Rhawn Gabriel Joseph, Ph.D.
The brainstem is an exceedingly ancient structure. It can be subdivided and consists of the medulla, pons and midbrain, the reticular formation, and monoaminergic neurotransmitter systems as well as the cranial nerves and associated nuclei. Straddling the brainstem is the cerebellum which is an outgrowth of the vestibular system.
The nuclei and fiber pathways of the brainstem subserve a variety of divergent as well as interrelated sensory and motor functions. These include the mediation and control of attention, arousal, the sleep cycle, heart rate, breathing, balance, gross axial movement, the initiation and coordination of eye, jaw, tongue, and head movement, as well as visual, somesthetic, gustatory, and auditory perception (Blessing, 2011; Cowie et al. 2009; Davidson & Bender 2008; Erickson et al. 2009; Johnson et al. 2009; Masino 1992; Rhode & Greenberg 2009). Many brainstem functions, however, are performed in a "clock-like" rhythmic, reflexive, or stereotyped manner, and in a fashion that does not necessitate thinking or planning, e.g., the beating of the heart, walking, chewing, breathing, sleeping, dreaming, and waking.
Given it's exceedingly long and ancient evolutionary history, not surprisingly, many brainstem functions occur without the aid of consciousness or awareness, or even forebrain/neocortical participation. That is, the motor programs which subserve many of these basic functions are essentially hardwired and need only be activated in order for the associated motor acts to be performed. Moreover, many brainstem activities are rhythmical and wax and wane in accordance with the their own intrinsic rhythms; e.g. sleep, dreaming, breathing, heart rate, etc..
OVERVIEW: GROSS ANATOMY
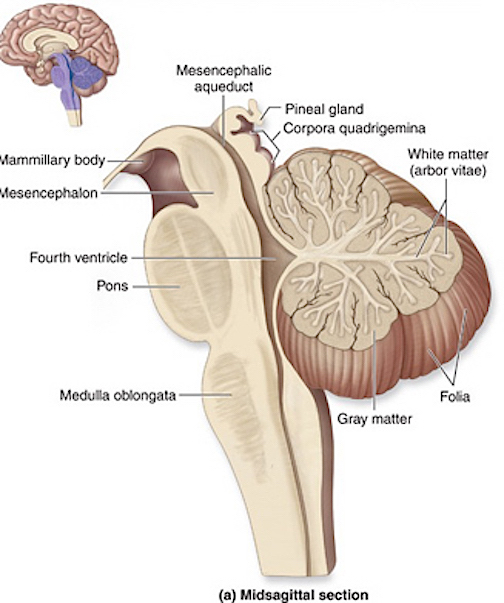
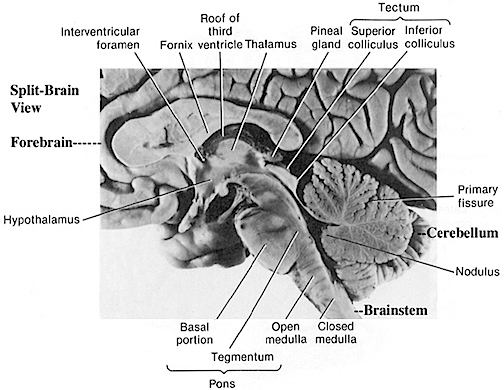
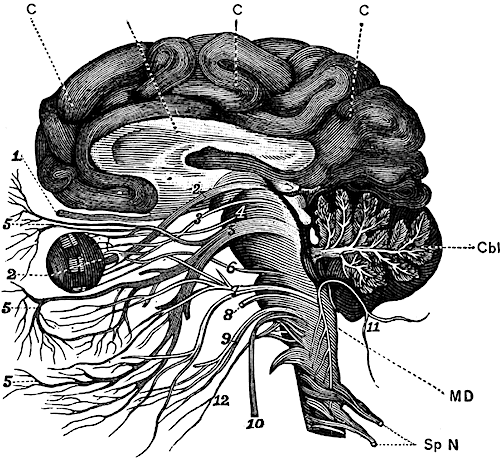
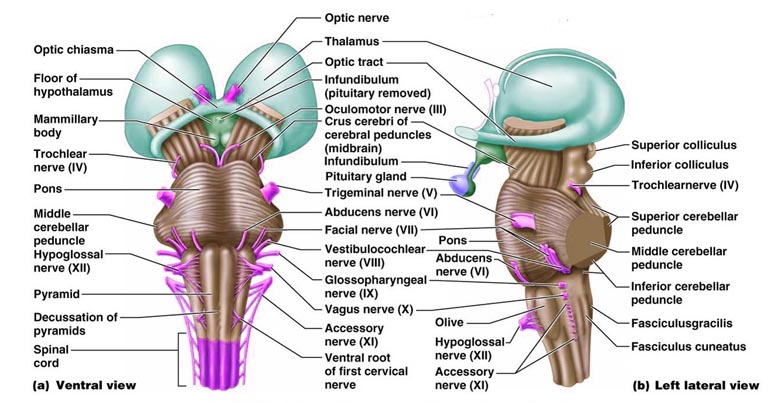
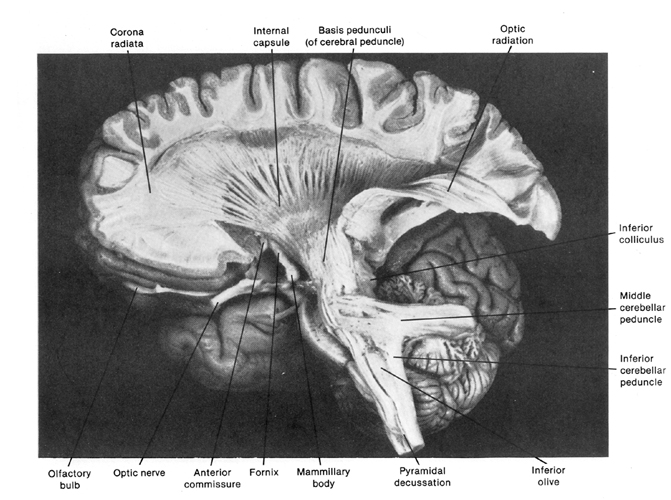
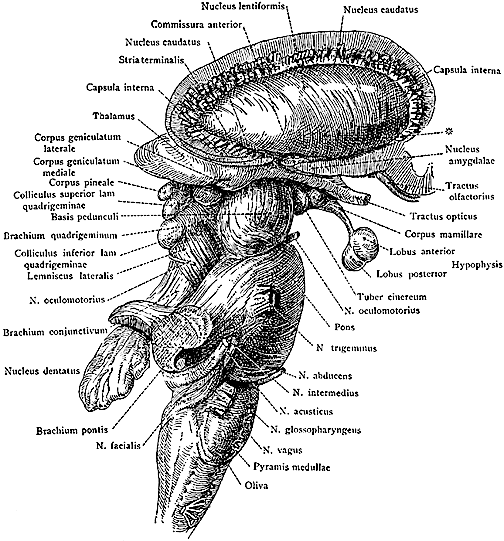
The brainstem consists of the medulla (myelencephalon), the pons (metencephalon), and the midbrain (mesencephalon). Straddling the dorsal surface of the pons is the cerebellum and spanning the length of the brainstem are the nuclei for cranial nerves 3-12 (see Carpenter 2008; Parent 1995) which will be briefly reviewed below.
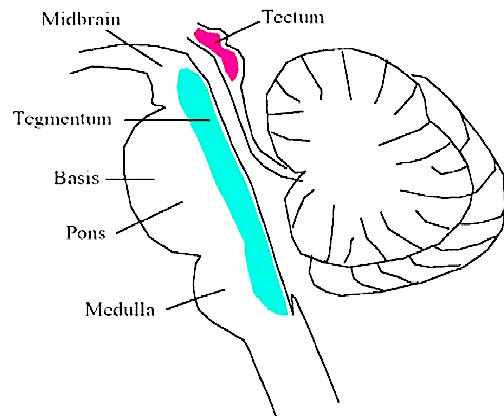
The most rostral portion of the brainstem, i.e. the midbrain merges with the diencephalon which in turn is surrounded by the cerebral hemispheres. The more posterior portion of the midbrain consists of the superior (visual) and inferior (auditory) collicululi which are directly related to the thalamus, and the red nucleus which receives motor fibers from the cerebellum and frontal and parietal lobe. The dorsal roof of the midbrain is referred to as the tegmentum (which produces dopamine) whereas more centrally located is the substantia nigra, a major source of corpus striatal dopamine.
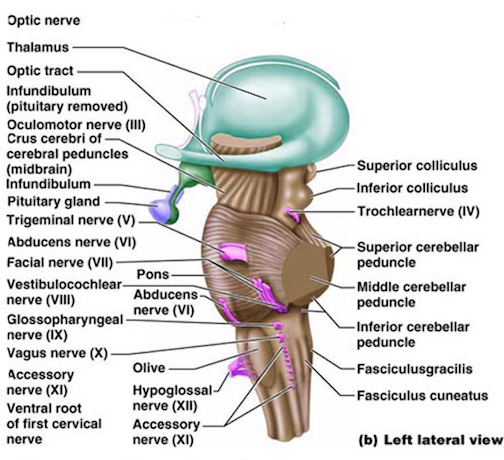
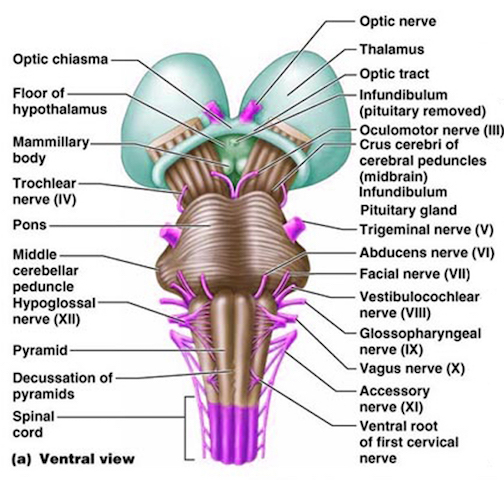
The pons represents the most rostral portion of the "hindbrain" and it is separated from the midbrain via the superior pontine sulcus, and from the medulla by the inferior pontine sulcus. The two components of the vestibulocochlear nerve (VIII), and the facial (VII), abducens (VI) -which also lies in the floor of the fourth ventricle- and the trigeminal nerve (V) are the cranial nerve nuclei associated with the pons.
The medulla is the most caudal portion of the brainstem. The transition from spinal cord to medulla, however, is not obvious, though located at the medulla-spinal junction are the paired medullary pyramids through which course the cortico-spinal/pyramidal tract as they cross over before descending into the spinal cord. Basically the medulla extends from the level of the foramen magnum to the inferior pontine sulcus. The hypoglossal (XII), accessory (XI), vagus (X), and glossopharygeal (IX), cranial nerves are associated with the medulla, whereas the vestibulocochlear (VIII) nerve emerges at the junction of the medulla, pons and cerebellum.
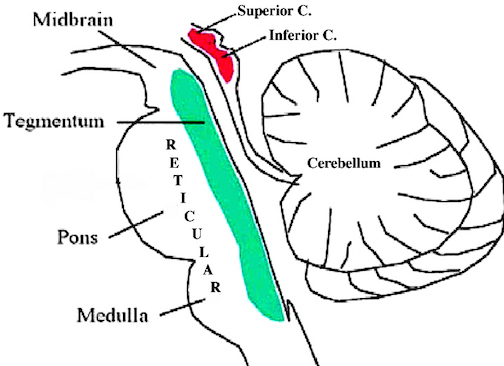
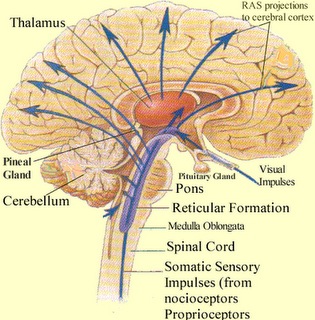
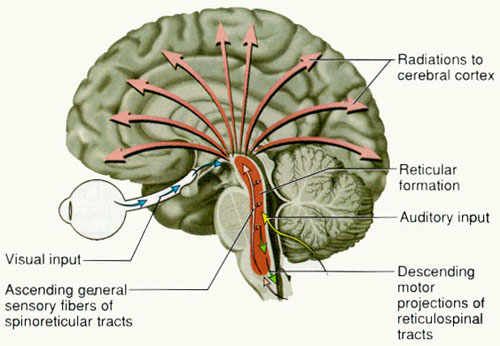
Spanning the medial and lateral lengths of the brainstem is a complex reticulum of richly interconnected cells with long ascending and descending axons, collectively referred to as the reticular activating system, which is concerned with generalized and selective arousal and activation of the neuroaxis. The pontine tegmentum and the more dorsal portion of the midbrain tegmentum is also considered part of the reticular formation through which course ascending sensory fibers and descending motor fibers.
Neurons of the reticular formation receive specific sensory input from the skin, muscles, joints, and vestibular system which they then integrate. That is, the reticular formation as a whole, is not only concerned with arousal, but the integration and coordination of behavior in response to arousal, such as through the integration of trunkal, limb, arm, head and eye movements. Hence, the reticular formation exerts both generalized and specific activating influences and is thus concerned with sensory-motor integration (mediated by the excitatory and inhibitory neurotransmitters, glutamate and GABA) and modulatory functions (mediated by norepinephrine and serotonin).
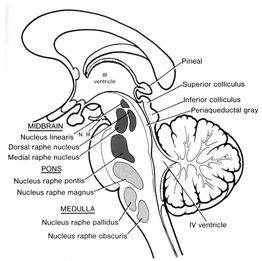
In part, the activating influences of the reticular formation are subserved by clusters of chemically active neurons which secrete and distribute norepinephrine (NE), serotonin, (5HT), and acetylcholine (ACh). Specifically, clusters of NE neurons (designated A1 through A7 by Dahlstrom & Fuxe, 19), are scattered throughout the lateral, ventral, and anterior reticular formation and tegmentum.
