen, substantia nigra, pulvinar of the thalamus, and dorsal hypothalamus (Beleydier and Mauguiere 1980; recently reviewed in Devinksy et al. 1995). In addition, the posterior cingulate projects to the red nucleus in the midbrain (which also receives frontal motor fibers) and to the spinal cord. Presumably the posterior cingulate acts to integrate visual input with motoric output and is not concerned with emotional stimuli per se, with the possible exception of nocioceptive functions (Devinksy et al. 1995).
However, the posterior cingulate may also be involved in visual-spatial and memory-cognitive activities, particularly as relates to the body and movement -hence the interconnections with the superior parietal lobe and the parahippocampal gyrus. The posterior cingulate may have, at least in part, evolved from the dorsal hippocampus (e.g. Sanides, 2014).
ANTERIOR CINGULATE GYRUS
The anterior cingulate (areas 24, 25, 33) is associated with processing and modulating the expression of emotional nuances, emotional learning and vocalization, the formation of long-term attachments and maternal behavior, including the initiation of motivationally significant goal directed behavior, as well as influencing and in part regulating endocrine and autonomic activities (reviewed in Devinsky et al., 1995; MacLean 2011).
The anterior cingulate maintains rich interconnections with the septal nuclei, amygdala, hypothalamus, mammilary bodies, hippocampus, dorsal medial nucleus of the thalamus and the periqueductal gray (Beleydier and Mauguiere 1980; Powell, 1978; Powell et al. 1974; Muller-Preuss and Jurgens 1976), as well as with the limbic striatum, caudate and putamen and the frontal motor areas.
The anterior cingulate thus appears to be a supra-modal area that is involved in the integration of motor, tactile, autonomic, and emotional stimuli, as well as with the production of emotional sounds (see below) and the capacity to experience psychological "pain and misery." In fact, the cingulate has long been associated with the experience of psychic and even physical pain (e.g. identifying the affective attributes of noxious and psychic stimuli).
Hence, during the 1930's and 1940's, bilateral cingulotomies were frequently performed to eliminate severe depressive and psychotic states as well as obsessive compulsive tendencies involving the hands (Le Beau, 1954; Whitty and Lewin 1957). However, following surgery, patients tended to become apathetic, emotionally blunted and/or socially and emotionally inappropriate or unresponsive.
Nevertheless, more recently it has been reported that 25% to 30% of patients with obsessive-compulsive disorder who were unresponsive to medication and behavioral treatment, significantly improve following cingulotomy (Baer et al. 1995), though these authors stress surgery is "a last resort treatment."
THE CINGULATE GYRUS AND EMOTIONAL FREE WILL
Electrical stimulation of the anterior cingulate can induce feelings of anxiety, pleasure and fear (Meyer et al. 1973) as well as changes in heart and respiratory rate and blood pressure accompanied by pupil dilation, gonadal and adrenal cortical hormone secretion, penile erection, and aggression (Buchanan and Powell 1982; Devinsky et al. 1995; MacLean 2011). Stimulation also induces a wide range of divergent vocalizations including growling, crying, high pitched cackling, and sounds similar to an infant's separation cry.
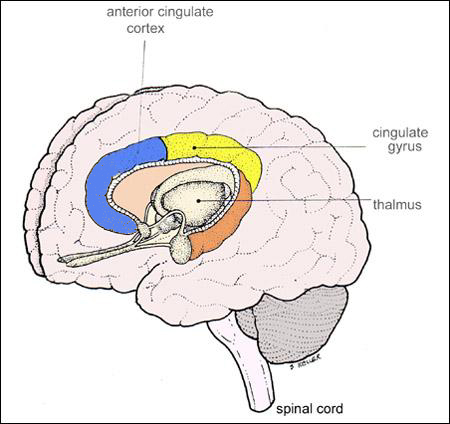
THE ANTERIOR CINGULATE GYRUS AND EMOTIONAL SPEECH
The amygdala is able to produce complex social-emotional vocalizations via the stria terminals and amygdalafugal pathways to the hypothalamus and periaqueductal gray which acts on the oral-laryngeal musculature. The amygdala also increasingly interacts with the rapidly maturing cingulate gyrus which is one of the most vocal structures of the brain (Jurgens, 2011, 2012; MacLean, 2011; Ploog, 2012; Robinson, 1967, 1972) and which becomes activated in response to and when producing human speech (Passingham, 2015; Paulesu et al., 2015; Peterson et al., 1988). For example, the anterior cingulate (as well as the left frontal lobe) become highly active when generating as many words as possible for a given category, e.g. words beginning with "F" (Frith and Dolan, 2015).
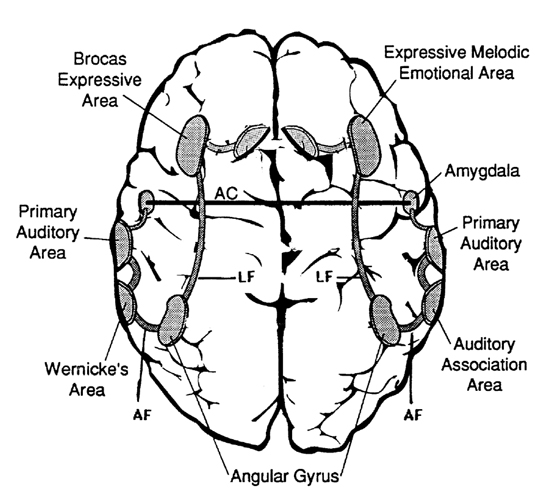
The anterior cingulate gyrus is also directly linked to the neocortical expressive speech areas located in the left and right frontal lobe, as well as with the hypothalamus and periaqueductal gray (Powell, 1978; Powell, Akagi, and Hatton, 1974; Jurgens, 2011, 2014)--which explains why the anterior cingulate and left frontal lobe become active simultaneously during language tasks (Frith and Dolan, 2015; Peterson et al., 1988). Indeed, Broca's expressive motor-speech (and oral-facial/hand) area in the left frontal lobe, and the emotional-melodic speech (and oral-facial/hand) area in the right lateral frontal lobe, appear to have evolved from the anterior cingulate gyrus/medial frontal lobe (Joseph, 1999e). Therefore, the right and left frontal lobes responds to cingulate (and posterior neocortical) impulses by vocalizing. If the anterior cingulate/medial frontal lobe were destroyed, the patient would become mute (Barris and Schuman, 1953; Devinksy et al. 1995; Joseph, 1999a; Laplane et al., 1981; Tow and Whitty, 1953).
Among its many functions, the anterior cingulate (Brodmann's areas 24, 25, 33) is associated with processing and modulating the vocal expression of emotional, melodic, and prosodic nuances, emotional learning, identifying the affective attributes of noxious psychic stimuli, maternal behavior, separation anxiety, and the formation of long-term attachments (Devinsky, Morrell, and Vogt, 1995; Joseph, 1999b; MacLean 2011; Powell, 1978). As based on functional imaging, the anterior cingulate also becomes activated by hot, painful, and noxious stimuli (Casey et al., 2014; Coghill et al., 2014), and, as noted, has been considered by some to be the seat of pain and misery.

