Rhawn Gabriel Joseph, Ph.D.
Brain Research Laboratory
BrainMind.com

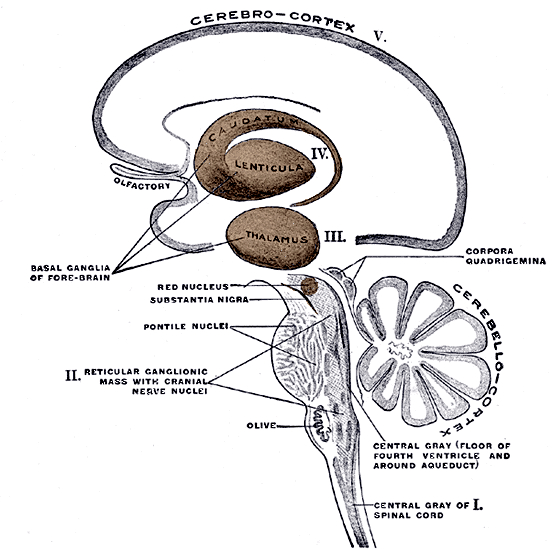
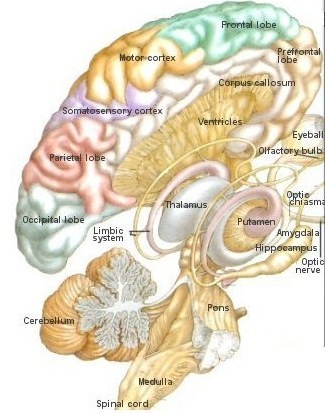
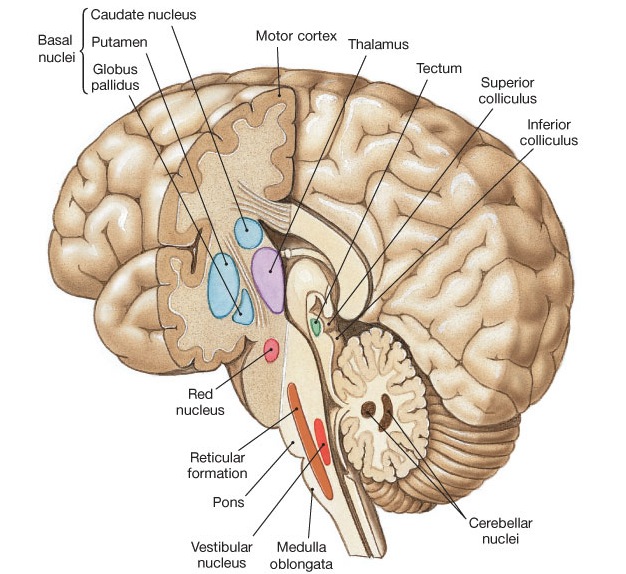
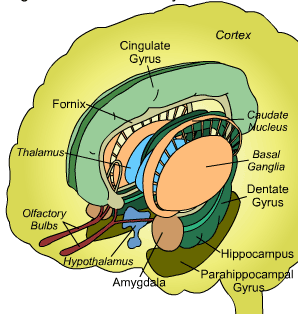
The basal ganglia, whose subdivisions include the brainstem, cerebellum, thalamus, and striatum, guide all aspects of gross muscular activity, such as kicking, hitting, running, walking, swimming, and even smiling. When injured, movement may become labored, stiff, clumsy, or even frozen as well as marked by uncontrollable tremors; as is the case with Parkinson's disease.
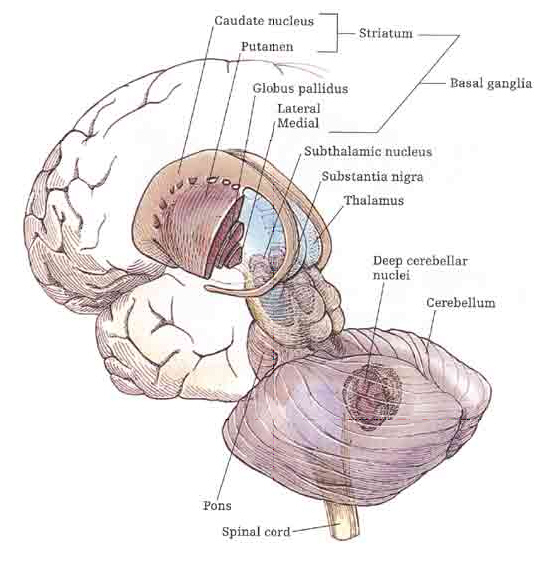
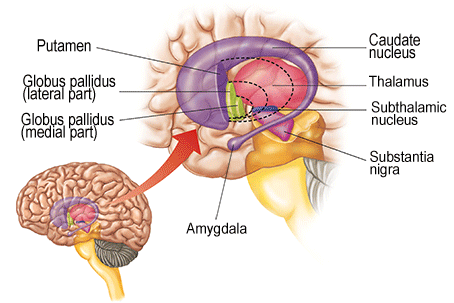
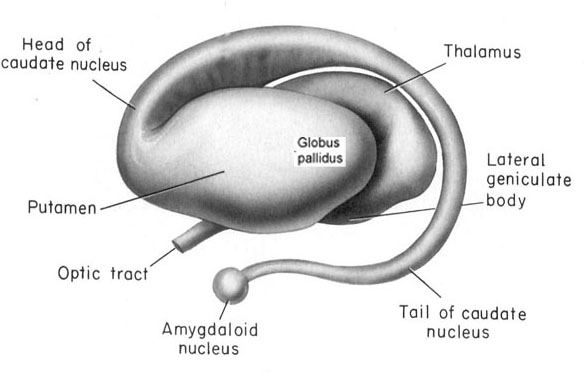
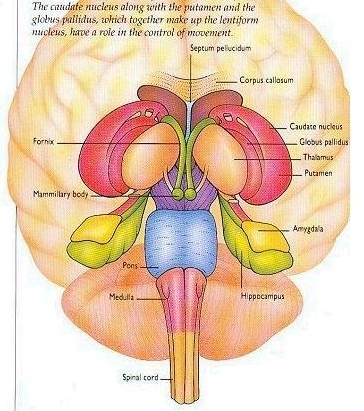
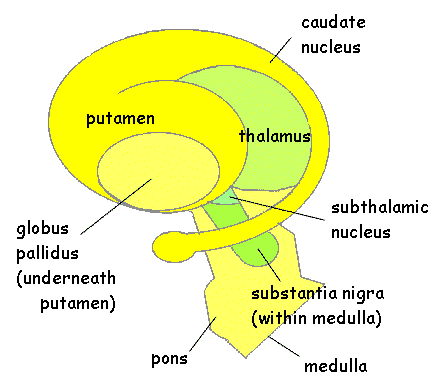
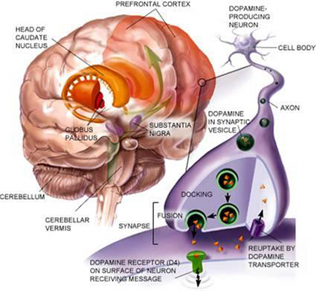
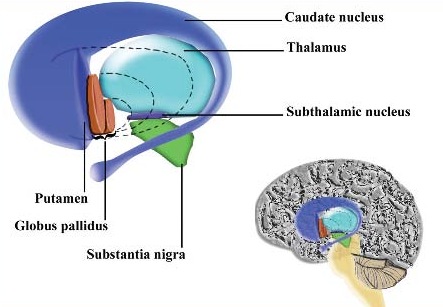
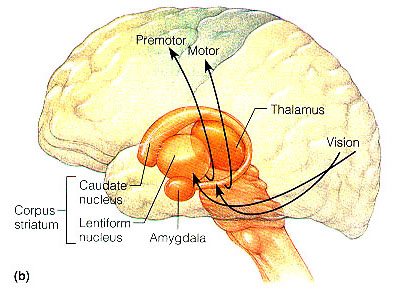
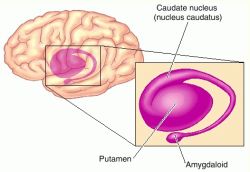
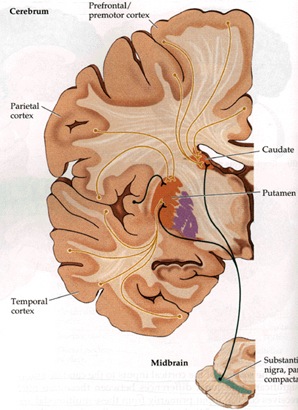
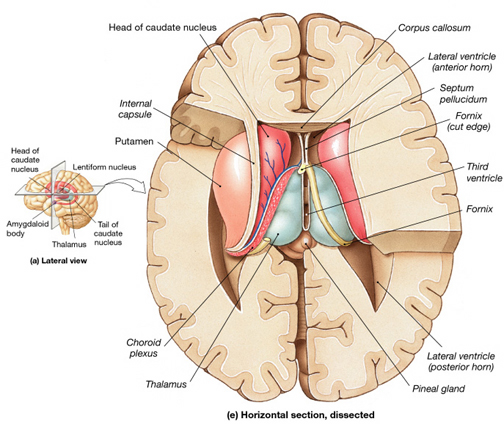
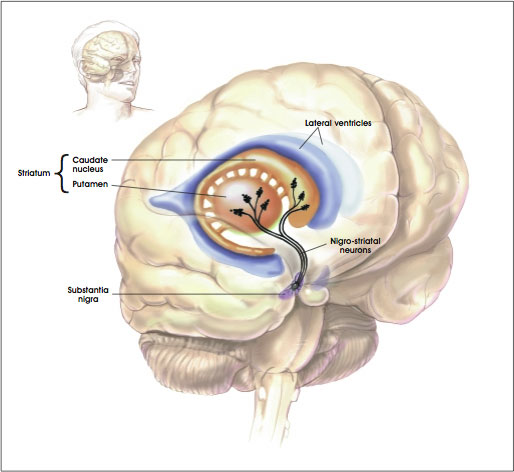