NE neurons project throughout the brain and spinal cord are concerned with emotional arousal, promote neuroplasticity, and also serve a neural protective function when subject to considererable and prolonged emotional stress (see chapter 30). Specifically, the more anteriorally located cell cluster, the locus ceruleus (A4 and A6) projects to the spinal cord, the brainstem, cerebellum, and cerebral cortex. Other NE cell clusters project throughout the brainstem, hypothalamus, limbic system and limbic striatum (A1-A3), as well as to the spinal cord and lower brainstem (A5 and A7).
5HT neurons are also concerned with emotional arousal, as well as with sensory filtering. 5HT neurons are clustered throughout the brainstem, forming the raphe nuclei in the midline, dorsal and median portions of the reticular formation (B1-B9). 5HT these neurons project throughout the forebrain.
Brainstem ACh neurons are clustered in the pons, forming the pendunculopontine nucleus and the laterodorsal tegmental nucleus. These neurons project throughout the brainstem, diencephalon, and limbic forebrain. Additional clusters of ACh manufacturing and secreting neurons are located in the septum and substantia innominata ("extended amygdala").
EVOLUTIONARY CONSIDERATIONS
As detailed in chapter 5, the brainstem in large part evolved from motor-sensory cells the had collected together to form like minded ganglia within the caudal regions of the head . Some of these ganglia came to constitute the portions of the spinal cord -where purely reflexive acts may be trigger (e.g. flexion or extension), whereas yet others formed the lower brainstem (and brainstem-spinal junction) and came to mediate a variety of vital vegetative functions, which for the most part occur completely outside conscious control; e.g. thermoregulation, salvation, cardiovascular functioning, swallowing, and respiration. This includes lower brainstem (medulla) motor nuclei which coordinate and program the movement of the tongue, palate, pharnyx, larynx, jaw, as well as swallowing, breathing, phonating, and "vocalization". These latter oral-facial- glutorial functions are mediated by brainstem nuclei and cranial nerves 12, 11, 10, 9, 7, and 5 (see Carpenter 2008; Parent 1995; Waxman & De Groot 2009).
Over the course of evolution, as motoric capabilities became more extensive and increasingly complex, the brainstem also evolved specific nuclei which could reflexively trigger and organize these movements. However, those nuclei concerned with the simpler aspects of movements, are also found within the lower brainstem.
The brain of a "fish" 350 mya.

Human brainstem and cranial nerves

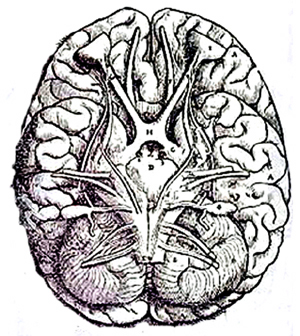
Ventral View of Human brainstem, cranial nerves, cerebellum, cerebral hemispheres
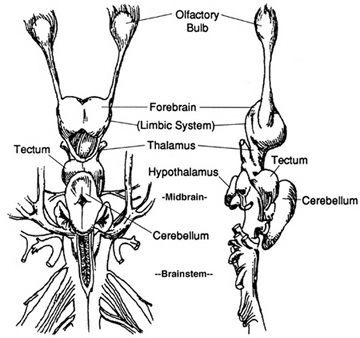
The brain of a "shark" 450 mya.
REFLEXIVE MOTOR ACTIONS & HIERARCHICAL FUNCTIONAL REPRESENTATION
When specific brainstem nuclei (and associated neural networks) are stimulated, sucking, chewing, swallowing, swimming, stepping, walking, and running movements can be induced (Barman, 2001; Cohen, et al. 1988; Klemm, 2001; Mogenson, 2001; Steriade & McCarley, 2001; Skinner & Garcia-Rill, 2001; Vertes, 2001). However, these movements decrease in complexity as the stimulation site moves in a caudal direction from the upper pons to lower brainstem; i.e. they are hierarchically organized with those requiring the least amount of organizational or conscious control being localized near the brainstem-spinal border. Hence, at the level of the spinal cord, movement programs are exceedingly simplified and usually consist of only fragments of the entire motor display.
Conversely, as one ascends from the spinal cord to the brainstem, the degree and extent of these various motor programs and behaviors, becomes more complex. For example, at the most caudal regions of the medulla, neurons when stimulated can induce stepping motions, whereas stimulation of the more anterior midbrain pontine junction (at the level of the inferior colliculus), can initiate eye movements, head turning, controlled walking, as well as running (Cowie & Robinson 2009; Cowie et al. 2009; Skinner & Garcia-Rill, 2001). Conscious and/or forebrain/telencephalic participation is not required for these motor response.
In addition, brainstem activation can also induce involuntary, stereotyped and routine motor acts such as yawning, opening and closing the mouth, wiping the face, or lifting the legs, taking steps, walking or running (Cohen, et al. 1988; Klemm, 2001), even when forebrain influences have been eliminated via transection (Skinner & Garcia-Rill, 2001). For example, if the brainstem is completely severed from the midbrain and forebrain (by a lesion below the superior colliculi at the midbrain-pons transition) mammals may respond to facial stimulation by raising one or both paws and wiping the face (see Klemm, 2001). Or, following transection, direct stimulation of the brainstem can induce walking and running movements. Because the brainstem stores these motor programs, even infants are capable of making stepping and walking and swimming movements if properly stimulated.
Presumably motor control is maintained via descending fibers which terminate on spinal motor neurons in the dorsal horns, and as such, components of the various motor programs that mediate these activities, are located within the spinal cord as well as the brainstem. Hence, in response to stimulation, the brainstem can act on the spinal and cranial nerves in order to trigger these complex motor acts in the absence of forebrain influences. Even the stereotyped motor components involved in sexual posturing and behavior appear to be under direct brainstem control.
SEX & THE BRAINSTEM
Portions of the caudal medulla (as well as the spinal cord) have been implicated in the motor control over sexual posturing (Benson, 1988; Rose, 2001). Specifically, there are medulla neurons which respond to hormonal influences, neurons which react to tactile-genital contact transmitted via the spinal cord, and neurons which integrate these messages as well as those transmitted by the amygdala and ventromedial hypothalamus and basal ganglia --nuclei which are also involved in sexual activities. Many of these same sexual neurons are located near cranial nerve nuclei which control phonation and respiration. Hence, when these latter neurons are activated, the subject may scream or cry out in orgasmic pleasure (see Rose, 2001).
Moreover, there are neurons in the medulla which when directly stimulated can induce females to assume the lordosis (or a receptive) posture. Similarly, there are brainstem nuclei, including 5HT neurons which act to suppress male sexual behavior and arousal and the capacity to sustain an erection -due to 5HT suppressive influences on related spinal motor neurons and the amygdala.
However, female sexual posturing appears to be more reflexive and "hard wired" as compared to male sexual behavior which is more complicated. That is, the female need only to stand or lay still while the male most pursue, stimulate the female, mount her, insert his penis, and discharge semen -activities which are highly dependent on sensory feedback and guidance, and more greatly under the influence of the limbic forebrain). It is because male sexual behavior is more complicated and more sensitive that it can be more easily disrupted not only by psychological factors, but lesions involving a wide variety of brainstem and forebrain nuclei.
Overall, it appears that these and related motor acts are stored in the brainstem, and do not require forebrain participation or voluntary or conscious control for their elicitation. However, most of the motor actions and "programs" stored in the brainstem can be activated by forebrain nuclei, in particular the amygdala and hippocampus, the basal ganglia, dorsal medial thalamus, and the motor areas of the frontal neocortex.
MULTI SENSORY-MOTOR BRAINSTEM NEURONS
Some brainstem neurons respond only to a single sensory system, others respond almost regardless of the nature of the stimulus. As one ascends from the spinal cord to the lower brainstem, and then from the medulla to the midbrain, neurons and nuclei become increasingly responsive to a number of different sensory modalities (Grillner & Shik, 1973; Schiebel, 1980).
For example, fibers from different sensory modalities sometimes terminate on the same neuron, indicating that many brainstem and reticular neurons have multimodal properties. Indeed, via these converging sensory fibers brainstem neurons are able to map multi-sensory space so as to create almost a three dimensional sensory representation of the environment (Schiebel, 1980), whereas others create a motor map that may be juxtaposed over the sensory map (Grillner & Shik, 1973).
Via the creation of these brainstem sensory-motor maps, the organism can respond in a reflexive manner to sensory stimuli, particularly those which are threatening, painful, or aversive, in which case reticular neurons induce generalized, or in some instances, selective arousal in response to a specific sensory signals. However, under less threatening (or reflexive) conditions, these brainstem sensory-motor neurons may preprocess stimuli in which case the corresponding region of the neocortex may subsequently become activated (see Klemm, 2001; Steriade & McCarley, 2001).
As noted, the brainstem mediates modulatory as well as sensory motor functions, and these are subserved by different transmitters. Specifically, modulatory functions are mediated by GABA and glutamate with exert inhibitory and excitatory influences, whereas sensory-motor activities are associated with NE, 5HT, and ACh. Moreover, modulatory and sensory-motor neurons differ in size and in their speed and rate of transmission.
For example, the axons of modulatory neurons tend to be small in diameter (10-25 um), have a slow rate of conduction ( 1 m s -1), slowly depolarize, and then spontaneously become self-inhibited. Presumably, this slower pace of modulatory excitatory followed by self-inhibiting activity is a consequence of the oscillation of GABA (inhibition) and glutamate (excitation) neurotransmitter release, which thus provide these neurons with "pacemaker" functions, which thus gives rise to the rhythmic nature of so many brainstem activities.
By contrast, the axons of sensory motor neurons are larger in size (50 to 75 um in diameter), fire at continuously high rates, with conduction velocities of over 100 m s -1. These neurons, therefore, are perfectly adapted for activating and arousing widespread areas of the cerebrum, and can quickly recruit (as well as inhibit) vast neural networks in response to life threatening or other emotionally or motivationally significant external events, including the presence of a potential sex partner.
As will be detailed below, modulatory and sensory-motor neurons and the neurotransmitters which serve them, also interact so as to regulate not just arousal and rhythmic motor activities, but sleep, including the generation of REM (rapid eye movement) and NREM sleep. That is, sensory motor neurons (e.g. NE and 5HT) play a significant role in the generation of dream (paradoxical) sleep, which is characterized by a high state of cerebral arousal, whereas the modulatory (pace maker) neurons (GABA and glutamate), play a significant role in the oscillation between REM and NREM.
THE MIDBRAIN
The midbrain is the least differentiated and the smallest segment of the brainstem and can be divided into three portions: a medial segment (the tegmentum) which includes DA manufacturing neurons and which represents the anterior continuation of the reticular formation (described below); the most recently evolved ventral segment through which pass descending cortical fibers; and the tectum which is dorsally located and includes the superior (visual) and inferior (auditory) colliculi. Located between and below the ventral segment and the DA producing (Mesolimbic) tegmentum is the substantia nigra which manufactures Dopamine. However, the most obvious structure of the midbrain is the pinkish colored "red nucleus", which receives descending motor fibers from the frontal lobe and gives rise to the rubrospinal tract which facilitates flexor muscle tone.
The Periaqueductal Gray.
Also located within the midbrain is the periaqueductal gray, a nucleus which receives extensive input from the amygdala and other limbic nuclei and is implicated in the motoric-vocal aspects of emotional expression. For example, stimulation of the periaqueductal gray can trigger the production of a variety of sounds that are suggestive of exceedingly negative moods (Jurgens, 2009; Larson et al. 2009; Zhang et al. 2009). However, these "sounds" are pre-programmed, and with the exception of reflexive responding to noxious or painful stimuli, have no bearing on emotional state, as they are not accompanied by complex behavioral displays -that is, unless signaled by the forebrain. However, if the periaqueductal gray is disconnected from the limbic system and neocortex (such as by a midbrain transection), stimulation of this nuclei will continue to evoke vocalization.
Specifically, the periaqueductal gray coordinates the activity of the laryngeal, oral-facial, and principal and accessory muscles of respiration and inspiration (Zhang et al. 2009). The coordinated activity of these tissues enables an individual to laugh, cry, or howl, even if the rest of the brain (excepting the brainstem) were dead and there was no evidence of consciousness.
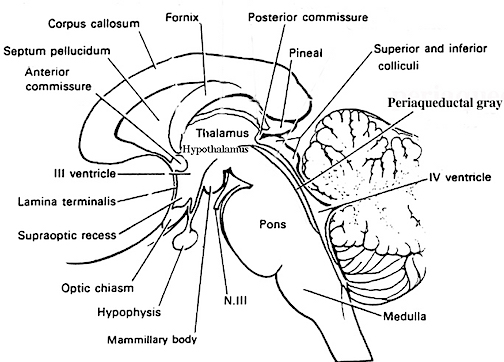
It thus appears that rather than a center for emotional vocalizations, the midbrain periaqueductal gray instead appears to be the site where particular vocalization motor patterns are stored. Hence, when the periaqueductal gray is activated by impulses received from the limbic system or neocortex (or via spinal nuclei involved in transmitting noxious stimuli), it activates the appropriate motor program and then organizes and coordinates the oral-laryngeal and respiratory muscles so that the appropriate sounds can be produced (Zhang et al, 2009). Presumably these neural motor programs are stored within the periaqueductal gray.
MIDBRAIN EVOLUTION
As pointed out in chapter 5, the brainstem (excluding the midbrain) may have evolved in a posterior-caudal and in an anterior direction so as to merge with the visual (midbrain) areas that probably initially supplied photopic derived energy to run the motor system and to arouse and energize the brain. In this regard, evolution parallels ontogeny (see chapter 23). As also detailed in chapter 5, the anterior most segment of the midbrain and anterior pons, appear to have evolved from sensory-motor, and photosensitive cells.
Due presumably in part to its photosensitive origins, and thus its concomitant ability to extract energy, the midbrain-pontine region of the brainstem developed the capacity to respond to light vs darkness, and to manufacture various neurotransmitters; e.g. serotonin, dopamine, and norepinephrine. In this manner the brainstem is able to exert widespread influences on cerebral arousal and neural transmission.