Depth electrode stimulation of the anterior cingulate can induce feelings of anxiety, pleasure and fear as well as a wide range of divergent vocalizations including growling, crying, high pitched cackling, laughing, and sounds similar to an infant's separation cry (Devinsky et al. 1995; Jurgens, 2011; MacLean 2011; Meyer et al. 1973; Robinson, 1967). The anterior cingulate also assists in setting thresholds for vocalization (Jurgens and Muller-Preuss, 1977; Robinson, 1967), including modulating some of the prosodic and melodic features which characterize different speech patterns, e.g. happiness vs sadness, and thus laughing vs crying. The vocalizing capabilities of the cingulate are made possible via subcortical connections with the periaqueductal gray (Jurgens 2011, 2014), and its axonal projections to the right and left frontal speech areas.


Whereas vocalizations triggered by excitation of the amygdala, hypothalamus, or septal nuclei are usually accompanied by mood-congruent behaviors (Gloor, 1960; Jurgens, 2011; Robinson, 1967; Ursin and Kaada, 1960) the cingulate is capable of producing exceedingly complex social emotional vocalizations which sometimes have no bearing on the organism's mood or true emotional state (Jurgens, 2011; Jurgens and Muller-Preuss, 1977; Meyer et al. 1973). In addition, completely different emotional calls can be elicited from electrodes which are immediately adjacent (Jurgens, 2011).
Thus the cingulate is capable of considerable vocal flexibility and apparently enables an individual to modulate the emotional-prosodic-melodic components of speech so that one's true feelings can be disguised or emphasized in order to produce sounds suggestive of, for example, sarcasm, incredulity, or hilarity. The anterior cingulate may well contribute to the "deceptive" vocalizations and behaviors demonstrated by innumerable mammalian and avian species (e.g., Hauser, 2015), such as when attempting to lure a predator away from one's helpless infants. Conversely, however, the anterior cingulate, coupled with the right frontal lobe, may also be responsible for the failure to hide one's true feelings, thus generating the complaint: "Its not what you said, but the way you said it!"
As noted, if the anterior cingulate were destroyed, of if the pathways linking this structure with the periaqueductal gray were severed, the individual would become mute (Barris and Schuman, 1953; Devinksy et al. 1995; Jurgens, 2011; Laplane et al. 1981; Tow and Whitty, 1953). However, if the cingulate and surrounding medial tissue were mildly injured, or became abnormally active, emotional-prosodic speech would become exceedingly abnormal and patients may stutter and repeat words such that, in the extreme, they may uncontrollably babble (Devinksy et al. 1995; Dimmer and Luders, 1995; Penfield and Welch, 1951).
THE CINGULATE, THE SEPARATION CRY AND "MOTHERESE"
The medially located cingulate gyrus begins to myelinate around the second postnatal month and achieves an advanced stage of myelination by the end of the first year (Debakan, 2000; Gibson, 1991; Yakovlev and Lecours, 1967); around the same time the amygdala increasingly vocalizes feelings of fear. However, in addition to fear, the anterior cingulate contributes to the experience of unpleasant feelings (Casey et al., 2014; Coghill et al., 2014) including separation anxiety and vocalizes a separation cry which is similar if not identical to that produced by a frightened infant (MacLean 2011; Robinson, 1967). In fact, abnormal activity in the anterior cingulate has in some cases induced not just anxious vocalizations, but infantile behavior, such as assuming the fetal position (Devinksy et al. 1995).
The anterior cingulate is thus responsible for producing complex emotional-prosodic vocalizations, including, perhaps, the prosodic variations which mothers employ when speaking to their babies and vice versa; i.e. "motherese." As is well known, considerable vocalizing typically occurs between mothers and their infants; and the infants of many species will often sing along or produce sounds in accompaniment to those produced by their mothers (Bayart et al., 2011; Hauser, 2015; Jurgens, 2011; Wiener, Bayart, Faull, and Levine, 2011). These interactions appear to be limbically mediated and reinforce and promote mutual vocalization, attachment behaviors, and may contribute to the development of language. Among animal and human mothers, much of this initial mutual sound production consists of exaggerated emotional prosody (Cooper and Aslin, 2011; Fernald, 1991, 2012; Fernald et al., 1989; Hauser, 2015; Jurgens, 2011); i.e. "limbic language."



These emotional-melodic vocalization greatly influence infant-emotional behavior and attention as infants not only produce but prefer and are more responsive to these exaggerated prosodic vocalizations (Cooper and Aslin, 2011; Fernald, 1991). In fact, by 5 months of age infants become quite adept at perceiving and distinguishing between different emotional vocalizations so as to determine the mood state and intentions of the speaker (Fernald, 2015; Haviland and Lelwica, 1987). Likewise, mothers are generally able to determine the mood and desires of her 5-month old offspring when it produces similar emotional vocalizations (D'Odorico, 1984; Wolff, 1969).
In many respects these mutual mother-infant social-emotional interactions appear to be a reflection of the limbic system of the mother communicating with the limbic system of her infant. The infant-neocortex is much too immature to comprehend non-emotional words and sentences.

The female limbic system (and the right frontal-temporal speech areas) are in fact adapted and organized so as to promote social-emotional communication with her young, and with each other. That is, the cingulate gyrus, amygdala and hypothalamus are sexually differentiated such that there is a "male" and a "female" limbic system (see chapter 13). Being in possession of a "female" limbic system presumably confers a superior ability to perceive and express social-emotional nuances and vocalizations (Joseph, 2000a); capacities at which females excell (Burton and Levy, 1989; Brody, 1985; Buck, 1977, 1984; Buck, Miller, and Caul, 1974; Fuchs and Thelan, 1988; Heller and Levy, 2012; Soloman and Ali, 1972; Strayer, 1980). Indeed, in contrast to males, females are not only more emotionally perceptive and expressive, but tend to employ 5-6 different prosodic variations and utilize the higher and fluctuating registers when conversing (Joseph, 2015, 1999e), especially with their infants (Fernald, 2012; Fernald, et al. 1989). Human (as well as non-human) infants are not only more responsive to the emotional-prosody conveyed by a female voice, but are most responsive to the higher as well as fluctuating registers (Fernald, 1985; Hauser, 2015).
However, in contrast to the infant, adult females (and males) also rely on the neocortices of the right frontal-temporal lobe to produce and comprehend emotional-melodic-prosodic vocalization. As noted, over the course of evolution the anterior cingulate appears to have contributed to the evolution of the frontal motor-speech areas, whereas the auditory areas in the superior temporal lobe appear to be evolutionary derivatives of as well as richly interconnected with the amygdala (Joseph, 1999e). The frontal and temporal auditory areas, however, do not begin to significantly mature until around the first year after birth; a process which can take 7 to over 20 years to complete (Blinkov and Glezer, 1968; Brody, et al. 1987; Conel, 1939, 1941; Flechsig, 1901; Huttenlocher, 2011; Yakovlev and Lecours, 1967).
MATERNAL BEHAVIOR & THE EVOLUTION OF INFANT SEPARATION CRIES
Sharks, teleosts, amphibians, and reptiles possess a limbic system, consisting of an amygdala, hippocampus, hypothalamus, and septal nuclei (see chapter 5). It is these limbic nuclei which enable a group of fish to congregate and "school", and which makes it possible for reptiles to form territories which include an alpha female, several sub-females, and a few juveniles. These nuclei promote social attachment and interaction.
However, sharks, fish, and the first amphibians and reptiles, like their modern counterparts lacked the four to five layered cingulate gyrus. Moreover, these creatures do not possess an inner ear or true middle ear, though amphibians and reptiles are attuned to hear low level vibrations and sounds, such as croaking, tails thumped on the ground, and a few distress calls and those of contentedness. Limbic language capabilities are not well developed in these creatures.