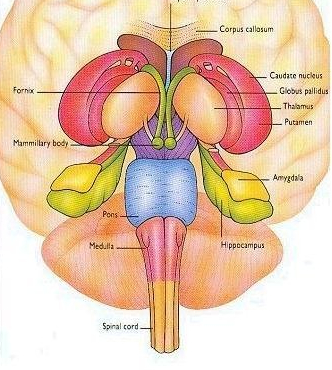
The striatum, along with the thalamus, are part of the forebrain, and in many respects, act as a nexus, or interface, between the more recently evolved neocortical motor centers in the frontal lobes, and the more ancient motor areas located in the brainstem. Important components of the striatum, include the caudate, putamen, globus pallidus, and the amygdala
For much of the course of vertebrate evolution, motor functioning was the province of the spinal cord, brainstem, cerebellum, and limic striatum, just as it is in modern humans. For much of evolution the forebrain consisted of the olfactory system and the amygdala, hypothalamus, and hippocampus. The striatum was dominated by the amygdala and was originally part of the amygdala. Moreover, the entire forebrain including the amygdala, hypothalamus, and hippocampus, was greatly influenced by the olfactory system, reacting to olfactory impulses by feeding, fighting, fleeing, or engaging in sexual behavior. Thus this part of the brain is sometimes referred to as the "rhinencephalon" or, "nose brain."