Over the course of evolutionary development, the capacity to respond to visual input increased beyond detecting light vs shadow, and soon came to include motion, as well as the ability to rotate the eyeballs and focus the retina via cranial nerves 2, 3, 4, 6. These complex functions in turn became subject to the control of the superior colliculus (or tectum).
However, with the evolution of "hearing" the midbrain became increasingly modified to subserve complex auditory functioning as well, which in turn became associated with the evolution of the inferior (auditory) colliculus. In this regard, just as the control and governance of motor functioning becomes more complex and hierarchically (caudal to anterior) organized, so too does sensory analysis and related sensory-motor integrative functions which take place within the midbrain. Multi-modal processing and complex behavioral reactions, and the integration and analysis of auditory, visual-tactile, and even motor stimuli are characteristics of the visual (superior) and auditory (inferior) colliculi of the midbrain.
THE MIDBRAIN SUPERIOR & INFERIOR COLLICULI
THE SUPERIOR COLLICULUS The superior colliculi are located in the anterior half of the midbrain. Although the midbrain per se is not well differentiated, these nuclei consist of gray and white layers and resemble cerebral cortex (Carpenter 2008). As noted in chapter 5, neocortical layers I and VII (between which are sandwiched layers II-VIa), in turn appear to be related to and/or an outgrowth of the midbrain.
The superior colliculi can be functionally divided into superficial and deep layers which subserve somewhat different functions. (Cowie & Robinson 2009; Davidson & Bender 2008; Weller 1989). For example, the superficial layers receive considerable input from the retina as well as temporal and occipital visual cortex, and respond to moving stimuli. The superficial layers also project to vision related cranial nerve nuclei.
By contrast, the intermediate and deeper layers receive converging motor, somesthetic, auditory, visual and reticular input, and in fact serves as an extension of the reticular formation, and maintains interconnections with the caudal medulla and those cranial nerves associated with movement of the head. Hence, these layers serve as a multi-modal assimilation area which is concerned with orienting toward external stimuli and movement; particularly movements of the head and eyes during gaze shifts (Cowie & Robinson 2009; Davidson & Bender 2008; Keller & Edelman 2009).
The deep and superficial portions are also intimately interconnected (Carpenter, 2008; Parent 1995) and both receive extensive optic input from the contralateral eye, and contralateral visual input from the ipsilateral eye. Moreover, they receive ipsilateral projections from the neocortical visual areas regarding the contralateral half of visual space. Hence, each colliculus is concerned with either the right or left half; i.e. the contralateral half of visual space. In addition, the colliculi receive input from the frontal eye fields.
As noted, cells within the colliculi are responsive to visual motion and many tend to respond only to movement in a certain direction (Davidson & Bender 2008). Most do not respond to stationary stimuli. Given that reptiles, amphibians, and fish are devoid of higher cortical centers, it thus appears that the colliculi evolved so as to detect the presence of prey (or predators) and to guide orienting reactions and thus movement related to escape or food procurement.
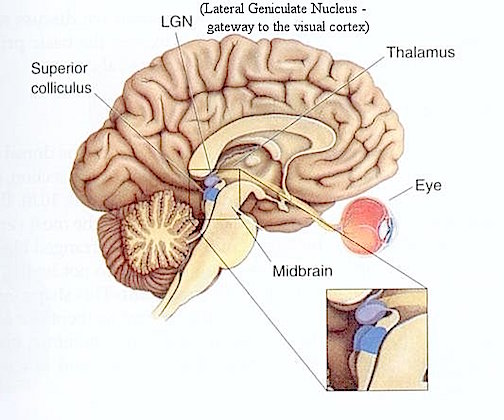
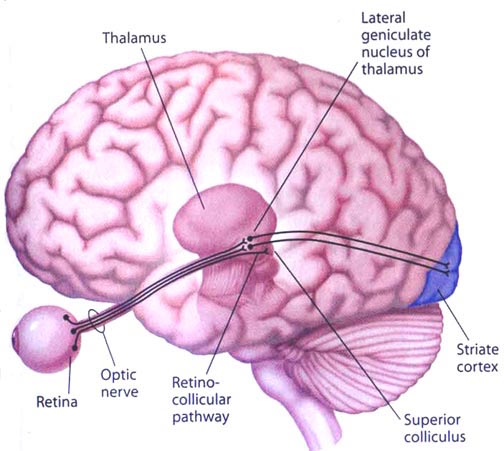
THE INFERIOR COLLICULUS
The inferior colliculus (IC) is predominantly concerned with detecting and analyzing auditory stimuli, and in fact is tonotopically organized; i.e. neurons are arranged in a laminar pattern which represents different auditory frequency bands (reviewed in Carpenter 2008; Brodal 1981). The IC can also respond to sounds arriving from either ear. This enables the IC to analyze and localize the source of various sounds and to correlate them with their various spatiotemporal characteristics. In this manner, sounds can be identified as coming from a certain direction and from a specific source.
The IC sends fibers to the pons and medulla as well as the superior colliculus, and relays auditory inpulses to the spinal cord, and the nuclei subserving the neck and facial musculature (Henkel & Edwards, 1978; Wright & Barnes, 1972). Hence, via the IC, auditory impulses can trigger head and body turning and thus orientation toward sound sources. In mammals, the IC also acts to relay auditory signals received from the lateral lemniscus to the medial (auditory) geniculate of the thalamus, and in this regard it serves not only to analyze and orient toward auditory stimuli, but as a major auditory relay nucleus. However, in this regard it is completely dependent on cranial nerve nuclei subserving the vestibular-cochlear system; cranial nerve VIII which is located at the pontine, medulla junction.
BRAINSTEM AUDITORY PERCEPTION
Long before amphibians left the sea to take up life on land and in the air, fish had already learned to detect and analyze sounds made in the water. This was made possible by a structure located along both sides of the fish's body, called the lateral line. The lateral line is very sensitive to vibrations including those made by sound. The mammalian auditory system, however, did not evolve from the lateral line but from the vestibular nucleus (chapter 5).
The human brainstem vestibular nucleus is very sensitive to vibrations and gravitational influences and helps to mediates balance and movement, such as in swimming and running. Over the course of evolution, it is through the vestibular nucleus (and the amygdala) where the first neuronal rudiments of "hearing" and the analysis of sound also took place. Among all mammals this is a function the vestibular nuclei continues to serve via the signals it receives from the inner ear; the brain's outpost for detecting certain vibratory waves of molecules and their frequency of occurence.
Initially, however, the auditory system also evolved so that organisms could turn and orient toward and localize unexpected sound sources. This was made possible by messages transmitted to the midbrain inferior colliculus. This includes things that go bump in the night such as the settling of the house and the creaking of the floor, all of which can give rise to alarm reactions in the listener (in their amgydala). This is a sense humans and their ancestors have maintained for well over several million years, its main purpose being to maximize survival via the detection of approaching predators. In fact, the inferior colliculus (as well as the amygdala of the limbic system) was the main source of auditory analysis two hundred million years before the evolution of neocortex (see chapter 5). This is a function it continues to perform among modern day reptiles, amphibians, sharks, as well as mammals.
AUDITORY TRANSMISSION FROM THE COCHLEA TO THE TEMPORAL LOBE
Within the cochlea of the inner ear are tiny hair cells which serve as sensory receptors. These cells give rise to axons which form the cochlear division of the 8th cranial nerve; i.e. the auditory nerve. This rope of fibers exits the inner ear and travels to and terminates in the cochlear nucleus which overlaps and is located immediately adjacent to the vestibular-nucleus from which it evolved within the brainstem.
Among mammals the cochlear nucleus in turn projects auditory information to three different collections of nuclei. These are the superior olivary complex, the nucleus of the lateral lemniscus, and, as noted, the inferior colliculi.
A considerable degree of information analysis occurs in each of these areas before being relayed to yet other brain regions such as the amygdala (which extracts those features which are emotionally or motivationally significant), and to the thalamus where yet further analysis occurs.
Among mammals, from the thalamus and amygdala, auditory signals are transmitted to the primary auditory receiving area located in the neocortex of the superior temporal lobe; Heschl's gyrus. Here auditory signals undergo extensive analysis and reanalysis and simple associations begin to be formed. However, by time it has reached the neocortex, auditory signals have undergone extensive analysis by the thalamus, amygdala, and the other ancient structures mentioned above.
THE BRAINSTEM & SPEECH
Brainstem mechanisms involved in vocalization includes control over the respiratory mucles; control over the vocal cords so that one can phonate; control over the pharyngeal, oral and nasal passages which effect resonance; and control over the palate, tongue, lips and mandible which control articulation. However, articulation also requires a mixture of nonvoiced and voiced (plosive) sounds (e.g. "puh, guh, kuh")-- which require a strong puff of air. In part, these functions are under the control of the midbrain periqueductal gray, as well as cranial nerves 12, 10, 9, 5, & 7.
In order to expel air through the mouth the soft palate must elevate to close off the nasopharynx from the oropharynx. If the subject articulates plosives poorly with air escaping through the nose, the palate is not properly elevating which suggests a lower brainstem lesion. Speech may sound hypernasal; or, conversely if there is insufficient air pushed into the nose, hyponasal (as if the nose has been pinched closed during speech); which may also be due to nasal obstruction. .
Absence of any phonation is called aphonia. Faulty articulation as a result of a forebrain or brainstem or muscular lesion and is called dysarthria. If the individual cannot speak due to a nerve or muscular lesion it is called anarthria. Because nerve 10 is associated with the palate (articulation), pharnyx (resonance), and larynx (phonation) disorders of speech and swallowing may be due to a lesion to this nerve or its bed nucleus.
However, not just phonation and articulation but swallowing and respiration would be effected by lesions in this vicinity. As such, individuals may articulate, phonate, and inhale and exhale inappropriately while speaking and may even sound as if they are laughing or crying when in fact inspiration and expiration are effected. This latter condition has been referred to as a bulbar palsy.
However, with severe bilateral lesions of the corticobulbar fibers, patients may lose the ability to speak or swallow and become mute and comatose. In the recovery phase or with gradual lesions of the corticobulbar tracts, the patient shows a characteristic, virtually pathognomic syndrome termed pseudobulbar palsy: He initiates speaking or swallowing very slowly, slurring words, with the voice having a peculiar strained pitch and quality. In addition, there is much emotional lability, crying one moment, laughing the next. The patients face, most of the time, appears immobile as though it were a wooden mask. However, when he laughs/cries, the facial movements return and become greatly exaggerated and prolonged. However, when questioned, the patient will claim that he does not feel the emotion being conveyed.
CRIES & LAUGHTER
As noted, the midbrain periaqueductal gray coordinates the activity of the laryngeal, oral-facial, and principal and accessory muscles of respiration and inspiration (Zhang et al. 2009). The coordinated activity of these tissues enables an individual to laugh, cry, or howl, even if the rest of the brain (excepting the brainstem) were dead and there was no evidence of consciousness.
Laughter and crying often involves activation of the entire musculature of the face, including the muscles of the throat, jaw, diaphragm, and to a lesser extent, the neck, chest, and abdomen. Laughter is also accompanied by respiratory expiration, whereas crying is associated with inspiration, such that rhythmic waves of air rush over the larnyx giving rise to the phonation of laughter, or the sound of sobbing.
Laughter and crying are therefore dependent on the functional integrity of the periaqueductal gray, as well as the pons and medulla, cranial nerves 12, 10, and 9, 5 and 7. However, the correspondence between the motoric-expressive and the felt aspects of emotion are maintained and dependent on two supranuclear pathways: the cortico-bulbar which arises in the frontal motor areas, and the fronto-limbic-pontine-medullary pathway which arises in the orbital frontal lobes and comes to include the medial frontal lobes and anterior cingulate, the hypothalamus, amygdala, periaqueductal gray and brainstem. It is this latter pathway which is responsible for conveying the felt aspects of emotional expression, such as is involved in laughter and crying (see chapter 13).
When the functional integrity of these interconnections are disrupted, such as due to a progressive bulbar (pontine-medullary) palsy, brainstem hemorrhage, tumor, amyotrophic lateral sclerosis (ALS), or related injuries to the hypothalamus and deep orbital area, pathological laughing and/or crying may be triggered or reflexively released via brainstem activation.
Specifically, crying is conveyed via prolonged bursts of respiration and laughter via sudden bursts of expiration. That is, what appears to be "laughter" is actually an uncontrolled and sustained clonus accompanied by an explosion of air passing over the larynx which repeats itself in a series of bursts. If the expiration is sustained, the sound produced becomes distorted and the patient may sound as if wailing or even screaming.
Essentially the periaqueductal gray and the lower brainstem have been released and disconnected from forebrain control such that normal brainstem activities, e.g. vocalization, become uncontrolled. Moreover, as the brainstem nuclei mediating facial expression are also effected, facial expression is also out of control. The face becomes contorted and seemingly expressive of extreme happiness or grief although patients will deny experiencing these feelings -although they may feel distressed and embarrassed by what is happening.
As described by a patient with ALS: "I begin to smile, but the smile becomes an exaggerated grin, which attaches itself to my face... [then]... I become angry, humiliated, and subject to uncontrolled tears. You have no idea how terrible it is when the crying is fully triggered and takes hold like a seizure. I can't control any of it. I simply disintegrate" (Lieberman & Benson 1977, p. 717-718).
THE BRAINSTEM RETICULAR FORMATION
The pontine and medullary brainstem significantly contributes to cortical arousal, attention, sensory and motor facilitation and inhibition, and the orienting reaction. This is made possible through ascending limbic, thalamic, and neocortical influences, and descending inhibitory influences on brainstem and spinal sensory and motor nuclei. In this manner the brainstem is able to selectively arrest and activate specific movements (NE/ACh), while simultaneously filtering out distracting or irrelevant sensory stimuli (presumably via 5HT influences) so that attention may be selectively directed and maintained (see Klemm, 2001; Steriade & McCarley, 2001).