As they also lack a cingulate gyrus, amphibians and most but not all reptiles show little or no maternal care, and rarely vocalize. They will also greedily cannibalize their infants who in turn must hide from their parents, and other reptiles, in order to avoid being eaten (MacLean, 2011).
When reptiles began to differentiate and evolve into the repto-mammals some 250 million years ago, and then, twenty-five million years later, when the first tiny dinosaurs (who diverged from a different line of reptiles, the theocondants) began to roam the Earth, major biological alterations occurred involving cranial and post-cranial skeletal structure, mammillary development, thermo-regulation, sexual reproduction, and limbic system function and structure (Bakker, 1971; Brink, 1956; Broom, 1932, Crompton & Jenkins, 1973; Duvall, 1986; Paul, 1988; Quiroga, 1980; Romer, 1966) --all of which coincided with tremendous advances in the ability to engage in audio-vocal communication and the capacity to nurse the young (chapter 5).

It was not until the appearance of the the therapsids, around 200 to 150 million years ago, that mammilary glands, and thus the capacity to nurse, came into being (Duvall, 1986). It was at this time that the middle ear began to undergo tremendous modification and the first rudiments of an inner ear developed (Broom, 1932; Brink, 1956; Crompton & Jenkins, 1973; Romer, 1966). Although the hypothalamus, septal nuclei, and amygdala continued to evolve, it was also around this time that the cingulate gyrus began to appear and increasingly enshroud the dorsal surface of the limbic forebrain -an event which corresponded with the appearance of nursing nipples (Duvall 1986) and the inner ear. When this began to occur, sounds came to serve as a means of purposeful and complex communication, not only between potential mates or predator and prey, but between a mother and her infant (Joseph, 2015, 2014; Maclean, 2011). This ability in turn was probably made possible by the amygdala and in particular, the evolution of the four to five-layered transitional neocortex, the cingulate gyrus.
As noted, it is the limbic system and the interactions of limbic nuclei such as the amygdala and the cingulate gyrus which not only stimulates the desire to communicate, but to form attachments, social groups, and eventually, the formation of the family. In fact, many of the late repto-mammals, as well as some dinosaurs and the later appearing therapsids, lived in packs or social groups, and presumably cared and guarded their young for extended time periods lasting until the juvenile stage (Bakker, 1971; Brink, 1956; Crompton & Jenkins, 1973; Duvall, 1986; Paul, 1988; Romer, 1966). Presumably long term attachments were made possible via the evolution of the anterior cingulate.
As also noted, the first appearance of rudimentary nipples coincided with therapsid development. Hence, one of the hallmarks of this evolutionary transitional stage, some 200 million years ago, was the cingulate gyrus and the first evidence of nursing, maternal feeling, and the creation of large social groups and hunting packs, and what would become the family.
MOTHER INFANT VOCALIZATION
Among mammals and primates the production of sound is very important in regard to infant care, for if an infant becomes lost, separated, or in danger, a mother could not quickly detect this by olfactory-pheromonal cues alone. These conditions would have to be conveyed via a cry of distress or a sound indicative of separation fear and anxiety; which would cause a mother to come running to the rescue. Conversely, vocalizations produced by the mother would enable an infant to continually orient and find its way back if perchance it got lost or separated. Hence, the first forms of complex limbic social-emotional communication may well have been first produced in a maternal context.
As noted, considerable vocalizing typically occurs between mothers and their infants (be they human, primate, or mammal); and the infants of many species will often sing along or produce sounds in accompaniment to those produced by their mothers. These mutual interactions reinforce and promote mutual vocalization which is often initiated by the mother.

In fact, primate females are more likely to vocalize when they are near their infants versus non-kin, and infants are more likely to vocalize when their mother is in view or nearby (Bayart et al. 2011; Jurgens, 2011; Wiener et al. 2011). Similarly, infant primates will loudly protest when separated from their mother so long as she is in view and will quickly cease to vocalize when isolated (Bayart et al. 2011; Wiener, et al. 2011). However, adult males are also more likely to call or cry when in the presence of their mother or an adult female vs an adult male (see Jurgens, 2011). It thus appears that the purpose of these vocalizations are to elicit a vocal response from mother, or an adult female, who in turn is likely to respond with soothing limbic language.
Hence, ontogentically and phylogenetically, the initial production of emotional sounds is limbically based, and increasingly, over the course of evolution, and as evident during early development, the production of these sounds is associated with maternal-infant care, and/or interactions with an adult female. As noted, the cingulate is sexually differentiated (MacLusky et al., 1987, 2014), and regardless of culture, human mothers tend to emphasize and even exaggerate social-emotional, and melodic-prosodic vocal features when interacting with their infants (Fernald, 2012; Fernald et al., 1989), which in turn appears to greatly influence infant emotional behavior and attention (Fernald, 1991). Similarly, human infants prefer listening to and are more responsive to these exaggerated limbic vocalizations, as compared to "normal" adult speech patterns (Cooper and Aslin, 2011) particularly when produced by a female; i.e. by a female cingulate gyrus.

FEMALE SUPERIORITIES IN LIMBIC LANGUAGE
In addition to the cingulate, the amygdala and hypothalamus are also sexually differentiated (Allen and Gorski, 2012; Allen et al., 1989; Blier et al., 1982; Gorski et al. 1978; Goy and McEwin, 1980; Raisman and Field, 1971; Swabb and Fliers, 1985; Swabb and Hoffman, 1988). In addition, the rope of nerve fibers which interconnect the right and left amygdala and inferior temporal lobes (the anterior commissure) is 18% larger in females than males (Allen and Gorski, 2012), which in turn likely contributes to sex differences in language, emotion, and maternal vs paternal behavior. Thus, females tend to produce a greater range of limbic (social-emotional) vocalizations than males (Glass 2012; Joseph, 2015, 2000a; Tannen 1991) and they tend to employ 5-6 different prosodic variations and to utilize the higher registers when conversing. They are also more likely to employ glissando or sliding effects between stressed syllables (Brend, 1975; Coleman, 1971; Edelsky, 2009).
Men tend to be more monotone, employing 2-3 variations on average, most of which hovers around the lower registers (Brend, 1975; Coleman, 1971; Edelsky, 2009). Even when trying to emphasize a point males are less likely to employ melodic extremes but instead tend to speak louder. Perhaps this is why men are perceived as more likely to bellow, roar, or growl, whereas females are perceived as more likely to shriek, squeal, coo, and purr. Nevertheless, although influenced by sex differences in the oral-laryngeal structures, these differential capacities are also reflected in the greater capacity of the female brain to express and perceive these nuances (chapter 7), which also appears to be the case in female primates. Thus female monkeys and apes are more vocal and engage in more social vocalizations, and in fact vocalize more often that males who in turn are more likely to vocalize when threatening or engaged in dominance displays (Cross and Harlow, 1965; Erwin, 1980; Fedigan, 2012; Goodall, 1986, 2011; Mori, 1975; Mitchell, 2009).
It has been repeatedly demonstrated that human females are also more emotionally expressive, and are more perceptive in regard to comprehending emotional verbal nuances (Burton and Levy, 1989; Hall, 1978; Soloman and Ali, 1972). This superior sensitivity includes the ability to feel and express empathy (Burton and Levy, 1989; Safer, 1981). From childhood to adulthood women appear to be much more emotionally expressive than males in general (Brody, 1985; Burton and Levy, 1989; Gilbert, 1969); abilities which confer upon her a greater emotional sensitivity to the needs and feelings of others, especially her babies. These superiorities assist her in being a good mother.