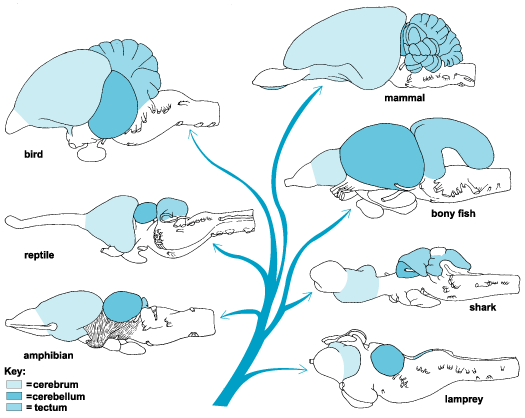
The amygdala-striatal system became almost completely separate structures once vertebrates crawled from the ocean onto dry land. (Gloor, 2007, MacLean, 2010; Stephans, 2004; Ulinsky, 2010). However, even in modern humans, the tail of the striatum becomes the amygdala, or convesely, the nose of the amygdala becomes the striatum. For this reason portions of the striatum is also referred to as the "limbic striatum" and the "extended amygdala."
Other important motor centers governing movement include the thalamus, and the primary, secondary and supplementary motor areas of the frontal lobes. The limbic striatum and the basal ganglia motor circuit, including the frontal motor areas are tightly linked and function as an integrated system which governs all aspects of emotionally triggered gross motor behavior,in conjunction with the brainstem, cerebellum, spinal cord and cranial nerve nuclei.


These are linked and function as an integrated system in the production of movement. For example, the basal ganglia provides input to the brainstem (via the "extra-pyramidal motor system") as well as to the motor thalamus and motor neocortex which also projects to the brainstem and spinal cord (Parent & Hazrati 2005). If injured, or if abnormalities develop secondary to excessive or diminished levels of neurotransmitters such as dopamine (e.g. Fahn, 2011; Turjanski et al, 2011; Wolters, 2011), a host of cognitive, affective, and motor disturbances may ensue, including Parkinson's and Alzheimer's diseases, chorea, hemiballismus (uncontrolled kicking, punching), restless leg syndrome, catatonia, schizophrenia, obsessive-compulsions, or depression (e.g. Aylward et al. 1994; Castellanos et al. 1994; Chakos et al. 1994; Deicken et al. 2005; Ellison 1994; Harrison, 2011; Joseph, 2011a; Turjanski et al, 2011). However, the nature of the symptoms depends on the nature, degree, extent, and laterality of destruction.
Schizophrenia is not thought of as a disorder of movement, but as a disorder of mind. However, subtypes of schizophrenia, such as catatonia, are typified by frozen motor functioning, such that patients may stay in the same positions for hours, days, and even weeks at a time.
Because the basal ganglia acts as an interface between the neocortex and the limbic system, disturbances in this system can effect the functioning of both areas. Thus, patients may become emotionally abnormal, lose control over their thoughts, or even control over their movements, as is the case with obsessive compulsive disorder (OBS). OBS is not a form of schizophrenia; however, some folks suffering from schizophrenia may develop obsessive thoughts and behaviors; and in these cases, the striatum is implicated.

For example, some subgroups diagnosed with schizophrenia have been found to have reduced striatal activity and reduced blood flow and metabolism (Buchsbaum, et al. 1992; Resnick et al. 2004; Wiesel et al. 1992). A schizophrenia-striatal association has also been demonstrated based on MRI, such that the limbic (ventral) striatum appears to be reduced in size (Shihabuddin et al. 2008) whereas the corpus (dorsal) striatum has been found to be increased in size (Heckers et al. 2001; Shihabuddin et al. 2008) especially the left striatum (Breier et al. 1992; Buchanan et al. 2003; Swayze et al. 1992). Left or bilateral caudate injuries are also associated with apathetic states, decreased spontaneous activity, slowed and delayed dysarthric and emotionally flat speech, with some patients responding to questions only after a 20-30 second delay (Caplan et al. 2010; Richfield et al. 2002). By contrast, injuries or abnormalities restricted to the right caudate are more likely to result in a manic-like psychosis (Castellanos et al. 1994; Robinson and Downhill 2005) --similar to right frontal lobe injuries (Joseph 1986a, 2004a, 2011a; Lishman 1973).
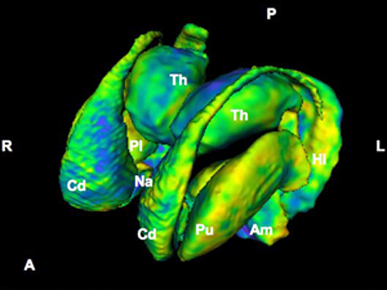
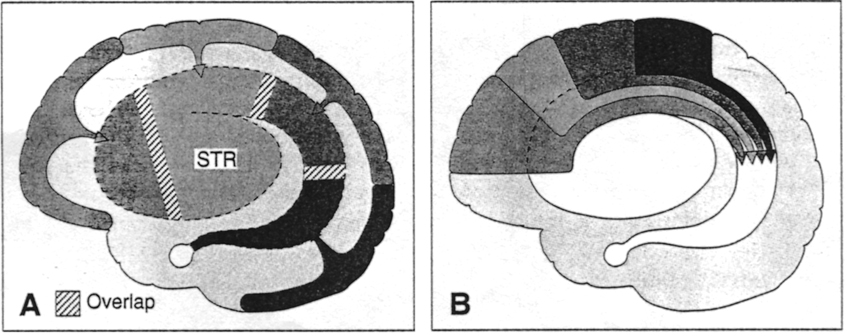
As noted the amydalostriatal gray consisted of both dorsal and ventral components, and so too does the human striatum--also referred to as the limbic (ventral) striatum. The substantia innominata, nucleus accumbens, olfactory tubercle constitute the limbic striatum. The inferior ventral globus pallidus (also referred to as the ventral pallidum or substantia innominata) is also part of the limbic (ventral) striatum and in fact eventually merges with the centro-medial amygdala and receives extensive projections from the lateral amygdala, the olfactory tubercle, and nucleus accumbens.