Brainstem motor and sensory filtering and inhibition is also made possible via reticular neuronal modulatory control over arousal; i.e. the reticular activating system. The brainstem reticular formation consists of a number of morphologically distinct nuclei and neurons (see Blessing, 2011; Steriade & McCarley, 2001; Vertes, 2001) which differ in regard to size, shape, number and orientation of dendrites, and axonal patterns of projection. It is via these nuclei that the reticular formation can exert widespread influences on motor and sensory functioning as well as arousal.
For example, cranial nerve and sensory fibers originating in the spinal cord give off collaterals as they ascend or terminate in the brainstem reticular formation (Brodal, 1981; Pompeiano, 1973). This includes auditory and visual fibers originating in the midbrain tectum (Edwards, 1980). The more lateral portions of the reticular formation consists of short axons which richly innervate adjacent brainstem nuclei and which are connected to the collaterals of the various sensory tracts.
Specifically, the ascending reticular activating system consists of a multi-neuronal, polysynaptic core that conveys, via long central axons with numerous laterally projecting collaterals, nonspecific impulses to the forebrain. These long axons ascend via the central tegmental tract which projects to the intralaminar nuclei of the thalamus. Many of these core reticular neurons are concerned with motor activation and inhibition as well as alerting and arousal functions so that motor functioning is coordinated with forebrain sensory processing. By contrast, fibers from the medial reticular formation project to the limbic system.
COMA, LETHARGY & THE BRAINSTEM
The sensory tracts, including those involved in transmitting noxious stimuli, are exceedingly important in maintaining arousal. If these sensory tracts are severed at the brainstem level, patients may become exceedingly lethargic, and even apathetic. Similarly, destruction of the olfactory, auditory, and visual pathways, even at a peripheral level, can result in prolonged lethargic states (see Steriade & McCarley, 2001). This indicates that sensory input, from the periphery to the brainstem, and up through the thalamic level is exceedingly important in maintaining arousal and cortical alertness, as well as the waking state.
However, destruction or interruption of these long ascending sensory pathways (i.e. the lemniscal systems) that span the spinal cord and terminate in the thalamus, does not eliminate the cortical arousal response. This is because forebrain arousal is dependent on the reticular formation which receives collateral sensory fibers from these ascending systems.
Thus, whereas lateral brainstem injuries may create lethargic and reduced states of arousal, destruction of the core brainstem reticular formation can result in a loss of forebrain arousal and create a permanent comatose state such that the patients or animals may not even respond to noxious stimuli (French & Magoun, 1952; Lindsely, et al. 1950). Indeed, transection of the brainstem just a few millimeters below the visual and auditory colliculus also induces coma.
However, this is not only a consequence of a loss of reticular input to the forebrain, but a loss of forebrain (and frontal lobe) and midbrain input to the brainstem reticular formation (Bremer, 1975; see also Steriade & McCarley, 2001). Just as the complexity of motor programming increases as one ascends the brainstem, so to does control over arousal.
For example, if the transection is in the upper brainstem (i.e. at the pons, trigeminal level) well below the tectum, the subject continues to demonstrates EEG signs of alertness, as well as visual tracking eye movements (reviewed by Steriade & McCarley, 2001), although they are otherwise in a coma. However, if the lesion is more anterior at the midbrain level, the subject becomes completely comatose and unresponsive --which indicates an important role for the midbrain reticular formation in maintaining cortical and forebrain arousal.
However, if the lesion is anterior to the midbrain, rather than coma and a loss of arousal, there results a loss of control over arousal such that environmental stimuli may be ignored or neglected. For example, if the immediately adjacent thalamus is lesioned (which is the terminal junction of many reticular fibers), this may result in complete neglect of auditory, visual, and tactual space, depending on the laterality of the damage. Presumably this is a consequence of an inability to transmit or relay sensory stimuli or to activate the neocortex, for with lesions to the thalamus, cortical EEG desynchronization is usually absent regardless of brainstem activity (Steriade & McCarley, 2001).
Conversely, if the lower brainstem is lesioned, the patient not only become comatose, but cardiovascular and respiratory disturbances will result. Hence, the patient may die.
FRONTAL-THALAMIC & AMYGDALOID CONTROL OVER BRAINSTEM AROUSAL
When thalamic output increases, the amplitude of cortical responses also increases (Steriade & McCarley, 2001). However, the thalamus, in turn is also under the control of the lateral and orbital frontal lobes which also exerts significant modulatory influences on the brainstem reticular formation while it simultaneously monitors neocortical and thalamic activity (Joseph, 2014a). Via the frontal lobe, the thalamus and the reticular formation can be activated or inhibited selectively or globally so that specific sensory modalities are attended to while others are filtered or suppressed so that the organism can engage in selective attention and information processing.
Similarly, the amygdala remains capable of inducing arousal as well as EEG desynchronization (Kriendler & Steriade, 1964; see chapter 14). This is because the amygdala is also intimately interlinked with the upper brainstem reticular formation (Takeuchi et al., 1982), as well as the frontal lobe and thalamus. Hence, when stimulated, the amygdala can induce alerting reactions and arousal (chapter 14), as well as PGO waves (Calvo, et al. 2005), which in turn are associated with the onset of paradoxical sleep and brainstem activity.
In addition, the medial and lateral portions of the posterior hypothalamus also project to the midbrain reticular formation (Steriade & McCarley, 2001), as does the preoptic regions of the hypothalamus (Swanson, et al. 2005) -a region intimately involved in sexual behavior and sexual posturing (chapter 14). The hypothalamus is also intimately linked with the orbital frontal lobes and the amygdala. Therefore the hypothalamus, being exceedingly involved in all aspects of rudimentary motivational and emotional functioning is also important in arousal and the waking state (Ranson, 1939), and if the posterior regions are damaged there can result severe apathetic states.
Hence, these findings inplicate the forebrain (i.e. the amygdala, frontal lobes, thalamus, hypothalamus) in maintaining forebrain arousal. Nevertheless, even in this regard the forebrain is dependent on the midbrain and brainstem. It is the brainstem which contain nuclei and cell clusters which manufacture specific neurotransmitters which are directly responsible for maintaining and promoting behavior, emotional, cognitive, and cerebral arousal including sleep and dreaming.
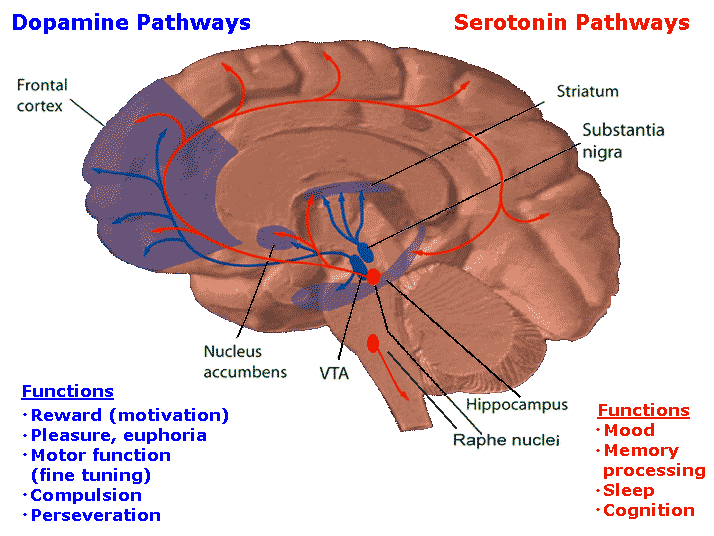
DOPAMINE, NOREPINEPHRINE & SEROTONIN
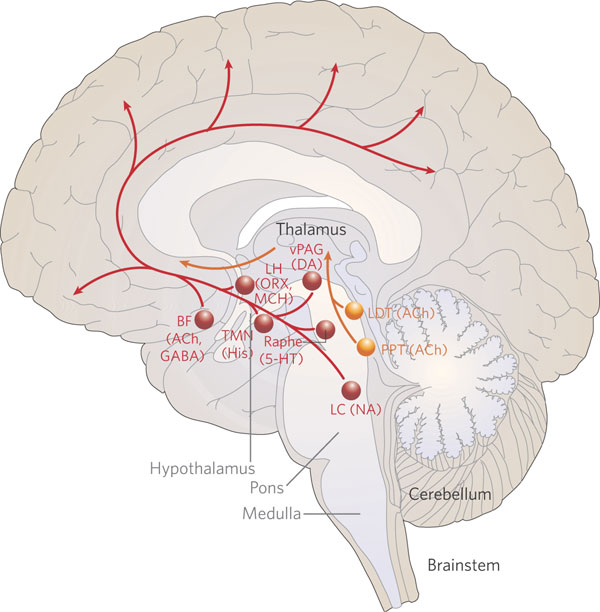
The midbrain and pons contain neurons and nuclei that are responsible for manufacturing three principle monamine neurotransmitters, i.e. dopamine (DA), norepinephrine, (NE) -also referred to as catecholamines, and serotonin (5HT) which is an indolamine. DA is produced predominantly within the midbrain substantia nigra and tegmentum, whereas NE is manufactured and distributed by neurons located predominantly in the locus ceruleus, which is located in the pons. 5HT producing neurons and cell groups are found in both the pons and medulla (e.g. the dorsal and median raphe nucleus).
DOPAMINE
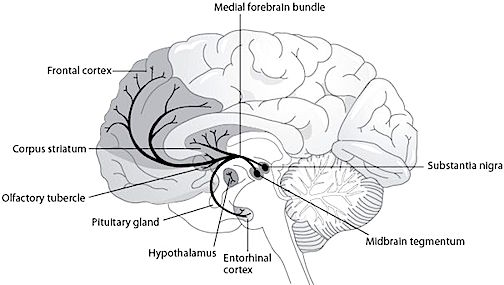
Dopamine is believed to play a dominant role in cognition and motor functioning, and disturbances in the DA transmitter systems are closely related to the development of Parkinson's disease and psychotic abnormalities (see chapter 16). For example, reductions in corpus striatal DA are associated with catatonia and Parkinson's disease, whereas excess DA has been linked to paranoid and psychotic disturbances (chapter 16). However, the nature of the disturbance is also dependent on which DA system is effected; i.e. substantia nigra-corpus striatal or tegmentum-limbic striatum.
For example, the midbrain ventral tegmentum (A10 DA cell group) provides dopamine to the amygdala, limbic striatum, and the ventral caudate and putamen and is believed to be related to emotion, mood and reward (referred to as the mesocorticolimbic/mesolimbic DA system). The mesolimbic DA system can therefore enhance or depress emotional-motoric expression.
The substantia nigra DA system is related to motor functions including the production of stereotyped and routine actions (Fibiger & Phillips, 1986; Robins & Everitt 1992; for related details). Prominent projections sites include the dorsal caudate, putamen and medial frontal lobes.
The nigrostriatal and mesolimbic DA system sometimes act in oppositional as well as in a complimentary and mutually supportive manner. For example, the release of DA occurs in response to specific biologically significant stimuli, which in turn may activate the limbic and corpus striatum and thus trigger motor activity such as approach or withdrawl (Fibiger & Phillips 1986; Robins & Everitt 1992). However, if one (or both) of these two DA systems becomes dysfunctional, counterbalancing influences are removed which may result in excessive activity within the supposedly "normal" DA system and related nuclei. Hence abnormalities in these DA systems may induce hyperactivity and mania or conversely a poverty of movement (e.g. Parkinson's symptoms and catatonia).
As noted in chapter 5, midbrain neurons, being derived, in part, from photosensitive cells, also display circadian and natural rhythms that may well have been induced via adaptions to changes in the light-dark cycle. Similarly, DA neurotransmission also displays circadian variations, which in turn is reflected by cyclic motor and DA activity within the caudate nucleus, and less so within the limbic striatum (Paulson & Robinson 2009).
NOREPINEPHRINE
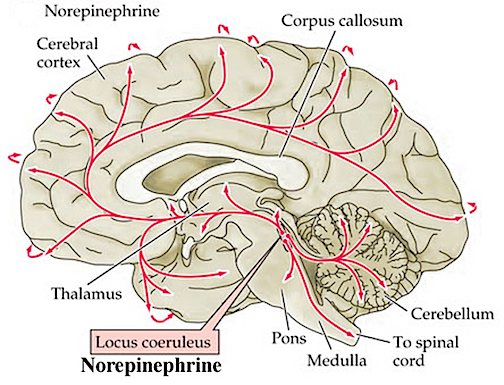
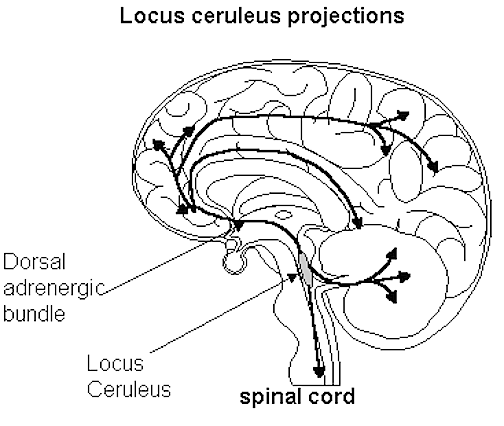
The NE neurons system is composed of three main neuronal clusters located in the pons and medulla; i.e. the dorsal medullary (A-2 cells), the locus ceruleus (LC, A-4, A-6 cells), and the subcoeruleus (A-1, A-3, A-5, A-7 cells). These nuclei give rise to ascending and descending monosynaptic projections which make diffuse and specialized selective contact with a variety of brain area via their highly branching axons (Dahlstrom & Fuxe 1965; Fuxe 1965; Levitt & Moore 1979). It has been estimated that via their diffuse projections a single NE neuron can make contact with up to 75,000 other neurons, and can contribute fibers to both the cerebral cortex, limbic system, and cerebellum (Cooper et al. 1974). However, this has also been disputed.