MATERNAL BEHAVIOR, ATTACHMENT, AND THE FEMALE LIMBIC SYSTEM
It has been proposed that these limbic system sex differences are responsible for and are an evolutionary consequence of woman's role in bearing and rearing children and the female desire to form long term attachments, and engage in maternal care and verbal communication (Joseph, 2015). Female humans, primates and mammals apparently find these activities rewarding in-themselves, due to these same limbic system sex differences.
That is, given that the sexually differentiated anterior cingulate, at least in part, evolved in a maternal context and promoted the development of maternal feelings and long term mother-infant attachment, whereas the amygdala and hypothalamus are also sexually differentiated, it appears that these structures may account for why human and non-human female primates differentially respond and desire to nurture, hold, cuddle, and stare at infants. Indeed, female humans, chimps, baboons and rhesus macaques cuddle more and more closely, and are cuddled more by their sisters, mothers and other females (Jensen et al., 1968a, Hansen, 1966; Mitchell, 1968; Goodall 1971, 2011), whereas males are much more resistant to being held, and will kick and fuss, and actively attempt to escape their mothers much more so than females (Elia, 1988; Fedigan, 2012; Freedman, 1974, 1980; Mitchell, 1968, 2009; Goodall 1971, 2011; Kummer, 1971). In part this sex differences also reflects a struggle against potential physical domination which most males find aversive (Joseph, 2015). Hence, be it a male dog, chimpanzee, baboon, or child, they are far more likely than females to struggle, squirm, or resist attempts to hold or pick them up, and may even respond as if they find it aversive.

Mothers are therefore more willing to hold female babies and for longer time periods as they are also easier to calm and are more fun to hold as they seem to enjoy it more than males. Since females demonstrate greater social responsiveness and are more likely to employ facial, vocal and social signals, mothers are more likely to physically, socially and vocally interact with their infant daughters and vice versa (Moss, 1974).
Being similarly socially inclined, mothers find it more socially rewarding and enjoyable to interact with their daughters who are also in possession of a "maternal" (albeit immature) limbic system and cingulate gyrus.
Be it a female chimpanzee, baboon, rhesus macaque, or human, females also begin to demonstrate an extraordinary interest in babies and in play-mothering during even the earliest phases of their own childhood (Devore, 2014; Elia, 1988; Fedigan 2012; Goodall 1971, 2011; Jolly 1972; Kummer, 1971, Mitchell, 2009; Strum, 1987; Suomi, 1972). When girls play together, much of their fantasy and conversation concerns fashion and making out and revolves around adult relationships, including the raising of a family and the behavior and misbehavior of children (their dolls). Babies are of enormous interest to females, be they human, ape or monkey, and social primates and female humans who have babies usually become tremendously popular and the center of attention (Fedigan 2012; Jolly 1972, Mitchell 2009, Strum ,1987). Even among women enslaved in a harem, once she becomes pregnant and has a child, her status is quickly and permanently elevated.
Mothers, grandmothers, young and adolescent females, and even women who describe themselves as "feminists" show much more interest in babies than do men, even when the baby is not their own (Azhn-Waxler et al., 2003; Berman, 2011; Berman and Goodman, 1984; Blakemore, 1981, 1985, 2011; Frodi & Lamb, 1978; Melson and Fogel, 1982; Nash and Fledman, 1981). Adolescent girls spend significantly more time talking about new baby's than boys (Berman, 2011), and mothers spend more time talking about the baby with their daughters than their sons (Berman, 2011).

Girls not only talk more but play and care for their infant sisters and brothers significantly more and show consider amounts of nurturant interest in the babies well being (Blakemore, 2011) even when there has been no request or pressure to do so. Indeed, girls often demonstrate an intrusive interest in babies (Berman, 2003), and will give infants much more care than they require (Ainsworth & Wittig, 1969), as if often the case with mothers (Stewart, 2011). These behaviors also appear to be limbically mediated, as they are demonstrated by females of other species.
Non-human female primates, be it gorilla, chimpanzee, baboon, rhesus macaques, lemur, and so on, will eagerly seek to groom, cuddle, and carry not only their own infants but those of other females (Jolly, 1985; Devore, 2014; Kummer 1971, Strum 1987; Suomi, 1972; Mitchell, 2009; Goodall, 1971). These primates may also spend all day passing them back and forth. Like human females, some will even steal these infants. Those female primates who show the greatest interest, however, are young females who had not yet had babies.
Moreover, among almost all social primates, the birth of a new baby has an extremely excitatory effect on all the other females of the troop who will gather around and touch, stare, hold, and cuddle it. This female interest, of course, is certainly quite adaptive, at least for those living in the dangerous condition of the wild for it insures that if a mother dies another will adopt her baby.
Such behavior is obviously not the result of sexist training for it is typical of almost all social female primates, whereas males, including young males show relatively little interest in babies. For example, boy chimpanzees show little interest in their younger infant siblings, whereas girl chimps become increasingly fascinated and will hold and cuddle them and will attempt to model their mother's interactions with the infant (Goodall, 1971). If a new mother dies but her baby has older male siblings, less than 25% will adopt the little orphan whereas females siblings are quite anxious and happy to take this role.
THE MALE LIMBIC SYSTEM AND INFANT CARE
With the exception of the baboon (Rowell et al., 1968; Kummer, 1968, 1971; Mitchell, 1968, 2009, Fedigan, 2012), lack of interest in infants is characteristic of most social male primates and almost all male mammals, reptiles, amphibians and fish, as well as human fathers and men and boys in general who generally have little or no interest in babies and generally provide little or nurturant care for their own or the children of others (Rossi, 1985; Gordon and Draper, 1982).
In fact, be it male chimps attacking another troop, or male humans attacking other humans, infants are often the victim of male aggression. Males will kill other humans including the babies of those who have done them no harm.