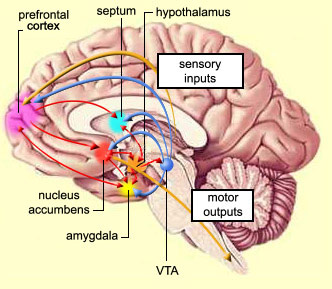
In addition, the "motor thalamus," the orbital and medial frontal lobes and medial supplementary motor area, hippocampus (as well as the central and medial amygdala), are richly interconnected with and constitute major components of the basal ganglia. However, as noted in chapter 12, the basal ganglia (i.e. the corpus and limbic striatum and globus pallidus) evolved out of the olfactory-amygdala and in many respects it could be considered part of the limbic system (Heimer & Alheid, 2001; MacLean, 2010).
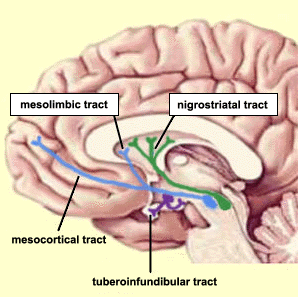
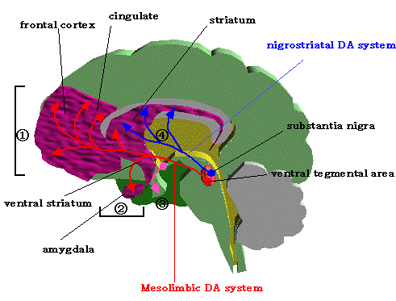
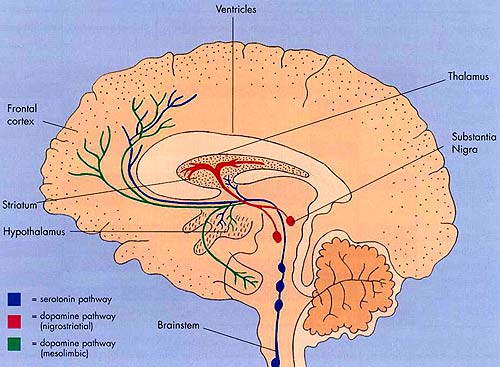
Intimately linked and thus part of the basal ganglia is the substantia nigra, reticular formation, and the midbrain tegmentum, which feed dopamine to the corpus and limbic striatum. Specifically, the nigrostriatal DA (A-9) cell group projects from the substantia nigra to the dorsal caudate and putamen and the medial frontal lobes (Ellison, 1994; Fibiger & Phillips, 1986; Parent & Hazrati 2005), although some fibers also innervate the limbic striatum. It is the nigro-(dorsal)-striatal system which is thought to be related to motor functions including the production of stereotyped and routine actions.
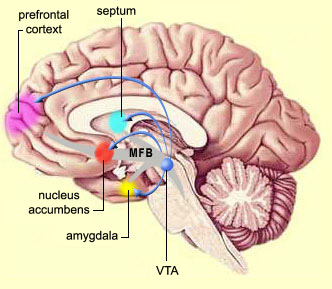
The mesolimbic DA systems originates in the ventral midbrain tegmentum (A10 DA cell group) and sends fibers to the amygdala, septal area, hippocampus, frontal cortical areas, including the ventral caudate-putamen, nucleus accumbens and substantia innominata (Le Moal & Simon 2001; Olton et al. 2001; Zaborszky et al. 2001), although some fibers also innervate the dorsal striatum. The mesolimbic DA system is believed to be related to emotion, mood, memory, and reward, including locomotor survival related activities such as running and even "galloping" (Ellison, 1994; Fibiger & Phillips, 1986; Le Moal & Simon 2001).
OVERVIEW
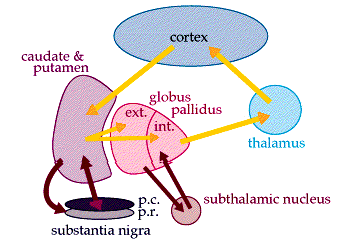
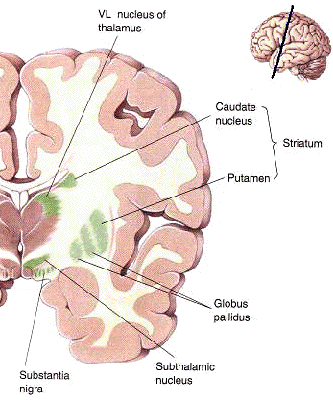
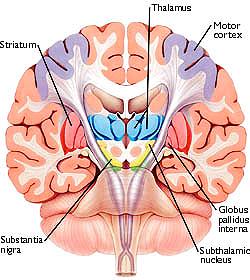
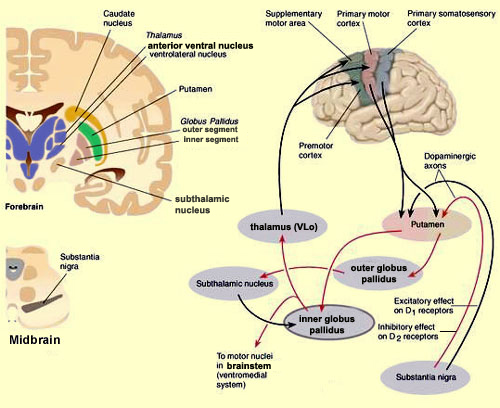
The "basal ganglia" (striatum, subthalamic nucleus) receives much of its input from the neocortex (Jones & Powell, 1970; Pandya & Vignolo, 1971), and the amygdala, and to a mimimal extent the hippocampus. The majority of these incoming fibers are excitatory and terminate in the corpus striatum and subthalamic nucleus. Specifically, the anterior portion of the frontal lobes projects to the head of the caudate whereas the more posterior putamen receives converging and overlapping input from the primary and secondary motor and somesthetic cortices (Jones & Powell, 1970; Pandya & Vignolo, 1971). This structure does not receive any direct input from the peripheral sensory or motor systems.