Broadly considered, the NE system projects to the midbrain colliculi and lateral and medial geniculate of the thalamus, the amygdala (which contains very high concentrations of NE), as well as throughout the brainstem where it influences auditory and visual functioning and is a major transmitter involved in facial expression. Indeed, the NE system subserved a wide range of functions, including regulation of cardivascular activity, micturation, respiration, sleep and dreaming, motivation, emotional expression, and mobilization in response to pleasure, fear, or stress (reviewed in chapter 30).
The locus ceruleus (LC)-NE system is clearly implicated in emotional functioning and contributes fibers (via the medial forebrain bundle) to the hypothalamus, amygdala, septal nuclei, hippocampus, thalamus, cingulate gyrus (Dalhstrom & Fuxe, 1965; Fuxe 1965) as well as the midbrain including the lateral geniculate and pulvinar of the thalamus, and the frontal and occipital lobe (see Foote et al. 1983; Morrison & Foote, 1986; Sakaguchi & Nakamura, 2005).
However, it maintains predominantly ipsilateral connections, including those which diffusely project to the neocortex, and there is some evidence which indicates that whereas DA is more greatly concentrated in certain left cerebral nuclei and brain regions, NE concentrations are greater in the right hemisphere, particularly within the right somesthetic nucleus of the thalamus (Oke et al. 1978). In this regard it is noteworthy that whereas left cerebral lesions will reduce left cerebral NE levels within the areas of injury, right hemisphere lesions can induce diffuse and bilateral NE reductions (Robinson 1979).
Locus ceruleus-NE neurons become highly active in response to novel or motivationally signficant somesthetic, visual, and auditory stimuli and NE levels are effected and rapidly metabolized by stressful and emotional experience (Foote et al. 1980), especially fear. Indeed, the neural circuitry involving the NE system makes it "uniquely situated to subserve all the known physiological correlates of fear" (Redmon & Huang 1979; p. 2154).
Hence, electrical stimulation of the LC can trigger severe anxiety and fear reactions. Conversely, ablation of the locus ceruleus results in a significant decrease in fear and anxiety reactions even in response to threatening stimuli (Redmod & Huang 1979). Similarly, NE-reuptake blockers (tricyclics) can reduce feelings of anxiety, whereas NE activators, such as piperoxane, can induce anxiety reactions in humans (Klein et al. 1978).
The NE system is also involved in the mediation of pleasure and reward, and is rapidly synthesized when subjects are engaged in pleasurable activities (Bliss & Zwanziger 1966). Conversely, low levels of NE are associated with a lack of pleasurable feeling; i.e. depression. Hence, NE is essential to the maintenance of the "pleasure circuit."
The NE system is also highly involved in arousal, including the sleep wake cycle, and the generation of REM, PGO activity, and dreaming (Chu & Bloom 1974). NE levels, therefore, fluctuate in cycles and demonstrate specific circadian rhythms which are also related to sleep and dreaming. For example, in humans and other primates, NE levels are lowest at 3 AM and gradually increase throughout the day until around 3 PM (Ziegler et al. 1976), which in some cultures corresponds to "nap" or "siesta" time.
SEROTONIN
Serontonergic (5HT) neurons exert widespread and often tonic and inhibitory influences on a variety of brain areas (Applegate, 1980; Jacobs & Azmita 1992; Soubrie, 1986; Spoont, 1992). This includes the modulation of fear and pain and emotional states such as depression, the inhibition of incoming sensory input, and the modulation of motor expression. That is 5HT restricts perceptual and information processing and in fact increases the threshold for neural responses to occur at both the neocortical and limbic level.
For example, in response to arousing stimuli, 5-HT is released (Auerbach et al. 2002; Roberts, 1984; Spoont, 1992) which acts to aid attentional and perceptual functioning so that stimuli which are the most salient are attended to. That is, 5HT appears to be involved in learning not to respond to stimuli that are irrelevant and not rewarding (See Beninger, 1989). These signals are filtered out and suppressed.
It has also been demonstrated that 5HT acts to suppress activity in the lateral (visual) geniculate nucleus of the thalamus and synaptic functioning in the visual cortex as well as the amygdala and throughout the neocortex (Curtis & Davis 1962; Marazzi & Hart 1955). By contrast, substances which block 5HT transmission -such as LSD- results in increased activity in the sensory pathways to the neocortex (Purpura 1956), which induces complex hallucinatory experiences.
As will be detailed below, 5HT is also implicated in the generation of sleep and dreaming, as well as the production of motor paralysis during sleep. For example, 5HT via descending influences, facilitates motorneuronal activity, while simultaneously decreasing or inhibiting sensory input (Morrison & Pompeiana 1965). In this manner, selective attention (via sensory filtering) can be maintained while the organisms is engaged in goal directed motor behavior. However, while sleeping and dreaming, internal sensory filtering is reduced (due to decreased 5HT) whereas motor functioning is inhibited -which prevents the subject from walking about and acting out their dreams (see below).
THE DORSAL & MEDIAN RAPHE-5HT NUCLEI
Serotonin (5HT) is produced by the raphe nuclei located in the pons and medulla, with the highest percentage located in the dorsal raphe (79%) with the bulk of the remainder produced by the median raphe. Thus, the main source for limbic and neocortical 5-HT is the dorsal (DR) and median raphe (MR) nuclei (Azamita, 1978; Azamitia & Gannon, 1986; Wilson & Milliver, 2008ab). Specifically, the amygdala, hypothalamus, basal ganglia, primary and association receiving areas, and the frontal lobe are innervated by the DR, whereas the hippocampus, cingulate gyrus, and septum receive their 5-HT from the MR nuclei (Azamita, 1978; Azamitia & Gannon, 1986; Wilson & Milliver, 2008ab).
The MR, however is diffusely organized and appears to exert a non-specific and global influence on arousal and excitabilty (Wilson & Molliver, 2008ab). The DR is much more discretely organized and can exert highly selective inhibitory or excitatory influences and plays a role in the coordination of excitation in multiple functionally related areas including the frontal lobes and amygdala (Wilson & Molliver, 2008ab). Because of the manner in which they are organized, the DR and MR can exert select inhibitory influences so as to engage in perceptual filtering in one or a variety of areas while simultaneously exciting yet other multiple regions which in turn aid selective attention and in the creation of specific neuronal networks.
NE & 5HT INTERACTIONS: FEAR, PAIN, STRESS
The 5HT and NE systems appear to interact in regard to a number of different functions including arousal, sensory and emotional processing, and sleep and dreaming, and (in conjunction with brainstem enkephalin) the experience and modulation of painful experience including depression. For example, it has been repeatedly and widely reported that altered brain 5HT turnover and reduced 5HT levels are often discovered postmortem among individuals who have committed suicide (e.g. Arranz et al. 2009). Moreover, some individuals commit suicide because of feelings of chronic stress and emotional turmoil, and an inability to experience pleasure in living -which in turn implicates the NE as well as 5HT system.
PAIN & STRESS
Somatic and visceral sensations are transmitted to the cerbrum by way of the spinal cord and brainstem. At the spinal level these signals are transmitted via the dorsal horns where collateral fibers from the peripherally located somatic receptors enter and then terminate near the substantia gelatinosa, thereby forming and giving rise to the lateral spinal tract which transmits pain and thermal impulses to the somesthetic (VPL) thalamus which contains high concentrations of NE -particularly the right VPL (Oke et al. 197).
However, descending LC-NE and raphe-5HT fibers also terminate in the substantia gelatinosa -which is also rich in opiate receptors- (Dalhstrom & Fuxe 1965). In response to nocioceptive impulses, both NE and 5HT (as well as the brainstem enkephalins) act to reduce and inhibit the transmission of these impulses (Headley et al. 1978). Similarly, stimulation of the raphe nucleus induces anylgesis and direct application of 5HT depresses dorsal horn reactivity to noxious stimuli.
In this regard, not only does NE and 5HT serve to counteract the reception of painful stimuli, they also act to arouse and mobilize the organism when experiencing pain or fear. As noted, stimulation of the LC can induce fear reactions as well as defensive behavior including flight (Redmond & Huang 1979). In this manner fear can also become associated with stimuli that induce pain and both can be associated with escape. However, in response to chronic fear, pain, or stress, 5HT and NE levels begin to rapidly decrease (see chapter 30).
STRESS & PSYCHOSIS.
In response to highly stressful, fearful, or painful stimuli, 5-HT levels, at least initially are increased which results in numbing, analgesia, and a loss of pain perception (Auerbach et al. 2002; Roberts, 1984; Spoont, 1992). Under conditions of prolonged stress, pain, or fear, 5-HT is rapidly depleted. In consequence, the individual may begin to feel overwhelmed and unable to appropriately process incoming sensory stimuli. Soon thereafter (i.e. following chronic stress) they may also demonstrate a heightened fear and startle response (Davis, 1984) as well as social withdrawal (Raleigh et al. 1983).
With continued high stress, sensory filtering is reduced, neurons in the amygdala, hippocampus, and inferior temporal lobe cease to be inhibited, which in turn can result in dream-like states, hallucinations (Joseph, 1988a, 1992a, 1998b, 2014d; Spoont, 1992), depersonalization (Sallanon et al. 1983) and associated psychotic and emotionally disturbed states (see chapter 30).
Under conditions of chronic emotional stress, 5-HT begins to be depleted. However, not just trauma, but repeated and chronic sexual activity is also associated with significant reductions and depletions in 5-HT (Spoont, 1992; Zemlan, 1978), which might explain why individuals who are repeatedly sexually molested or assaulted, may develop PTSD, dissociative and amnesic states, and psychosis (chapter 30).
In addition, stress, be it due to combat, cold, prolonged swimming, or pain, is associated with the depletion of NE (Bliss et al. 1968). With the exception of strenuous physical activity, fear and stress can reduce NE concentrations by up to 50%.
In fact, even a single trauma may result in alterations in NE and 5HT neurtransmitter turnover and release, which in turn can influence and increase the size of both the pre and postsynpatic substrates (Goelet & Kandel, 1986; Krystal, 2001) and even gene expression. This may induce not only alterations in learning and memory, but increased emotional reactivity and alarm responses that may persist indefinitely (Charney et al. 1993; Goelet & Kandel, 1986).
Under chronic conditions, effected neurons and associated neural networks mayalso become adapted to reduced 5-HT and NE levels, thereby increasing the likelihood of enhanced startle reactions, social withdrawal, feelings of depersonalization, hallucinatory states, sleep disturbances, increased REM latency, and other associated symptoms associated with a chronic post traumatic stress disorder (Carlson & Rosser-Hogan, 2008; Cassiday et al. 1992; Charney et al. 1993; Foa et al. 2008; McNally et al. 2001; Putnam, 2002; Wilcox, et al. 2008; Van der Kolk, 2005). It is thus noteworthy that blockage of 5-HT reuptake (which increases the amount of 5-HT in the synapse) acts to reduce the symptoms of post-traumatic stress disorder (Hollander et al. 2001).
DEPRESSION, 5HT & NE
As is now well known, major factors in the pathogenesis of depression include disturbances in the LC-NE and 5HT systems (Arranz et al. 2009; Delgado et al. 2009). For example, a disturbance in NE synthesis and metabolism, regardless of its cause, will disrupt limbic and cortical functioning and arousal, reduce neuroendocrine activity, and thus the organisms ability to mobilize its defenses in response to stress, adverse experience, or even self-defeating thoughts. Every component of social-affective expression will be altered, especially that related to the experience of pleasure and reward -a condition conducive to depression.
Similarly, when 5-HT levels are reduced individuals may become depressed. There is also an increased tendency to respond to non-rewarding situations (Sourbrie, 1986) and to continue to respond regardless of punishment (Spoont, 1992). Effected individuals may fail to avoid or may even be drawn to abusive or potentially frightening or traumatic situations, and may seem helpless to alter their behavior, e.g. learned helplessness (see Charney et al. 1993; Krystal 2001). Indeed, reduced 5-HT has been repeatedly noted in the brains of those who have committed suicide and taken their lives in a violent fashion (e.g. Brown et al. 1982; Cronwell & Henderson 1995).
Although it is difficult to classify a depressive episode or psychosis strictly in terms of a biochemical abnormality, it has been known for over two decades that there are at least two subgroups who differ in regard to depressive symptoms and NE and 5HT activity levels (Goodwin et al. 1978; Van Dongen 1982; Van Praag 1982). For example, those with an NE depression may behave in a more emotionally labile and expressive manner, whereas those with a 5HT depression may demonstrate a much more profound motor retardation coupled with social withdrawal and confusion due to sensory overload.
Moreover, both groups respond differently to pharmacological treatment. For example, some respond to best to impramine which inhibits NE reuptake (thus increasing NE levels in the synapse), whereas others react best to amitriptylline which potentiates 5HT (Goodwin et al. 1978; Van Praag 1982) and inhibits NE reuptake. This suggests that among some individuals NE and 5HT may simultaneously contribute to a distinct form of depression.
It has also been argued that reduced 5HT does not cause depression per se and is not linearly related to the level of depression (Delgado et al. 2009). Rather 5HT may be a predisposing factor in depression which in turn may be related to a postsynaptic deficit in 5HT utilization or binding affinity (Arranz et al. 2009; Delgado et al. 2009).
DREAMING, SLEEP & RHYTHMIC & OSCILLATING BRAINSTEM ACTIVITY
As noted, many of the functions performed by the brainstem are not only reflexive and stereotyped but characterized by specific oscillating rhythms. For example, the sex act involves the temporal-sequential insertion and movement of the male penis and rhythmic motion of the hips, whereas walking involves the rhythmic control of the extremities. As noted in chapter 5 (and above) over the course of neuronal evolution dorsally situated neurons, including those which came to form the pineal body, and portions of the thalamus and midbrain, were highly responsive to light and in consequence developed circadian and rhythmic cycles of activation.
Hence, a considerable number of neurons located in the midbrain and pons display a bursting, oscillating rhythm -a pattern that also accompanies controlled locomotion and a host of other cyclic activities. This includes 5HT and NE neurons which are also involved in controlling a wide variety of rhythmic functions, including respiration, chewing, and micturation (Skinner & Garcia-Rill, 2001) as well as sleep and dreaming. Presumably, these interconnected monoamine, cholinergic and catecholaminergic neuronal clusters are out of phase which induce cyclic oscilations (Skinner & Garcia-Rill, 2001) including those involved in sleep and dreaming.