Of course, there are always exceptions; particularly among males who may possess a "female" limbic system. Rather, like other social primates, boys seek boys for playmates and engage in considerable amounts of rough housing, wrestling, and hitting; behavior that is completely inappropriate in regard to infant interactions. When boys or male primates begin to separate from their mothers, they show no interest in younger siblings but seek out adolescent and adult males to play with. Although they may on occasion seek nurturance they seldom provide it in return.
Human males and fathers rarely behave in any manner that approximates normal female maternal behavior (Belsky et al., 1984; Clarke-Stewart, 1978; Frodi et al., 1982) as this is simply not an activity they find interesting, pleasurable or rewarding. This is why, for example, child care professions and those jobs involving high levels of child interactions, such as elementary school teacher, are overwhelmingly made up of women (Gordon and Draper, 1982); a function not of pay but lack of heterosexual-male interest (Blakemore et al., 1988). Rather, fathers and adult heterosexual males tend to express interest in younger males (and females) only when they reach adolescence, and this is also true of most male primates.
Given that these sex differences are obviously innate, it could therefore be argued that in contrast to male humans, primates, and mammals who have little or no interest in child care, that the female limbic system is designed to promote these interests. Just as the male limbic system rewards males for engaging in competitive and aggressive actions (see chapter 13), the female limbic system probably generates rewarding feelings, coupled with appropriate emotional vocalizations, when females look at, hold, care for, and form attachments to their babies, infants, young children. Although this has yet to be determined, the female limbic system probably contains nuclei, neural networks, and individual neurons which respond selectively to infant visual and auditory related stimuli; e.g. baby faces, infant cries.
Again, consider that the anterior cingulate, in part, evolved in a "maternal" context and acts to promote the development of maternal behavior and mother-infant communication. Indeed, sex specific structural differences in the limbic system probably account in large part for most all sex differences in emotionality and related behavior, including childcare, the desire to have and nurture babies, and the greater female propensity for developing affective and mood disorders. However, these sex differences also make her a more communicative mother.
CINGULATE MATERNAL INFANT COMMUNICATION
The anterior cingulate gyrus, in conjunction with the amygdala and right frontal lobe, appears to provide the foundation for mother-infant communication, the generation of separation anxiety, as well as the desire to provide as well as receive prolonged maternal care (Davidson and Fox, 1989; Joseph, 2015, MacLean, 2011). Long-term mother-infant communication and prolonged maternal care is unique to human and non-human primates, as well as some mammals (e.g. Hauser, 2015), and appears to be directly associated with the rather recent evolution of the five-layered neo-limbic mammalian cingulate gyrus (Joseph, 2015, MacLean, 2011). Again, animals lacking the more recently evolved cingulate gyrus, but who possess a hypothalamus, amygdala, and brainstem (e.g. such as reptiles, amphibians, teleosts, and sharks) fail to provide even short-term maternal care and sometimes cannibalize their young.
As noted, with the evolution of the cingulate and mammal-like therapsids, it appears that vocalization came to serve as a means of complex communication, not only between potential mates or predator and prey, but between mother and infant (for related discussion see MacLean, 2011; Ploog, 2012). Hence, in humans, whereas the anterior cingulate is one of the most vocal structures of the brain and becomes highly active when speaking (Frith and Dolan, 2015; Passingham, 2015; Paulesu et al., 2015; Peterson et al., 1988), destruction of the anterior cingulate abolishes emotional speech production, and results in severe abnormalities in social and emotional behavior and a loss of maternal responsiveness (Barris and Schuman, 1953; Laplane et al. 1981; Maclean, 2011; Tow and Whitty, 1953). Behavior, in fact, becomes reptilian, and human and non-human primates become mute, cease to groom or show acts of affection, and treat their infants as if they were inanimate objects that might be walked on and discarded. In non-human primates, the majority of infants whose mothers have suffered anterior cingulate destruction, die from lack of care (MacLean, 2011).
BABBLING, LIMBIC LANGUAGE, AND NEUROANATOMICAL MATURATIONAL EVENTS
The capacity to vocalize is initially the province of the brainstem and midbrain periaqueductal gray which responds reflexively to the immature hypothalamus. Thus, for the first 30-days following birth, infants tend to cry, cough, belch, grunt, and express displeasure and distress. These initial sounds are likely produced reflexively by the periaqueductal gray, perhaps in reaction to the hypothalamus which may trigger crying when experiencing hunger or thirst. However, as the brainstem, hypothalamus, and then the amygdala and cingulate continue to mature the infant begins to babble and increasingly expresses feelings of pleasure and other social-emotional nuances.
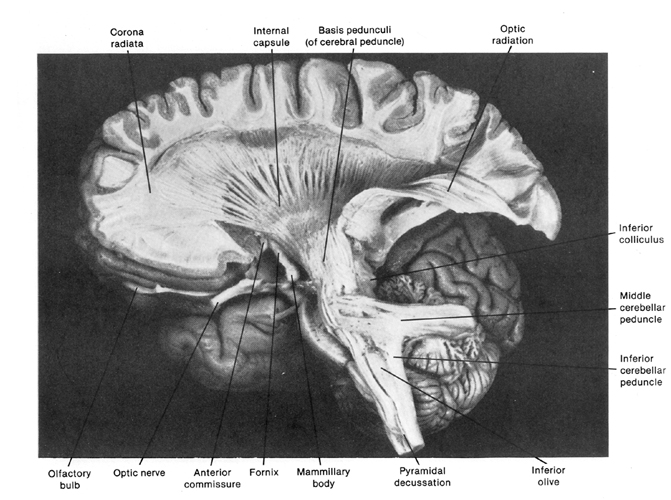
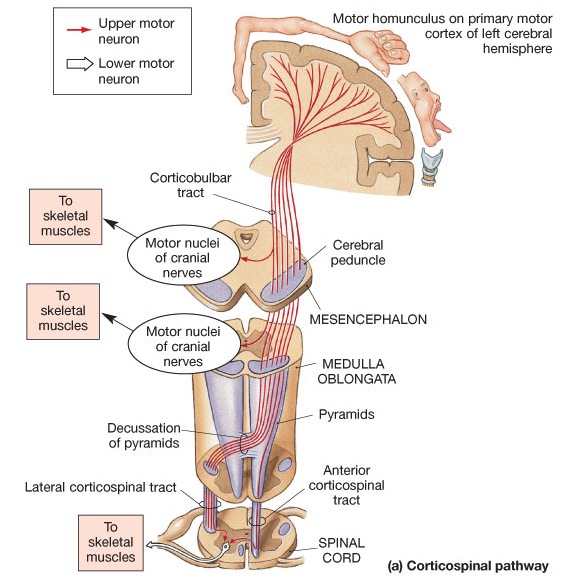
For the first six months of life, with the exception of the somatomotor areas, much of the neocortex is so immature that its influences are negligible. However, the somatomotor areas begin to mature quite early; reflected in dendritic and pyramidal neocortical development (Flechsig, 1901; Joseph, 1982; Gibson, 1991; Gilles et al. 2003; Scheibel, 1991) and the growth and myelination of the corticospinal tracts which invades the brainstem several months before birth (Kertesz and Geschwind, 1971; Yakovlev and Rakic, 1966). However, the corticospinal tracts (which project from the motor areas to the brainstem and spinal cord), take well over a year to reach advanced levels of myelination (Yakovlev and Lecours, 1967).
The myelination of the corticospinal tract coincides with the descent of the larynx, the myelinization and development of the amygdala and the amygdalafugal brainstem pathway, and later, the maturation of the cingulate gyrus and its pyramidal brainstem pathways (Debakan, 2000; Langworthy, 1937; Yakovlev and Lecours, 1967; Yakovlev, and Rakic, 1966). These overlapping maturational and physical events also coincide with vocal development and the onset of early and late babbling followed by canonical and jargon babbling.
Early Babbling, Probable Meanings, and Prosody
By 2-3 months of age amygdala-brainstem pyramidal fibers as well as corticospinal axons have already begun to myelinate. These maturational events coincide with an initial shift in the emotional utterances of the infant which become progressively complex and prosodic and increasingly subject to sequencing and segmentation. The infant begins to "coo," "goo," and babble.
Specifically, as the amygdala (followed by the anterior cingulate) matures and begins establishing hierarchical control over the hypothalamus, midbrain periaqueductal gray, and the brainstem masticatory centers with which it maintains a massive fiber pathway (Takeuchi et al., 1988) the infant will laugh, becomes increasingly oral, displays genuine pleasure, stares and smiles at the human face, and while so doing, will phonate and babble.