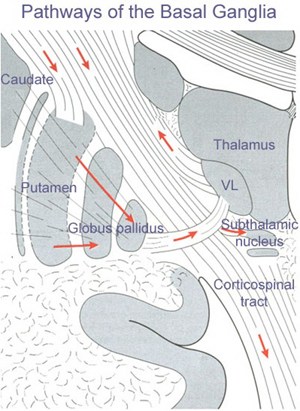
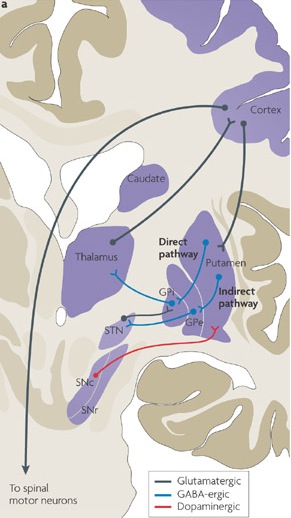
The main avenue of output is via the globus pallidus (and in the brainstem, the substantia nigra) which projects to the thalamus and the brainstem via the "extra-pyramidal system". The basal ganglia does not project directly to the spinal cord. The majority of these outgoing axons arise from "spiny" neurons, and these are responsible for transmitting striatal impulses to the globus pallidus (GP). These are predominantly inhibitory and employ GABA (and various peptides, e.g. substance P) as neurotransmitters.
The GP transmits to the brainstem and to the motor thalamus and subthalamic nucleus (Mink & Thach, 2001; Parent & Hazrati 2005). The motor thalamus in turn projects to the frontal motor areas i.e. the primary, secondary, supplementary motor areas and frontal eye fields, including the anterior frontal lobe.
The subthalamic nucleus, is directly linked with the hypothalamus and midbrain, and receives excitatory input from all frontal motor areas; Unlike the globus pallidus, output from the subthalamic nucleus is excitatory, and it in fact projects back to the globus pallidus.
The GP, therefore, receives diffuse excitatory input from the subthalamic nucleus, and converging inhibitory input from the striatum, and the transmits the bulk of its inhibitory impulses to the motor thalamus which acts on the frontal neocortical motor areas. Thus a complex feedback loop involving inhibitory and excitatory circuits is maintained in this area--feedback which presumably assists in the guidance of movements directly controlled by the frontal motor areas, as well as those initiated internally vs externally (Schultz & Romo, 1992).
Conversely, disturbances in this feedback loop, or in the neurotransmitters which maintain it, can result in a host of motor disturbances, ranging from the rigidity of Parkinson's disease and catatonia to chorea, hemiballismus, or "restless leg syndrome." Restless leg syndrome, for example, is a chronic condition which presumably afflicts about 5% of the population, and is characterized by a constant, sometimes painful urge to move the limbs which is only relieved by walking (e.g. Tergau, et al., 2011; Turjanski, et al., 2011). Functional imaging indicates that this disorder is directly related to disturbances in striatal dopamine binding and uptake, particularly in the caudate and putamen (Turjanski, et al., 2011). Presumably, this disturbances is thus a function of abnormal activity in these structures which begin to fire in the absence of any desire to move, thus inducing an urge to move, coupled with excessive activity in the frontal motor areas (e.g., Tergau, et al., 2011)
In this regard it is noteworthy that neurons in the striatum begin firing prior to movement, 20 ms on average. They increase their rate of firing at the start of specific movements, and cease to fire following movement (Montgomery & Bucholz, 2001; Schultz & Romo, 1992). Moreover, neurons related to movement are somatotopically organized such that those that represent the leg, face or arm, cluster together within the striatum, and increase their activity prior to and during movements of the leg, face or arm, etc.,
The corpus striatum, GP, and subthalamic nucleus, however, represent only the dorsal aspect of the "basal ganglia." Ventral to and intimately associated with the dorsal striatum is the limbic striatum, portions of which have also been referred to as the "extended amygdala." Indeed, the amygdala is a major component of the "basal ganglia," and these structures function as a cohesive interacting unit for the purposes of defensive and other affective behaviors regarding gross body movements, such as kicking, flailing, running, and kicking.
The striatoamygdaloid gray (or "dorsal pallium") performed motor functions that in some respects mirrored those of the brainstem which was dominated by the vestibular system and to a lesser extent, the visual system; one of the major differences being that the olfactory-forebrain was provided with a crucial "pregnant interval" before it need respond, which enabled it to beocme a thinking machine whereas the brainstem remained reflexive.
As animals emerged from the sea and began to live on dry land, the amygdala and striatum were pushed apart due to the increased important of motor functioning and as the olactory bulb expanded as did its forebrain pathways, so as to adapt to living in a world of smell. With the evolution of amphibians (the tailless anura) the amygdala and the striatum (and much later the hippocampus and striatum) became semi-separate structures (Herrick, 1925; Nieuwenhuys & Meek, 2010ab; Stephan & Andy, 1977; Ulinksi, 2010) which nevertheless remained tightly linked, the striatum responding to amygdala impulses which were directed to the brainstem. As these structures expanded and were pushed further apart, the dorsal aspect became a rudimentary dorsal striatum, and the ventral aspect became the ventral (limbic) striatum.
Ontogeny often replicates phylogeny and this amygdala-striatal separation is also repeated over the course of embryological development. For example, around the sixth week of fetal development immature neuroblasts migrate in massive numbers from the ventricular lining, and congregate in the more caudal portion of the emerging forebrain, thus forming an arc shaped "striatal ridge" from which the primordial amygdala will emerge (Gilles et al., 1983; Humphrey, 1968). Approximately one week after the formation of the amygdala, this primordial striatum begins to differentiate and balloon outward. Thus both the striatum and amygdala are derived from the arc shaped "striatal ridge," the caudal portion giving rise to the primordial amgydala at about the 6th week of gestation, and the basal portion later giving rise to the primordial striatum which initially overlies and is contiguous with the amygdala (Gilles et al., 1983; Humphrey, 1968). Over the ensuing weeks, these structures are pushed further apart thus again, replicating phylogeny.