REM-OFF & REM-ON NEURONS
It is now well known that the visual-emotional hallucinatory aspects of dreaming occur during REM, whereas more thought-like and verbal ideational patterns are produced during NREM. A variety of nuclei and brain regions appear to be involved in the production of REM, i.e. the amygdala, hippocampus, right temporal lobe, and especially brainstem nuclei located in the lateral and medial pons.
Specifically, cholinergic neurons located in the lateral pons, and neurons located in the medial pontine reticular formation appear to be the locus for REM-on neurons which initiate and/or maintain the production of REM sleep (see Steriade & McCarley, 2001; Vertes 2001). That is, during the production of REM and paradoxical sleep, there is increased cholinergic activity in these regions, whereas with the termination of REM, these same neurons have greatly reduced their activity.
Similarly, when cholinergic agnonists are injected into the medial pons, REM sleep is produced (Steriade & McCarley, 2001). When cholinergic agnonists are injected into the lateral pons, muscle atonia is produced -a common characteristic of paradoxical sleep. Thus, REM on cells tend to be cholinergic and become very active during paradoxical sleep, induce muscle atonia, but markedly decrease their activity during slow wave and non-REM sleep at which point atonia also tends to disapear.
In contrast, REM-off neurons, which tend to be located in the medial raphe nucleus (which contain 5HT neurons) and in the locus coeruleus (located at the midbrain-pons junction and which contain NE neurons) are highly active during waking but then begin to reduce their activity during slow wave sleep and then significantly decrease their activity with the initition and onset of REM and the production of pontine, lateral geniculate, occipital activation; i.e. PGO waves (see Steriade & McCarley, 2001; Vertes 2001). Conversely, experimental cooling (and thus deactivation) of the LC consistently and repeatedly induces REM sleep (Cespuglio et al. 1982).
Hence, these REM-Off neurons appear to suppress REM and PGO activity and interfere with the onset of dreaming and paradoxical sleep, whereas REM-on neurons initiate the opposite sleep phase in which case the brain appears to be highly active (e.g. the amygdala, pons-geniculate,-occipital lobe) and the individual begins to dream.
REM-on and REM-off neurons, therefore, appear to oscillate in a rhythmic fashion thus inducing sleep, dreaming, and waking. When the REM-off neurons cease to fire, the REM-on neurons (which are predominately cholinergic) become highly active until a REM episode is produced. However, as REM-on neuron activity decreases, REM-off activity increases, thus setting into motion a continuous 90 minute cycle of REM - Non-REM sleep alteration.
In addition, and as noted above, whereas NE and 5HT (i.e. sensory motor neurons) play a significant role in the generation of dream (paradoxical) sleep, GABA and glutamate (modulatory) pace maker neurons, also play a significant role in the oscillation between REM and NREM, in part by acting on REM-off neurons. That is, REM-off neurons are turned off by modulatory inhibition (GABA), whereas REM-on neurons are turned on by modulatory excitation (glutamate).
However, these latter oscillating on/off activities appear to take place not only in the brainstem, but in the hypothalamus. That is, because the hypothalamus (via the suprachiamatic nucleus) is also involved in pace maker functions, whereas the posterior hypothalamus is involved in sleep (Sherin, et al., 1996) there is some evidence to suggest that this GABAergic inhibition may actually begin in the hypothalamus thus initiating sleep onset and the first sleep stage (NREM). GABAergic inhibitory actions then slowly spreads to and then throughout the brainstem only to become self-inhibiting and replaced by glutamate (excitatory) secretion which triggers REM-on neurons, and thus the next stage of sleep (REM).
Because of these on/off excitatory/inhibitory activities, during NREM NE and 5HT levels decline (Hobson, et al., 1975; Lydic, et al., 2008). However, ACh levels actually increase (Hobson, et al., 1975; Lydic, et al., 2008) and in fact remain at high levels during REM and the production of pontine, lateral geniculate, occipital activation; i.e. PGO waves (see Steriade & McCarley, 2001; Vertes 2001). As ACh is also implicated in memory, this may well explain whey recent memories tend to become incorporated in dreams.
PGO WAVES
Paradoxical sleep and the production of dream states are associated with the development of high levels of activity within the pons, the lateral geniculate nucleus of the thalamus, the occipital lobes, and association and motor cortices (Steriade & McCarley, 2001) -activity collectively referred to as PGO waves (see Hobson et al. 1986). Presumably, as these neurons are activated, visual imagery is produced as these regions are concerned with analyzing visual input as well as visual scanning of the environment.
However, PGO waves, although generated in a variety of brainstem reticular neuronal clusters, are also induced by amygdala activation (Calvo, et al. 2005). In addition, during REM, the hippocampus begins to produce slow wave, theta activity (Jouvet, 1967; Olmstead, Best, & Mays, 1973; Robinson et al. 1977), which is associated with long-term potentiation which is associated with learning and memory (see chapter 14).
The amygdala, in concert with the hippocampus, therefore appears to contribute emotional and visual imagery and experiential impressions to the dream state--a theory first proposed by this author (Joseph 1982, 1988a, 1992a) and which has subsequently been embraced by numerous investigators. Indeed, via the extensive interconnections the amygdala maintains with the brainstem and the geniculate and occipital lobes, and its capacity to induce high levels of arousal, this nucleus is ideally situated to initiate if not produce (again in conjunction with the hippocampus) the auditory-visual imagery characteristic of the dream state including all aspects of related emotion (see chapter 13). Moreover, the amygdala also receives 5HT and NE from the brainstem (with which it is intimately interconnected), thereby creating an elaborate feedback circuit.
The lateral pontine reticular formation is also involved in producing hippocampal theta during REM (Vertes, 1984). As detailed in chapter 14, hippocampal theta is associated with arousal, and theta activity may represent the introduction and transformation of hippocampal based memory-imagery into dream imagery. However, the production of hippocampal theta may also help insure that whatever is experienced during REM sleep is stored in such a fashion that dream stimuli do not become committed to memory.
Nevertheless, in regard to dream sleep, the amygdala and hippocampus appear to be largely dependent on the brainstem, for when disconnected (see below), although the brainstem continues to demonstrate a sleep wake cycle, the forebrain will cease to demonstrate paradoxical sleep activity. Moreover, although the amygdala can induce PGO activity, in general, neurons in the upper medial pontine reticular formation begin to fire well in advance of the development of PGO waves (McCarley & Ito, 1983). It is also these pontine neurons (in conjunction with the cranial nerves and midbrain) which initiate eye movements during REM. Typically, PGO waves reach their maximum amplitude in the lateral geniculate nucleus of the thalamus which (like the amygdala) are also cholinergically responsive (Steriade & McCarley, 2001).
It is noteworthy that PGO-on neurons also become active during waking when eye movements are made (Nelson et al. 1983). Therefore, PGO waves are not strictly a phenomenon associated with sleep, but can occur when the subject is emotionally aroused and perhaps looking anxiously around for the source of stimulation -which again implicates the amygdala in their production.
Characteristically, however, PGO waves and their transfer to the thalamus has two distinct states (Steriade & McCarley, 2001). That is, they first appear during the last stages of synchronized sleep just prior to REM, and then again, accompanying the development of desychronized sleep and REM.
THE MIDBRAIN RETICULAR FORMATION & REM
Although NE and 5HT neurons appear to make a major contribution to the development of sleep and dreaming, REM is also dependent on activity within the midbrain reticular formation -activity which presumably includes excitation of the superior (visual) colliculus which in turn can trigger eye movements and orienting reactions to sensory stimuli.
Midbrain reticular activity during waking and REM is twice that during slow wave sleep (Steriade & McCarley, 2001). Midbrain reticular neurons also markedly increase their activity during the transition from slow wave to REM sleep.
Conversely, if the midbrain is transected, REM does not appear in the forebrain, although all associated components are preserved in the brainstem, including REM (although reduced), muscle atonia, and the pontine contribution to PGO waves (Hobson et al. 1986; Steriade & McCarley, 2001; Vertes 2001). Transection of the lower (medulla) brainstem does not significantly effect REM sleep (Siegel et al. 1986), which again implicates the midbrain and pons as the dream-sleep generators. However, they also contribute to the waking state as well.
For example, midbrain reticular neurons tend to significantly reduce their activity immediately prior to spindle production as well as during the transition from wakefulness to sleep and during drowsiness (Steriade & McCarley, 2001). However, reticular activation can also abolish the thalamic production of spindles, such as occurs during waking and paradoxical sleep. Hence, brainstem stimulation or activity can either inhibit or promote spindle wave production and induce paradoxical as well as slow wave, synchronized sleep.


SYNCHRONIZED, SLOW WAVE SLEEP
The defining feature of synchronized (or quiet, Non-REM) sleep is large amplitude rhythmic EEG waves of varying frequencies (from slow to fast). Even during waking, however, and when highly aroused or alert, some cortical areas may display synchronized EEG activity, whereas even during REM sleep some cortical areas display synchronized activity (rviewed in Steriade & McCarley, 2001). Presumably in that some cortical areas demonstrate slow wave activity whereas yet others are highly aroused, is a reflection of selective attention and discrete neocortical activation. Some cortical regions becomes suppressed thus narrowing the range of incoming stimuli whereas other specific regions remain activated in order to process incoming information and/or for the purposes of contributing to whatever mental process may be taking place.
Steriade and McCarley (2001) propose that the development of synchronized sleep is dependent on the removal or lessening of brainstem input and is a passive rather than an active process. That is, fluctuations and decreases in cholinergic and non-cholinergic activity within the reticular formation decreases thalamic activity thus producing sleep spindles in the reticular thalamic nucleus followed by synchronous activity. However, with increases in cholinergic and non-cholinergic activity, thalamic activity increases, and synchronous EEG activity is replaced by desynchronization and the production of REM (see also Hobson et al. 1986; Vertes 2001).
THALAMIC CONTRIBUTIONS
Given that the thalamus acts to relay sensory input to the neocortex, not surprisingly thalamic neurons are also involved in synchronized and paradoxical sleep. Specifically thalamic neurons inhibit sensory transfer to the neocortex during the synchronized sleep stage as well as during the transition from wakefulness to sleep and when drowsy; otherwise the individual would keep waking up due to neocortical activation.
Some thalamic neurons, however, act to enhance sensory transfer during desynchronized sleep (Steriade & McCarley, 2001) as well as during waking. Presumably this allows for sensory stimuli to become incorporated into and to in fact induce dream states.
Specifically, the reticular thalamic nucleus appears to also act as a "pacemaker" center for the development of sleep spindles (i.e. EEG waves which wax and wane between 7 and 14 Hz) and which herald the onset of sleep (Steriade & McCarley, 2001). Spindles are typically associated with loss of consciousness and thalamic inhibition and thus loss of information transfer to the cortex. Moreover, spindles originate in the reticular thalamus, which in turn projects to other thalamic neurons. These thalamic nuclei are indirectly under the control of the frontal lobe and dorsal medial thalamus (see chapter 19).
MOTOR INHIBITION DURING SLEEP
The frontal lobe as well as brainstem motor areas become activated during REM (see Steriade & McCarley, 2001). However, although motor commands may be initiated and even transmitted from the neocortical motor centers, they cannot be acted out during REM due to the production of muscle atonia. Muscle atonia is produced, in part, via descending 5HT influences on the spinal cord (see above).
Specifically, during the transition from slow wave to paradoxical sleep motor neurons in the brainstem and the spinal cord come to be actively inhibited (Chandler, et al. 1980; Chase & Morales 2002). The result is muscle atonia, or what has also been referred to as sleep paralysis. This condition is almost exclusively associated with REM sleep (Chase & Morales 2002).
Presumably these inhibitory influences arise within the pontine and bulbar brainstem reticular formation and via descending 5HT influences so as to prevent an individual from motorically acting out their dreams and possibly injuring themselves (e.g. sleep walking). Indeed, the dorsal lateral portion of the pontine tegmentum has been repeatedly implicated as responsible for muscle atonia during sleep (see Steriade & McCarley, 2001).
By contrast, lesions of the pontine reticular formation (with sparing of the locus coeruleus) abolishes muscle atonia (Hendricks, et al. 1982; Jouvet, 1979). In consequence, during REM sleep, the animal will move about and engage in semi-purposeful behaviors, including walking, orienting movements, and attack -as if the animal were acting out its dream (Hendricks, et al. 1982).
BRAINSTEM SLEEP DISORDERS: NARCOLEPSY & CATAPLEXY
NARCOLEPSY
Narcolepsy is a syndrome that is often chateracterized by excessive daytime sleepiness, and may be accompanied by partial or complete and sudden attacks of REM sleep where the patient was quite awake just moments before (Broughton 2001; Guilleminault, 1989). In some instances patients may also experience sleep paralysis, hypnagogic hallucinations, and cataplexy. These daytime attacks sometimes follow the 90 minute cycle characteristic of REM sleep. Abnormalities involving rapid eye movements (REM) and motor disturbances such as muscle twitching and persistence of muscle tone are not uncommon (Schenck & Mahowald 1992), and the latency of REM sleep is often very short.
This condition is often treated with amphetamines and with tricyclic anti-depressants. It is also believed to be secondary to abnormal concentrations of NE and DA. However, narcolepsy has several different components.
Excessive Daytime Sleepiness (EDS). EDS involves the sudden sleep onset of REM involving dreaming. These sleep isodes tend to last from a few minutes up to half an hour. Sleep Paralysis (SP). SP usually occurs upon waking, such that the person experiences a paralysis of the voluntary muscles. They cannot open their eyes, move their arms or legs, and cannot speak. Some are terrified by these experiences. Presumably, many people have experienced this at least once in a mild form. The condition tends to last just a few minutes.
Presumably SP is due to the abnormal prolongation of activity within the dorsal lateral portion of the pontine tegmentum, a region which is normally responsible for producing muscle atonia during sleep (see above).