This early babbling stage generally involves the repetition of pleasant friction and voicing sounds which tend to be produced while making face-to-face and eye-to-eye contact and while engaged in social interaction (Kent and Miolo, 1995). Moreover, whereas the expression of pleasant sounds are in the ascendant, crying tends to become less frequent but more variable in tone, and can be differentiated into requests, calls, and sounds of discomfort (D'Odorico, 1984; Wolff, 1969).
As the amygdala, corticospinal tracts, and cingulate continue to mature, and the larynx continues to assume an adult pattern of orientation, the infant not only babbles but vocalizes a variety of sounds which increasingly convey probable meanings which may signify to the listener a variety of diffuse feelings and needs (D'Odorico, 1984; Wolff, 1969). Infants produce different noncry vocalizations depending on context and in reaction to people vs objects. The 3-4 month old infant can in fact produce at least four different pitch contours differening in fundamental frequency, each of which conveys probable meanings regarding affective state (Fernald, 2012; Hauser, 2015).
For example, if the 4 month old infant coos and babbles "mama," to the primary caretake (and depending on context, facial expression, and prosody/fundamental frequency) this may be interpreted to mean: "mama come here," "mama I hurt," "mama I thirst," etc. (e.g., D'Odorico, 1984; Fernald, 2012; Joseph 1982, Piaget, 1952; Vygotsky, 1962; Wolff, 1969). Hence, although the infant's utterances are not referential and may at times represent little more than the random universal babbling produced by all infants, they can also convey meaning and serve as a means of communicating with the primary caretaker (see Fernald, 2012; Hauser, 2015, for related discussion).
MATURATION OF THE AMYGDALA, CINGULATE, AND EARLY AND LATE BABBLING
As detailed above, the increased complexity of the infant's utterances likely reflect the maturational influences of the amygdala, and later, the cingulate, coupled with physiological/anatomical changes in the position and orientation of the larynx. These same maturational events are also correlated with different babbling stages.
Activation of the amygdala can trigger lip smacking, rhythmic jaw movement, babbling, and manidibular-teeth "chattering" (Gloor, 2015), which, when coupled with sound production may induce babbling. Infant's display similar behaviors, which is presumably a direct consequence of the immaturity of the amygdala and its projections to the masticatory centers in the brainstem. Indeed, early babbling appears to be a direct function of reflexive and spontaneous jaw movement (e.g, MacNeilage and Davis, 2011; Moore and Ruark, 1996; Weiss, 1951) and lip smacking. Hence, early babbling may reflect immature amygdala (as well as amygdala-striatal and motor neocortical) influences on the brainstem masticatory centers and the periaqueductal gray which reflexively triggers the oral musculature thereby inducing rhythmic movement of the jaw.
"Early" babbling is soon replaced by "late" babbling which has its onset around 4 months of age (de Boysson-Bardies et al.,1981; Oller, 1980; Oller and Lunch, 2012). Late babbling is sometimes described as "repetitive babbling" (Mitchell and Kent, 2011), and at later stages of development may include the repetitive production of CV syllables in which the same consonant is repeated, such as "dadada." As noted, electrical stimulation in the cingulate and surrounding medial frontal tissues can trigger the repetitive babbling of certain words and sounds, such as "dadadada" (Dimmer and Luders, 1995; Penfield and Welch, 1951).
The development of late babbling also occurs in conjunction with the infant's increased ability to produce sophisticated social-emotional nuances, and appears to be associated with increasing cingulate (as well as amygdala) influences. For example, around 4-months, the infant's intonational-melodic vocal repertoire becomes more elaborate and tied to a variety of specific feeling states (Piaget, 1952); which may reflect increasing amygdala maturational dominance.
However, over the ensuing months vocalizations also begin to assume an imitative quality (Nakazima, 1980) which are often context specific but which do not necessarily reflect the infant's internal state--a characteristic also of cingulate vocalizations. Some infant vocalizations are produced in mimicry and in play (Piaget, 1952), and the cingulate is also associated with mimicry and play behavior (MacLean, 2011). The late babbling stage has also been repeatedly described as a form of "sound play;" an activity which increasingly contributes to phonetic development (de Boysson-Bardies et al. 1981; Ferguson and Macken, 2003). As the cingulate is associated with mimicry and the onset of play behavior (MacLean, 2011) and since the production of these sounds do not necessarily reflect the infant's true emotional state, the cingulate, therefore, is implicated in all aspects of the late babbling stage.
As noted, late, repetitive babbling consists of sequences of CV syllables in which the same consonant is repeated, e.g. dadadada. However, infants will also produce what has been referred to as non-duplicated, variegated, and concatenated babbling (see Oller, 1980; Mitchell and Kent, 2011). That is, they will increasingly vocalize phonetically-varied-multisyllables which are periodically inserted into what is otherwise a repetitive sequences of CV syllables. These latter tendencies in fact, appear to be present even at the onset of the late babbling stage (Mitchell and Kent, 2011).
Repetitive, late babbling increases in frequency until around the seventh to tenth month of postnatal development (de Boysson-Bardies et al. 1981; Ferguson and Macken, 2003; Nakazima, 1980; Oller, 1980; Oller and Lunch, 2012), at which point the tendency to produce phonetically varied multisyllables becomes dominant. Thus the late babbling stage comes to be largely replaced by what has been termed "variagated" or "canonical" babbling (Oller, 1980; Oller and Lunch, 2012) which in turn is followed by "jargon" babbling (around 12 months).
Likewise, during these latter babbling periods the infant is also able to express fear, separation anxiety, and a variety of subtle social-emotional nuances. Although it appears that there is considerable overlap between these so called stages (e.g. Mitchell and Kent, 2011), the onset and increased frequency of late babbling followed by the increased production of variagated/canonical and then jargon babbling (Nakazima, 1980; Oller, 1980; Oller and Lunch, 2012), coincides with and appears to reflect the (overlapping) maturational influences of the amygdala followed by the anterior cingulate and then the frontal motor neocortice (which produce true speech).