Nevertheless, they do not completely separate, and the medial amygdala remains extensively interconnected with the limbic (ventral) striatum. As noted, the substantia innominata (ventral globus pallidus/nucleus basalis) merges with the centro-medial amygdala and receives extension projections from the lateral amygdala. In addition, the lateral amygdala id directly connected with the corpus striatum, via the so called "tail of the caudate." However, as these connnections are predominantly from the amygdala to the striatum and not vice versa, the caudate could be considered the bulbous "tail" end of the amygdala.
In fact the human corpus and limbic striatum are sandwiched between the posterior/ventral medial/lateral "tail" and the extended centro-medial "nose" (or internal shoulder) of this nucleus. And, these structures, being derivatives of the amygdala, respond to amygdala impulses, as they receive extensive amygdala projections (Gloor, 1955; Klingler & Gloor, 1960; Krettek & Price, 1978)--projections which are not reciprocated. The the right and left amygdala povides ipsilateral connections.
The amygdala (as well as the anterior cingulate, lateral hypothalamus, and hippocampus), therefore is able to exert considerable influence on the basal ganglia which appears to have evolved out of the amygdala in order to serve as an emotional-motor interface so that amygdala needs and impulses may be acted on in a flexible manner These are functions the basal ganglia (the limbic striatum in particular) continues to perform in humans as well as other creatures (e.g., MacLean, 2010; Mogenson 2001).
For example, the basal ganglia is exceedingly important in the stereotyped and species specific motoric expression of social and emotional states such as running away in fear, biting defensively, or via ballistic movements (hitting, kicking) or as manifested through facial expression, posture, muscle tone, or gesture (Mogenson & Yang, 2001; MacLean, 2010; Rapoport 2001). Because humans possess basically the same basal ganglia and limbic system, when happy, sad, angry, and so on, the facial and body musculature assumes the same readily identifiable emotional postures and expression regardless of culture or racial orgins (Ekman 2003; Eibl-Ebesfedlt, 2010; However, see Russell 1994).
Because of this similarity in basal ganglia functional architecture, regardless of culture or race (and in many respects, mammalian species) if frightened, angry, or in the process of being assaulted, animals or humans may similarly engage in (ballistic) hitting and kicking, or biting, and/or all of the above, depending on context, mood and situational variables (including play). However, in contrast to the brainstem and cerebellum which provides reflexive and stereotyped motor programs that can be performed without thinking, the basal ganglia is capable of considerable flexibility in regard to motor-emotional expression, and is exceedingly responsive to the organisms motivational and emotional state -via its extensive interconnections with the limbic system.
Thus, the striatum contains cells which selectively respond to motivationally significant stimuli, including novel and familiar variables that are rewarding or punishing (Rolls & Williams, 2002; Schneider & Lidsky, 1981). Striatal neurons can also react differently to familiar stimuli depending on their reinforcement properties, and many neurons will in fact increase their responsiveness as stimuli approach the mouth, and/or touch the face and mouth (Rolls & Williams, 2002; Schneider & Lidsky, 1981). Some striatal neurons also respond when making tongue and lip movements (e.g. licking) and during arm movements toward a food item (Rolls & Williams, 2002).

These findings suggest the striatum is involved in orienting and guiding movements toward the mouth presumably so that a desired object can be licked, sucked on, chewed, and consumed. In this regard the striatum could be considered a primary motor center which enables various limbic desires, needs, and impulses such as hunger, to be satisfied.
The limbic striatum (e.g. the nucleus accumbens) and the mesolimbic DA system which projects to these nuclei also appear to be highly involved in mediating feelings of pleasure including the rewarding effects of amphetamine, cocaine and opiates (see Ellison, 1994; Hakan et al. 1994; Koob et al. 2001). These effects (including the aversiveness of opiate withdrawal) are probably due not only to the presence of opiate, DA, and related receptors, but the rich interconnections maintained with the amygdala as well as the lateral hypothalamus (Kelsey & Arnold 1994; Olton et al. 2001; Zaborszky et al. 2001) which constitute part of the "pleasure circuit" maintained by the medial forebrain bundle (Olds & Forbes, 1981; see also chapter 13).
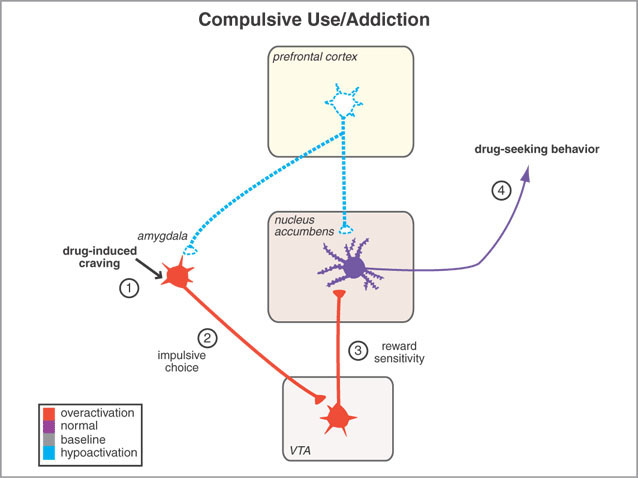
However, animals will also work in order to self-administer opiates directly into the accumbens (Koob et al. 2001; Olds & Forbes 1981). Conversely, lesions to the nucleus accumbens may disrupt the capacity to experience pleasure or the rewarding effects of opiates and cocaine (Koob et al. 2001); or to engage in complex coordinated defensive acts (see below).
However, when chemical or structural lesions extend beyond the basal ganglia and come to include the medial frontal lobe, not only might an individual suffer motor rigidity, they may become catatonic and experience extreme difficulty responding to external or internally mediated impulses (Joseph, 2011a). Various aspects of this symptom complex also characterize those with Parkinson's disease (see below).
Other disturbances associated with striatal abnormalities include Huntington's chorea, ballismus, restless leg syndrome, catatonia, sensory neglect and apathy, obsessive compulsive disorders, mania, depression, "schizophrenia" and related psychotic states (Aylward et al. 1994; Baxter et al. 1992; Caplan, et al. 2010; Castellanos et al. 1994; Chakos et al. 1994; Davis, 1958; Deicken et al. 2005; Ellison, 1994; Rauch et al. 1994; Richfield, et al. 2002; Turjanski, et al., 2011).