Hypnogogic Hallucinations. Some patients exerience hypnogogic hallucinations upon waking and while seemingly completely conscious. These can be exceedingly vivid and realistic and involve auditory, kinesthetic, and somesthetic as well as visual hallucinations. The effected individual may see what they believe to be ghosts or even intruders attempting to attack them. Hypnogic hallucinations (and sleep paralysis) appear to represent the intrusion of REM into wakefulness.
SOMNAMBULISM
Sleep walking is most common among children, and presumably up to 15% of all children sleep walk at one point in their lives (see Kavey, et al. 2001). Among adults, it occurs in less than 1% of the population.
Somnambulism occurs during the first third of the night during NREM sleep. Usually patients have no recollection or only a vague memory of walking about (Kavey, et al. 2001). While sleep walking some patients may behave in an aggressive or agitated manner, scream and engage in fighting or fleeing behaviors and may fall or strike and injure themselves. Fragmentary memories may include being chased.
Adults who sleep walk often have a history of sleep walking during childhood (Kavie et al. 2001). Some patients demonstrate epileptiform EEGs and/or suffer form atypical nocturnal complex partial seizures (Pedley & Guilleminault 1977).
REM Sleep Behavior Disorder.
REM sleep behavior disorder is associated with narcolepsy and REM sleep (Schenck & Mahowald, 1992), and is a form of motor disinhibition accompanied by often violent behaviors. Patients appear to be acting out their dreams. It usually effects men over 60, though it is also seen in children with obvious brainstem dysfunction (see Schenck & Mahowald, 1992). However, when it accompanies narcolepsy this disorder tends to have it's onset in men in their late 20's (Schenck & Mahowald, 1992).
Presumably somnambulism and REM sleep behavior disorder are a consequence of disinhibition of the brainstem and spinal motor neurons which are normally inhibited during REM. That is, it is likely that the dorsal lateral portion of the pontine tegmentum, a region which is normally responsible for producing muscle atonia during sleep, fails to induce muscle atonia such that the person begins to walk about and act out their dreams. As noted above, destruction of this nuclei also results in motor disinhibition during REM such that the subject begins to move about as if actively engage in purposeful behavior.
CATAPLEXY
Cataplexy involves a sudden loss or decrease of muscle tone; atonia. Hence the effected individual may suddenly fall to the ground, sometimes injuring themselves. In mild cases the jaw may suddenly become slack and the knees become weak and may buckle. In severe cases the patient immediately falls to sleep, and enters REM which is accompanied by dream mentation. Often cataplexic attacks are induced by sudden surprise or attacks of emotion, including anger or laughter.
Cataplexy is generally considered a component of narcolepsy and the production of REM and the loss of muscle tone indeed suggest a brainstem loci for this disorder. However, the influence of emotion in inducing cataplexic attacks and the fact that some individuals become rigid and stiff, almost catatonic, suggests that the amygdala and the basal ganglia and perhaps, indirectly, the medial supplementary motor areas (SMA) of the frontal lobe may also be contributory. That is, in some respects this disorder is reminiscent of abnormalities within the basal ganglia and SMA -nuclei which are significantly influenced by the amygdala.
CRANIAL NERVES OF THE MEDULLA SHOULDER, HEAD, JAW, TONGUE MOVEMENT, BREATHING, PHONATING, HEART RATE
CRANIAL NERVE 12: HYPOGLOSSAL The hypoglossal nucleus located in the caudal medulla gives rise to the 12th nerve which controls tongue movements via innervation of the somatic skeletal muscles. A lesion, tumor, or infarct invading this area can give rise to weakness or atrophy of the tongue. For example, if the tongue is weak and deviates to the right, the ipsilateral lower motor neuron (LMN) is involved, indicating that the right genionglussus muscle is weak. Weakness can be tested by having the patient place his tongue in one side of cheek and ask him to press against your finger (on the outside of cheek). The clinical and EMG signs of an LMN lesion of the 12th nerve are hemiatrophy, unilateral fasiculation or fibrillation, and usually severe paralysis with obvious deviation to the paralytic side when the tongue is protruded.
CRANIAL NERVE 11: SPINAL ACCESSORY
Cranial nerve 11 consists of two distinct segments; a cranial portion which, along with the vagus nerve, forms the inferior laryngeal nerve which innervates the muscles of the larynx; and a spinal portion which innervates the sternocleidomastoid and upper trapezious muscles and therefore aids in turning the head and elevating the shoulders. Injuries to this nerve may result in a sagging of the shoulder on the affected side, or weakness in turning the head -particularly when resistance is applied.
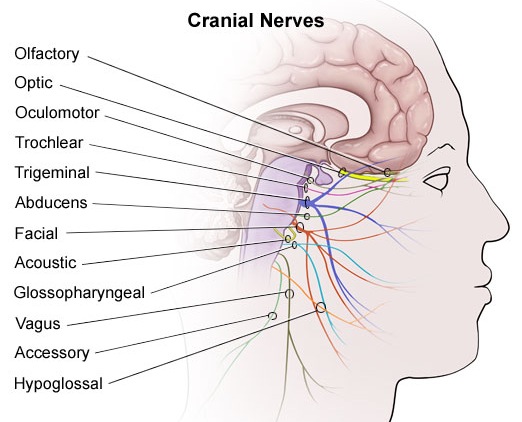
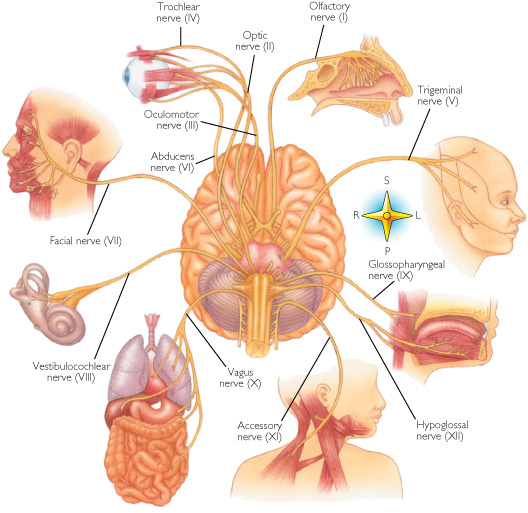
CRANIAL NERVE 10: VAGUS
[ The vagus nerve is actually a complex mix of nerves which innervate a variety of structures including the trachea, larynx, pharnyx, esophagus, and abdominal and thoracic viscera, the epiglottis, and the external auditory meatus. Hence, this nerve (in conjunction with the 9th nerve -both of which are derivetives of the skeletal muscles that orginaly formed the brachial (gill) arches) is important in regard to oral activities, including swallowing, breathing, and speaking, pharyngeal constriction and thus with movement of the palate, pharnyx and larynx. The vagus nerve is therefore responsible for swinging the soft palate upward and backward to contact the posterior wall of the pharynx, sealing off the oropharynx from the nasopharynx when one swallows, whistles, or speaks.
An injury to this region can result in severe and enduring palatal weakness as well as a condition referred to as pseudobulbar palsy. Unless the soft palate elevates properly liquid will escape into the nose when one drinks, and when one speaks resulting in nasal speech. Indeed, damage to these nuclei can severely effect speech.
CRANIAL NERVE NINE: GLOSSOPHARYNGEAL
The 9th nerve is closely related to the vagus and both have similar functional components, including general somatic and visceral afferents and efferents. The glossopharyngeal nerve thus receives tactile, thermal, and pain sensations from the tongue, and contributes fibers to the solitary nucleus thereby forming the gustatory nucleus. In addition, the glossopharyngeal nerve receives fibers from the carotid sinus which transmit impulses regarding increases in carotid arterial pressures. The ninth nerve then transmits this data to the solitary nucleus which in turn contributes fibers to the vagus nerve. In this manner the 10th and 9th nerve can induce reductions in arterial blood pressure and heart rate.
Lesions to the 9th nerve generally results in a loss of taste and sensation in the posterior third of the tongue, and a loss of the gag reflex and the carotid sinus reflex. In some cases intense pain may be triggered by swallowing or coughing.
LATERAL MEDULLARY SYNDROME
The lateral medullary syndrome is typified by dysphonia (hoarse voice), persistent hiccup, disordered equilibrium, vertigo, nausea, and loss of thermal sense and pain involving the contralateral half of the body and the ipsilateral half of the face. Usually this condition is due to vascular abnormalities involving the vertebral arteries, or occlusion of the posterior inferior cerebellar artery.
THE CRANIAL NERVES OF THE PONS
THE EIGHT CRANIAL NERVE & THE COCHLEAR SYSTEM
TINNITUS, DEAFNESS, DIZZINESS, VERTIGO
A portion of the 8th nerve arises from the cochlear nuclei so as to form the auditory branch of the 8th nerve which in turn bifurcates and innervates the ampulla, utricle, saccule and cochlear duct of the semi circular canals of the inner ear. It is via the acoustic nerve that auditory stimuli are relayed to the ventral and dorsal cochlear nuclei for further transduction prior to transfer to the inferior colliculus of the midbrain, and the medial geniculate nucleus of the thalamus. Hence, lesions involving this nerve or the cochlear nucleus can give rise to significant hearing problems, including deafness and tinnitus --ringing in the ears which may be reported as buzzing, humming, whistling, roaring, hissing, or clicking.
Tinnitus
There are two general types of tinnitus, vibratory, and non-vibratory. Non-vibratory tinnitus is actually quite common, being due to contraction of the muscles of the inner and middle ear, but is usually masked and may only be heard at night or when in a very quiet environment. In contrast, vibratory tinnitus is most often due to disorders of the inner ear, ossicles of the middle ear, tympanic membrane, or 8th nerve, and may be accompanied by deafness in one ear.
Deafness
If a patient complains of deafness, it must be determined if this is due to otosclerosis, chronic otitis, occulsion of the external auditory canal or eustachian tube, in which case the problem is conductive and is not related to nerve damage. By contrast, disease of the cochlear or of the cochlear division of the 8th nerve, or its central connections results in sensorineural (or nerve) deafness.
These two distinct disorder can be easily differentiated with the help of a tuning fork. When struck, it is placed at the center of the forehead or on the mastoid bone behind the ear so that the vibration can bypass the middle ear and can be mechanically conveyed to the inner ear so as to excite auditory impulses.. If the condition is due to nerve deafness, the sound is localized in the normal ear and fails to be perceived by the effected ear. If the disorder is conductive, the sound is heard as louder in defective ear. If there is an obstruction or a middle ear abnormality (vs a nerve disorder) then the sound is heard louder in that ear due to dampening of noise via the air conduction block. If there is a lesion of the nerve then the sound is heard in the opposite of normal ear only.
THE EIGHTH (VESTIBULAR) NERVE
The vestibular division of the 8th nerve arises from the vestibular nerve which innervates the labyrinth and the maculas of the saccule and utricle and the ampullae of the semicircular canals. As noted in chapter 5, the vestibular system was derived from the lateral line system, and in turn gave rise to the evolution of the cerebellum.
The major concern of the vestibular division is not hearing or vibratory perception, however, but determination of the body's position in visual-space so as to maintain equilibrium during movement. All sensory receptors for the vestibular system are located in the membranes of the labyrinth of the internal ear, and in the vestibular ganglion which is in the internal auditory canal. Thus it can determine changes in position via alterations in fluid balance within the semi-circular canals.
Lesions of the vestibular receptors, their nerves, or central connections cause abnormal sensations of movement, vertigo, nausea, tendencies to fall, dizziness, and motion sickness, etc.
The vestibular system also provides information about the position of the head and correlates head and eye movements with somatic muscle activity. Together with the descending medial longitudinal fasciculus (MLF) and vistibulospinal tracts, the vestibular system is able to mediate the postural reflexes. In part it is able to accomplish this via rich interconnections with those cranial nerve nuclei (via the MLF) which subserve eye movement (nerves VI, IV, & III). Disease involving these tissues can therefore cause nystgamus.
Following injuries to this system, patients may complain of to-and-fro or up-and-down movements of body, or floors, and noted that the walls seem to tilt or sink or rise. When walking there may be feelings of unsteadiness such that they veer to one side. Or there may be a feeling of being pulled or drawn--a feeling of impulsion. There also may be a disincilination to walk (particularly during an attack), a tendency to list to one side, and the condition may be aggrevated by riding in a vehicle. Some disturbances may occur only for a few seconds, or after lying down or sitting up, turning, etc. When less severe the patient may merely veer to one side while walking.
The vestibular system is also concerned with eye movement, for it is also via ocular signals that the position of the head and body in space can be determined. Hence, vestibular dysfunction can include difficulty focusing or fixating on objects while walking, or when the object is moving. This is due to a loss of stabilization of ocular fixation by the vestibular system during body movement and is caused by an inability to integrate visual with vestibular input. These functions are normally made possible through rich interconnections (via the MLF) between the vestibular system and the 6th, 4th and 3rd nerves which subserve eye movement, as well as the bilateral interconnections between these regions and the cerebellum.
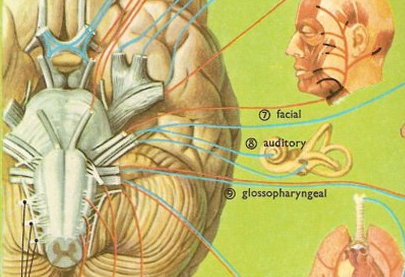
ORAL-FACIAL MOVEMENT & SENSATION
THE SEVENTH CRANIAL (FACIAL) NERVE
The 7th nerve, and the brainstem nuclei which it innervates, is concerned with facial movement, including elevation of the eyebrows, retraction of the lips, closure of the auditory canals, as well as with gustatory sensation. Injuries to the 7th nerve can therefore produce a lip retraction, eyebrow lifting or eyelid closure paralysis; i.e. Bell's palsy. Patients have difficulty or are unable to wrinkle the forehead, purse their lips and show their teeth,and the corner of the mouth may droop.
In addition, whereas cranial nerves 9 and 10 innervate the taste budes of the posterior 3rd of the tongue, cranial nerve 7 innervates the anterior 2/3s. Hence, a lesion of the 7th nerve can result a disturbance of taste sensation.