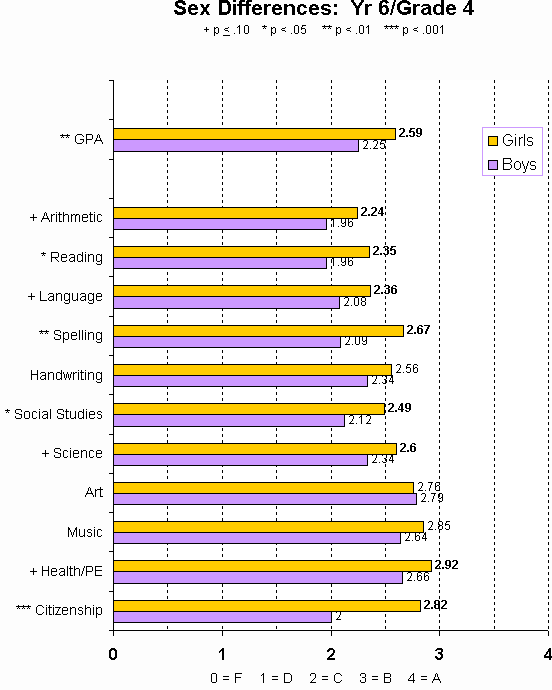
BABBLING AND AMYGDALA, CINGULATE, NEOCORTICAL MATURATION
The maturation of the amygdala is associated with the onset of early babbling, whereas the anterior cingulate likely contributes to the development of late (repetitive) babbling, and the increased production of canonical/variagated babbling. These latter maturational events, however, also correspond to those taking place in the motor areas of the neocortex and may represent increasing pyramidal influences on the brainstem and oral-laryngeal musculature. In fact, jargon babbling appears to be a function of the immature neocortical somatomotor areas slowly gaining control over the limbic system, midbrain inferior-colliculus, and periaqueductal gray (see also Herschkowitz et al. 2015).
For example, pyramidal fibers from the somatomotor neocortex to the brainstem become increasingly well myelinated from 4 to 12 months of age (Debakan, 2000; Yakovlev and Lecours, 1967). Likewise, the somatomotor areas of the neocortex begin to rapidly mature around the first postnatal year (Brody et al. 1987; Chi, Dooling, and Gilles, 1977; Gilles et al. 2003; Scheibel, 1991, 2015). Hence, the neocortex likely increasingly contributes to the development of jargon babbling, especially around one year of age.
Moreover, just as the pyramidal/corticospinal tracts as well as the somatomotor areas continue to mature and myelinate over the first and second years (Conel, 1937, 1941; Debakan, 2000; Yakovlev and Lecours, 1967) and beyond (Paus et al., 1999), babbling continues throughout the first and second years. It is during these same time periods in which the child gradually acquires and develops the phonetic structure which underlies speech production (de Boysson-Bardies et al. 1981; Oller, 1980; Oller and Lunch, 2012). This implies considerable forebrain as well as right and left neocortical influences over vocal behavior (see below).
With increasingly neocortical control, what appears to be a "new and unique motor skill" slowly emerges (Moore and Ruark, 1996) which directly contributes to the development of speech. That is, once the neocortical speech area establish hierarchical control, and begin to program the oral-laryngeal motor areas, a new form of (neural-muscular) vocalization emerges which appears somewhat distinct from its precursors (e.g. Moore and Ruark, 1996). The infant begins to speak their first words.
IMMATURITY OF THE NEOCORTICAL SPEECH AREAS AND JARGON BABBLING
The overlapping transition from the initial amygdala-brainstem-babbling stage to late babbling, around 4 months of age, may represent the onset of an amygdala to cingulate transition in vocalization control. Around 7-10 months, and as the cingulate become ascendant, the late babbling stage is transformed into the overlapping (cingulate-) canonical babbling stage. Canonical babbling, such as "dadada," involves the rhythmic and repetitive production of identical consonant vowel (CV) syllables (da, ba). Temporal sequencing motor capabilities are the province of the left hemisphere as well as the anterior cingulate gyrus. As the neocortex becomes ascendant and increasingly exercises (albeit immature) motor control, (cingulate-) canonical babblings is followed by (neocortical-) jargon babbling around one year of age.
The somewhat latter to appear "jargon" (or "conversational") babble resembles actual speech. At a distance it sounds as if the infant were talking, although the infant is in fact spouting prosodically sophisticated nonsense. Jargon babbling appears to reflect the increasing influences of the still exceedingly immature right and left frontal-temporal neocortex.
Jargon babbling is generally produced in a social context and while making eye contact and in many respects is similar if not identical to Wernicke's ("jargon") aphasia. Jargon aphasia is associated with severe injuries to Wernicke's area and the temporal-parietal junction which transmits abnormal streams of neologistic jargon to Broca's area (via the arcuate fasciculus) which then spouts nonsense words; a condition also referred to as "fluent aphasia." However, rather than a function of brain damage, jargon babbling is probably an indication of the extreme immaturity of the superior temporal lobe and Wernicke's area (as well as the left frontal lobe).
In general, "jargon" babbling (like jargon aphasia) consists of normal stress and intonational patterns that have been abnormally sequenced and chunked together. Hence, the infant expresses normal emotional-melodic prosody (probably via the limbic systm and right frontal lobe) coupled with nonsense words (via Broca's area), which makes it sound as if the child is truly attempting to communicate. Indeed, while jargon babbling the infant may appear to be engaging in a two-way discussion, or they may seem to be making requests for help or a desire to direct the other's attention to some object or activity (Kent and Miolo, 1995). Patient's with Wernicke's aphasia behave in an identical fashion. As with Wernicke's aphasics, children who jargon babble are attempting to communicate yet appear oblivious (or agnosic) to the fact they what they are spouting is emotionally meaningful verbal nonsense.
As jargon babbling coincides with the emergence of the infant's first words, this babbling stage heralds a hierarchical shift from the limbic system to the neocortex which increasingly acts to perceive and express social-emotional nuances and to punctuate and impose temporal sequences on what had been purely emotional speech. That is, beginning around 12 months and over the following years, the neocortex of the right hemisphere increasingly subserves the perception and expression of emotional-melodic-prosodic speech--as it does in adulthood (Gorelick and Ross, 1987; Heilman et al. 1975; Lalande et al. 2012; Ross, 2015; Shapiro and Danly, 1985; Tucker et al. 1977). By contrast, the left hemisphere motor areas increasingly act to punctuate, segment, and impose temporal sequences on sound production and perception (Joseph, 1982, 1988a, 1999e). Thus, as the neocortex of the left hemisphere gains motor control over the brainstem and oral musculature, what had been purely limbic language becomes grammatical, vocabulary-rich human speech.
RIGHT AND LEFT HEMISPHERE LANGUAGE
ACQUISITION
During the last phase of fetal development the corticospinal tract of the left hemisphere descends and establishes brainstem and spinal synaptic contact in advance of the right (Kertesz and Geschwind, 1971; Yakovlev, and Rakic, 1966). These include pyramidal axons from the left amygdala (and amygdala-striatum), which is more concerned with motor control than the right amygdala, as reflected in its heavier concentrations of dopamine (Bradbury, Costall, Domeney, and Naylor, 1985; Stevens, 2012). Moreover, the left frontal primary motor areas representing the oral-facial and laryngeal muscles and cranial nerves, matures more rapidly than the right frontal primary motor areas (Joseph, 1982; Schiebel, 1991, 2015). In consequence, the left hemisphere is provided with a competitive expressive-motor advantage over the right, which is demonstrated not only in control over the oral-laryngeal musculature, but handedness.
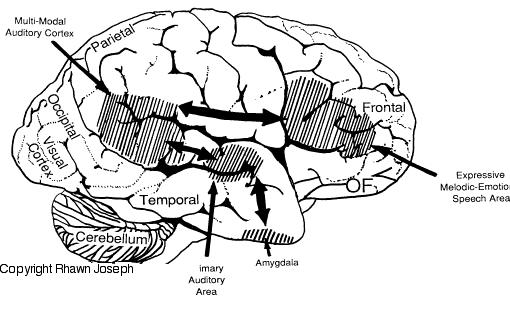