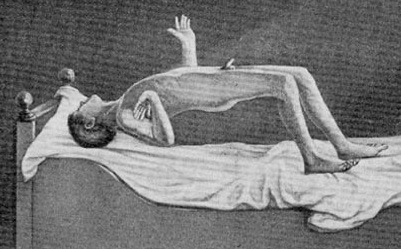
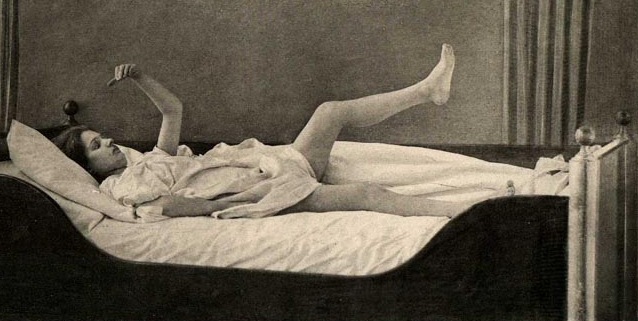
Severe memory loss and social-emotional agnosia and an inability to recongize friends or loved ones is also characteristic of striatal abnormalities, particularly disturbances involving the limbic striatum.
Thus although the basal ganglia is often viewed and described as a major motor center (detailed below), the functional capacities and symptoms associated with this group of nuclei are quite diverse, and vary depending on the nuclei and chemical neurotransmitters involved as well as the laterality, location, and extent of any lesion.
THE CORPUS STRIATUM
PATCHES & MATRIX
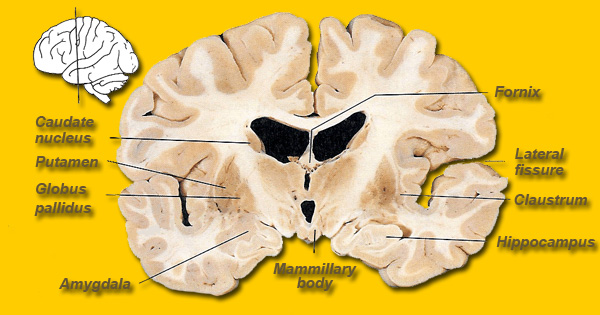
The caudate and putamen are tightly interlinked and in some respects are indistinguishable and possess a similar internal compartmental structure of patches and matrix (Graybiel 1986; Gerfen, 2004). This is why a gross analysis of the the mammalian caudate and putamen reveals a striated (patchlike) appearance.
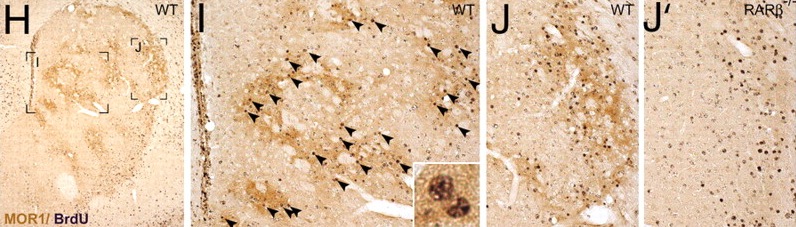
The patches and matrix are biochemically distinct and receive projections from different regions of the neuroaxis (Gerfen, 2004, 2002; Graybiel 1986). For example, the patches contain dense concentration of opiate receptors (Graybiel, 1986) and receive projections from the amygdala, hippocampus, and other limbic tissue and maintain interconnections with the DA neurons in the substantia nigra. The patches in fact form a continuous labyrinth which snakes throughout the striatum.
The surrounding matrix also receives projections from the cingulate gyrus, the motor thalamus, and from throughout the neocortex and maintains interconnections with GABA and DA neurons in the substantia nigra (Gerfen, 2002). The matrix also contains large amounts of acetylcholinesterase.
MOTOR FUNCTIONS
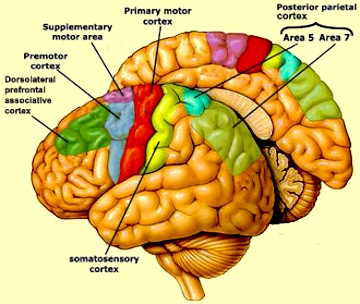
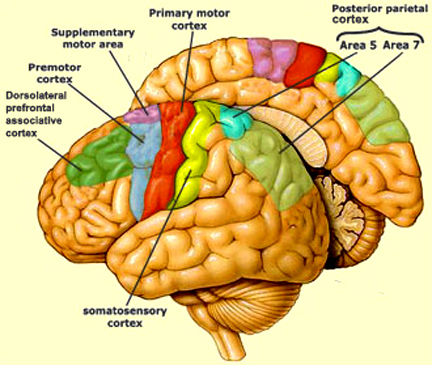
Although tightly linked and similar in structural organization, the caudate appears to exert more influence and provide more input to the putamen, than vice versa. However, like the caudate, the putamen also receives considerable input from the medial frontal, supplementary, secondary and primary motor cortex, as well as areas 5 and 7 of the parietal lobe (Jones & Powell, 1970; Pandya & Vignolo, 1971).
As per motor functioning, presumably the putamen, in conjunction with the caudate, transmits this information to the globus pallidus which in turn projects to the motor thalamus, brainstem reticular formation, as well as to the motor neocortex, thus creating a very elaborate feedback loop (see Mink & Thach, 2001; Parent & Hazrati 2005) whose origin may begin in the medial frontal lobes (Alexander & Crutcher, 2010; Crutcher & Alexander, 2010) or perhaps the limbic system, e.g. anterior cingulate, amygdala.