In addition to the muscles of the face, the 7th nerve also innervates the stapedius muscle, which acts to dampen excessive sound via inhibition of the movement of the ossicules. If the 7th nerve is injured, the stepedious may become paralyzed and the patient may report that sounds are uncomfortably loud. However, disturbances of hearing are most usually associated (at least at the brainstem level) with damage involving the 8th cranial nerve.
As to oral and facial movement including swallowing and speaking, the functional integrity and participation of the 5th nerve is also important for it controls the jaw. In this regard, the 5th, 7th, 9th, 10th, and 12th nerve and associated nuclei frequently act in concert regarding oral-facial, jaw, head and shoulder movement, and are richly interconnected.
THE FIFTH CRANIAL NERVE: TRIGEMINAL
The 5th nerve is the largest of the cranial nerves and innervates the trigeminal nucleus within the medulla. It is concerned with jaw closure, as well as chewing, grinding, and lateral movement of the jaw. In this regard the 5th nerve also acts in concert with the 7th nerve which innervates all the muscles involved in facial expression. These muscles control the size of every facial aperature, including the auditory canals. A lesion involving this nucleus, therefore, can result in difficulty chewing, and if severe, atrophy and complete paralysis of the left or right temporal and masseter muscles. Paralysis and atrophy are always ipsilateral to the lesion.
The general somatic afferent components of the 5th nerve also mediate the general sensory modalities for the face, teeth, and mouth, and the mucous membrances of the nose, check, tongue, and sinuses. These general sensory functions include proprioception, touch, pain, and temperature.
EYE MOVEMENT THE SIXTH CRANIAL NERVE: ABDUCENS
The abducens is a motor nerve that innervates the lateral rectus muscle of the eye, and is part of a collection of fibers which are found with the loop of fibers that also form the facial nerve. However, fibers from the abducens nuclei also ascend the brainstem and terminate on neurons belonging to the occulomotor complex which innervates the medial rectus muscle. It is therefore responsible for horizontal eye movements. In large part, the pontine center for lateral gaze and the abducens nuclei form a single entity (Carpenter 2008). In addition, the abducens is linked to the pontine/midbrain center for vertical gaze (the rostral interstitial nucleus of the MLF).
Thus abducens nerve and nuclei are the pontine centers which control lateral eye movements outward to the right or to the left. Injuries to the 6th nerve can therefore produce a lateral gaze paralysis as well as a paralysis of the lateral rectus muscle which results in double vision (horizontal diplopia).
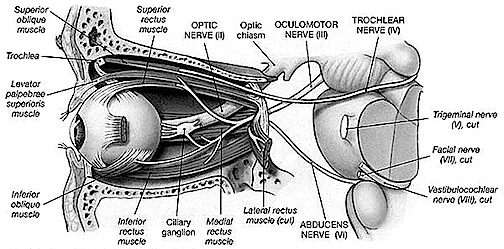
CRANIAL NERVES OF THE MIDBRAIN EYE MOVEMENT
Movement of the eye is dependent on the functional integrity of the posterior parietal neocortex, the frontal eye fields of the lateral frontal lobes, the midbrain visual colliculi, cerebellum, and the cranial nerves and nuclei of the upper pons (cranial nerve VI) and midbrain (nerves IV & III). All pathways mediating saccadic, pursuit, and vestibulocular movements, including those originating in the forebrain and midbrain, then converge onto the pontine centers for horizontal gaze.
THE 4TH NERVE: TROCHLEAR
As noted, the 6th (abducens) nerve and nuclei are the pontine centers which control lateral eye movements outward to the right or to the left. By contrast, the 4th (trochlear) nerve and nucleus is located just caudal to the inferior colliculi, and innervates the superior oblique muscle of the eye; a muscle which has three actions: depression, abduction, intorsion. The 4th nerve therefore assists in moving the eye downward or inward.
THE 3RD NERVE: OCULOMOTOR
The 3rd (oculomotor) nerve is responsible for rotating the eye upward, downward, as well as inward and innervates all ocular rotary muscles (except for the lateral rectus and superior oblique). These include the medial, superior, inferior recti and inferior oblique.
The 3rd never also innervates the intraocular and smooth muscles of the pupil; i..e. the ciliary and pupilloconstrictor muscles. Lesions, therefore, may result in an inability to rotate the eye upward, downward, or inward, and the pupil (on the side of the lesion) may fail to respond to direct light.
In addition, the 3rd nerve innervates the levator palprebrae muscle which elevates the eyelid. When the third nerve has been injured this may result in levator palpebrae weakness and thus ptosis of the eyelid.
DISTURBANCES OF EYE MOVEMENT
Eye movement nuclei are all located in the pontine brainstem and receive input from (and transmit information to) the superior (visual) colliculus within the midbrain (Grantyn & Grantyn, 1976), and the frontal eye fields (Leichnetz, et al. 1984), which also projects to the superior colliculus (Segraves & Goldberg, 2005). As is well known, the frontal eye fields and the superior colliculus become activated immediately prior to the onset of an eye movement, and stimulation of these regions elicits eye movements and visual orienting reactions as well.
Hence, damage to these cortical areas or to any of these nerves or nuclei can result in significant visual disturbances. For example strabismus (or squint) is due to a brainstem lesion that results in muscle imbalance and thus improper alignment of the visual axes of the two eyes. A complete lesion of the oculomotor nerve causes ptosis (drooping of the upper eyelid) and an inability to rotate the eye upward, downward, or inward. Lesion of the 4th nerve results in weakness of downward movement of the affected eye and patients may complain of difficulty reading or walking downstairs. Head tilting to opposite shoulder is especially characteristic. Lesion of the 6th nerve results in paralysis of lateral or outward eye movement.
Most common causes of 3, 4, 6th nerve damage are tumors of the base of the brain, trauma to head, ischemic infarction of one of these nerves, and aneurysms of the circle of Willis. Sixth nerve palsies in children are due to neoplasm-pontine glioma. The fourth nerve is most commonly injured by head trauma. Third nerve damage is often due to compression by aneurysm, tumor, or temporal lobe herniation--enlargement of the pupil is an early sign.
As noted, cranial nerves 3, 4, and 6 are linked to the 8th (vestibular) nerve via the MLF. It is due to these rich interconnections that a lesion involving these regions and the MLF can cause vertigo, dizziness, as well as nystagmus and impairment of vertical fixation and pursuit. However, disturbances such as these also raise the possibility of cerebellar dysfunction. (reviewed in Stein & Glickstein 1992).
THE OPTIC NERVE
The optic nerves are responsible for transmitting all visual impulses form the retina to the brain, first crossing over at the optic chasm, and then penetrating the brain to form the optic tracts which terminate in the lateral geniculate nucleus of the thalamus. Specifically, beginning at the retina, the rods and cones project to horizontal and bipolar cells which in turn project to X, Y and W ganglion cells within the retina. Axons of the retinal ganglion cells run in parallel along the surface of the retina and converge at the optic disc (the blind spot) where they gather together and punch through the retina thereby forming the optic nerve.
The right and left optic nerves project into the cranial cavity via the optic foramina and then unite to form the optic chiasm where a decussation occurs, thereby forming the optic tracts. Subsequently, visual input from the left half of visual space projects to the right hemisphere, and input from the right half of visual space projects to the left cerebrum via the optic tracts which in turn project predominantly to the lateral geniculate nucleus of the thalamus, and to a much lesser extent, to the superior colliculus, and probably the hypothalamus, amygdala, and inferior temporal and superior parietal lobes. Because the optic nerve does not project directly to the brainstem, it is not a true cranial nerve -at least in mammals- although some fibers (via the optic tract) are received in the midbrain visual colliculi.
From the lateral geniculate nucleus these visual fibers form the optic radiations which project predominantly to the striate cortex within the occipital lobe, and give off collaterals to the inferior temporal and superior parietal lobe where the upper and lower visual fields are also represented.
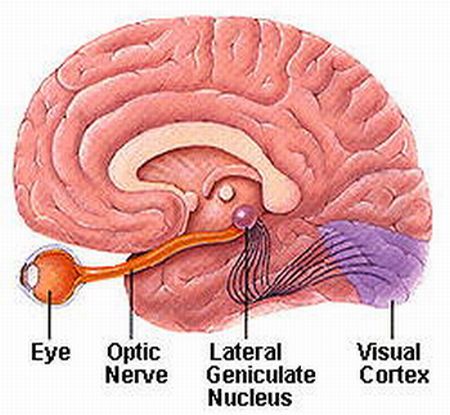
Injury to the optic pathways necessarily produce visual defects and are referred to as homonymous if they are restricted to either the right or left visual field, or heteronymous if both visual fields are disrupted to some degree. Heteronymous defects suggest a lesion involving both cerebral hemispheres, or a lesion to retina or the optic nerve before it crosses over at the decussation.
If the visual defects are homonymous, then the lesion can be localized to one side of the optic tract or radiations and thus to the right or left hemisphere. Complete destruction of the optic tract or its terminal zones induces a homonymous hemianopsia to the left or right, whereas a partial injury may induce a quadratic homonymous defect: upper quadrant being associated with temporal lobe defects, and lower quadrant defects associated with the superior parietal lobe.
THE OLFACTORY NERVE
The olfactory nerve also bypasses the brainstem, and is not a true cranial nerve, but a complex axonal pathway which projects directly to a wide variety of forebrain structures, including the amygdala, entorhinal cortex, hypothalamus, orbital frontal lobes, and the dorsal medial nucleus (see chapter 12). Nevertheless, the olfactory nerve is associated with brainstem functions as originally it was via the olfactory system that information regarding not just smell but taste were derived. That is, early in the course of evolution, the olfactory pathway split apart and became segregated (perhaps with the emergence of the vomeronasal organ) so as to form distinct pathways that subserve smell and taste. Nevertheless, smell remains important to taste, which is why when suffering from a severe cold, there is a generalized dampening of the sense of taste.
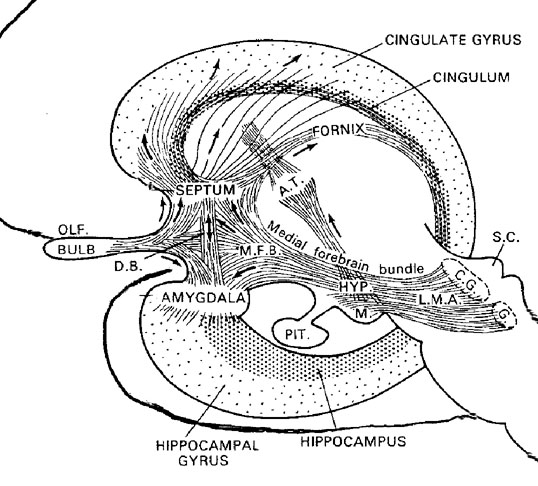
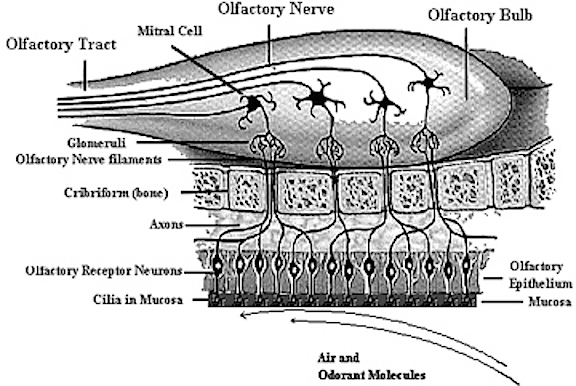
The olfactory nerve originates via bipolar cells located in the olfactory epithelium which in turn is composed of very primitive cells that have a life span that last only days. New axons and new synapses are continually forming. These nerve fibers perforate and pass through the cribriform plate (making them very susceptible to injury and shearing -see chapter 20) and project directly to the olfactory bulb. From the olfactory bulb the olfactory tract is formed which projects to pyriform, periamygdaloid, and entorhinal areas -which in turn constitute the primary olfactory cortex. Projections continue to the amygdala, hippocampus, thalamus, orbital frontal lobes, and insula.
Following a head injury the cribriform plate may fracture, the olfactory nerve may be severed, and the meninges may rupture. If this occurs, in addition to loss of smell, cerebrospinal fluid may continually drip or gush into the nose -which suggests a cerebrospinal fluid fistula. To determine if nasal drip is due to CSF leakage or normal mucous secretion, the fluid can be subject to a glucose test tape (as used for urinalysis). CSF contains glucose, whereas mucus does not. If glucose is indicated, call a neurosurgeon.
However, in some cases, a head injury may cause these nerves to be severed although the cribriform plate remains intact. In these instances, loss of smell may result: anosmia. However, this loss may be unilateral or bilateral (involving both nostrils). If unilateral, the patient will not notice the loss, in which case each nostril must be assessed separately.
Dysnosmia refers to a perversion of the sense of smell, which in turn may be due to partial injuries of the olfactory bulbs, or due to tumor of the nasal sinuses or the temporal lobes in which case food may also be said to have an extremely unpleasant odor and/or taste. Similarly, olfactory hallucinations are associated with tumors, seizure activity, as well as head injuries involving the inferior temporal lobes.
THE CEREBELLUM
It is perhaps a matter of convenience to differentiate the most caudal regions of the medulla from the spinal cord, as they are in fact contiguous. At the level of the spinal cord cranial nerves becomes spinal nerves, whereas ascending spinal pathways terminate in specific brainstem nuclei.
The cerebellum is also contiguous with the brainstem, not merely because it is anchored to the pons, through which is receives and transmits a variety of impulses, but because it is an outgrowth of the brainstem. In some respects, in fact, the emergence and differentiation of the cerebellum from the brainstem, parallels the emergence and differentiation of the striatum from the amygdalo-striatum, and then continues to "evolve" in parallel with the motor areas of the frontal lobes--with which it maintains rich interconnections. Thus, whereas the limbic forebrain has "its" hierarchical motor centers, so to does the brainstem and cerebellum