By contrast, right frontal non-motor and secondary-motor gyral development, and right frontal dendritic growth within the emotional, melodic, prosodic speech area, initially develops faster than Broca's area (Chi et al. 1977; Gilles et al. 2003; Scheibel, 1991, 2015). Although the right hemisphere remains functionally dominant in the non-motor, non-linguistic, sensory-affective modalities (Chiron et al., 2015; Joseph, 1982), neuronatomically, Broca's area overtakes its right-sided counterpart around one year of age (Scheibel, 1991, 2015) and the infant increasingly produces jargon babbling, and then later, its first words.
Specifically, due to the earlier development of the right cerebral sensory receptive and non-motor areas, which in turn correspond to the development of the limbic system, sensory-limbic-receptive and expressive emotional functions come to be hierarchically represented in the right half of the brain (Joseph, 1982, 1988a). By contrast, the motoric and associated temporal-sequential aspects of expressive functioning, including right hand dominance, becomes the province of the left hemisphere whose motor areas and corticospinal tract develop more rapidly, which enable it to overtake, and thus gain a competitive advantage over the right.
As the left hemisphere matures and establishes dominance over motor control and expressive speech, temporal sequences and syllabication are imposed on emotional, intonational, and melodic vocalizations which come to be puctuated, segmented, reorganized, classified, and shaped so that vowel, consonantal elements, and words are produced.
Specifically, Broca's expressive speech area, upon receiving linguistic input from the inferior parietal lobule and Wernicke's area (chapter 11) and emotional-prosodic input via the cingulate/medial frontal lobe (Joseph, 2015, 1999e), acts on the adjacent primary and secondary frontal motor areas which subserve the oral-laryngeal musculature (Foerster, 1936; Fox, 1995; LeBlanc, 2012; Petersen, Fox, Posner, Mintum, and Raichle, 1989). In this manner, speech units are motorically organized and vocalized. Thus, left hemisphere speech comes to be superimposed over limbic (and right hemisphere) speech and by one year of age a second form of language emerges, initially one word at a time.
However, as the left and right frontal and temporal areas interact interhemispherically via the corpus callosum, and intra-hemispherically through the arcuate fasciculus, and as these neocortices are linked with the amygdala and cingulate gyrus, language remains emotional and melodic even as it becomes increasingly grammatical and descriptive. Thus even among adults, language remains emotional, melodic, and prosodic, as these nuances continue to be produced not only by the limbic system, but by the right frontal lobe.
CONCLUSIONS
Based on an extensive review of animal and human studies, the preponderance of evidence indicates that the roots of language and social emotional and maternal behavior, originate within the limbic system. Laughter, fear, cries, warning calls, and a variety of emotional vocalizations have been produced via electrode stimulation of wide areas of the limbic system including the hypothalamus, amygdala, cingulate gyrus, and septal nucleus; and these same areas often become activated in response to certain emotional sounds. The limbic system is more vocal than any other part of the brain.
Nevertheless, the type of vocalizations elicited, in general, depends upon which limbic nuclei has been activated. This is because different limbic nuclei, and in fact, different divisions within these nuclei, subserve unique functions and maintain different anatomical interconnections with various regions of the brain. In addition, some areas are more evolutionarily advanced such as the five-layered cingulate gyrus, and/or have expanded and added additional nuclei, such as the lateral amygdala. The vocalizations and emotional behaviors mediated by these recent evolutionary acquisitions are also more complex than those produced by more ancient nuclei such as the medial hypothalamus and brainstem periaqueductal gray.
In some instances ontogeny replicates phylogeny and over the course of early development the medial hypothalamus (and periaqueductal gray) becomes functionally active and produces primitive vocalizations indicating distress, and later, pleasure. The later to develop medial and lateral amygdala experiences, perceives, and vocalizes joy, wariness, anger, and fear, whereas the neo-limbic cingulate gyrus is capable of producing a wide range of complex social emotional vocalizations such as separation anxiety and those which disguise the individual's true feelings.
As these nuclei and structure interact and mature at different and overlapping rates, over the course of early development the infant's emotional and babbling repertoire expands as does its ability to perceive, vocalize and express social-emotional nuances. The acquisition of infant speech, and the progressive expansion and increased complexity of limbic speech and social emotional behaviors parallels the maturation of these limbic structures.
Likewise, since all humans are in possession of a limbic system which is organized and which develops in a similar manner, the emotional and babbling precursors to language also appear to develop and are expressed in a similar manner regardless of culture. Thus early babbling is associated with the amygdala, late and canonical babbling with the cingulate, and jargon babbling with increasing, albeit immature neocortical influences.
As the neocortex matures and the amygdala and cingulate gyrus establish interconnections with the superior temporal and frontal lobe, emotional speech and limbic language is slowly transformed into segmented units of prosodic speech and grammatical utterances. That is, the maturing right frontal lobe, via the emotional-melodic speech area, hierarchically comes to express emotional-melodic and prosodic nuances.
By contrast, the left frontal motor and Broca's expressive speech areas and the left cerebral inferior parietal lobule increasingly punctuate, fractionate, and impose temporal sequences onto the stress, pitch, and melodic intonational contours of the infant's speech output. Hence, vowel, consonantal elements, and then words are produced. Left and right hemisphere speech comes to be superimposed over limbic speech and by one year of age a second form of language emerges, initially one word at a time.
Although language is commonly associated with the left hemisphere and is discussed in terms of grammar and vocabulary, the underlying foundations are and remain emotional and have their source in the limbic system. It is limbic (and right hemisphere) emotional mediation which explains why patients suffering from severe left hemisphere injury and profound receptive or expressive aphasia retain the capacity to express and comprehend these nuances and the meanings they imply (Boller et al., 2009; Boller and Green, 1982; Joseph, 1982, 1988a; Smith, 1966; Smith and Burklund, 1966).
It is due to these same limbic linguistic nuances which enable speakers to convey, and listeners to comprehend, the connotative and contextual implications of what is being said, even when words have been filtered or eliminated (Blumstein and Cooper, 1974; DeUrso, Denes, Testa, and Semenza, 1986; Dwyer and Rinn, 1981; Kramer, 2014), or when interacting with those from other cultures and who speak foreign dialects (Beier and Zautra, 1972; Fernald, 2012; Joseph, 1988a, 2015; Kramer, 2014). Again, consider the famous aside: "I don't know what they're saying, but I sure don't like the sound of it."
Language is both emotional and grammatically descriptive, limbic and neocortical. It is precisely because all humans possess a limbic system which is organized, and which develops in an identical fashion, that a listener is able to comprehend not only the content and grammar of what is said, but the emotion and melody of how it is said --what a speaker feels, even when that speaker is a cooing, gooing, babbling infant.