For example, when anticipating or preparing to make a movement, but prior to the actual movement, neuronal activity will first begin and then dramatically increase in the medial supplementary motor areas (SMA), as well as in the striatum (Montgomery & Buchholz, 2001; Schultz & Romo, 1992) followed by activity in the secondary and then the primary motor area (see chapter 19), and then again in the caudate, putamen and globus pallidus which become increasingly active prior to and then during movement and ceasing their activity once the movement is completed (Alexander & Crutcher, 2010; Mink & Thach, 2001; Schultz & Romo, 1992).
Hence, these areas, including the "motor" thalamus, in many respects act in a coordinated fashion so as to mediate purposeful movement. As noted in chapter 12, this was a principle role of the basal ganglia long before the evolution of the neocortex and frontal motor areas.
It is perhaps important to point out that some investigators have argued there are at least five different motor circuits involving the basal ganglia which are segregated to varying degrees (Alexander et al. 2010). The dorsal striatal motor circuit also consists of at least two separate systems involving the putamen and medial (internal) globus pallidus, and the putamen and lateral (external) globus pallidus (reviewed in Marsden & Obeso, 1994; Parent & Hazrati 2005).
FUNCTIONAL DIFFERENTIATION OF THE CAUDATE & PUTAMEN
Over the course of evolutionary metamorphosis, the corpus striatum and the motor thalamus began to develop in tandem and became increasingly interlinked in order to subserve motoric and related activities, including the processing and analysis of sensory information and the expression of feeling states via specific motor activities. That is, the corpus striatum initially served not only the motor functions and related information requirements of the limbic system but in many respects performed (at a rudimentary levels) some of the same "analytical" and perceptual functions that would later be subsumed by the neocortex.
With the continued expansion of the the neocortex and the exponential increase in the capacity to analyze and respond to divergent sensory information, the corpus striatum essentially was split in two by the tremendous proliferation of thalamic axons (i.e. the internal capsule, or rather, the thalamic radiations) that not only terminated on striatal dendrites, but which swept forward and radiated outward to innervate the frontal lobes (Kemp & Powell, 1970). Thus the putamen and caudate nucleus were formed and began to receive differential input from the thalamus as well as from the neocortex and therefore began to subserve somewhat different functions.
For example, although both nuclei contain significant amounts of DA, 5HT, ACH, and GABA (reviewed in Ellison, 1994; Parent & Hazrati 2005; Stoof et al. 1992) and receive input from the amygdala, hippocampus, and motor and somatosensory perceptual data (Haber et al. 2001; Van Hoesen, et al. 1981; Whitlock & Nauta, 1956), the putamen is the recipient of considerable bilateral and topographical input, such that a motor and sensory map of the body (particularly the face, mouth, leg, and arm) is maintained in this region (Delong et al. 1983; Parent & Hazrati 2005). Axonal projections from the motor and sensory neocortex which are concerned with the arm converge in one area of the putamen, whereas those concerned with the leg converge in another.
When coupled with the symptoms and experiments describes below, it is suspected that the putamen is concerned with integrating sensory with intended motor actions and coordinating the movement of the limbs and body in visual space via projections maintained with the medial and lateral globus pallidus as well as the parietal lobe.
In contrast, the caudate nucleus is dominated by axons from association cortices including the inferior temporal lobe and anterior cingulate (Percheron, et al. 2002), the amygdala (Ammaral et al. 1992; Heimer & Aheid, 2001) and the frontal motor areas. The caudate appears to be more involved in multi-modal motor, emotional and sensory integration, analysis and inhibitory functions. Consequently lesions to the caudate can produce sensory neglect and unresponsiveness, or conversely, loss of inhibitory control over the musculature; depending on the extent and laterality of the lesion.
THE CAUDATE: MANIA, APATHY, CATATONIA
The head of the caudate nucleus is concerned with multi-modal information processing and inhibition. Via inhibition the caudate is able to exert modulatory effects on motor activity and facial-gestural posture and expression, and aids in the maintenance of selective motoric attention, e.g. standing still and observing. In consequence, in primates and humans, lesions, destruction, or shrinkage of the head of the caudate can result in sensory neglect, agitation, hyperactivity, distractibility and in some cases what appears to be a manic or "schizo-affective" psychosis (Aylward et al. 1994; Caplan, et al. 2010; Castellanos et al. 1994; Chakos et al. 1994; Davis, 1958; Richfield, et al. 2002) depending on the extent and laterality of the destruction.

For example, Richfield et al. (2002, p. 768 ) report a 25 year old female honor student (soon to be married) who after complaining of headaches and nausea disappeared for 3 days. "When found, she had undergone a dramatic personality change manifested by alterations in affect, motivation, cognition, and self-care." These changes were largely permanent. "Her abnormal behaviors included vulgarity, impulsiveness, violent outbursts, enuresis, indifference, hypersexuality, shoplifting and exposing herself. She was inattentive and uninterested in her surroundings but could be encouraged to concentrate for short periods of time. She would frequently lie down to sleep. Her affect was flat." CT-scan indicated bilateral damage to the head of the caudate nuclei.

Some patients will alternate between hyperactivity and apathy- a function perhaps, of the laterality and extent of the lesion as well as associated biochemical alterations. For example, injuries or abnormalities restricted to the right caudate are more likely to result in a manic-like psychosis (Castellanos et al. 1994) --quite similar to what occurs after right frontal lobe injury (chapter 19). Similarly, right caudate hypermetabolism and blood flow has also been associated with obsessive compulsive disorders (Baxter et al. 1992; Rauch et al. 1994) whereas frontal abnormalities may induce perseverative disturbances (chapter 19) as well as obsessive- compulsions (Rauch et al. 1994).
Conversely, left caudate injuries (particularly those which extend to the mesial and left frontal lobe) may induce severe apathy as well as speech disturbances. As noted, left frontal injuries are associated with similar abnormalties (chapter 19).
With extensive left or bilateral caudate injuries it is not uncommon for patients to appear agitated, apathetic, with decreased spontaneous activity and slowed and delayed, dysarthric (or stuttering) and emotionally flat speech, with some patients responding to questions only after a 20-30 second delay (Caplan et al. 2010). This condition is particularly likely if the medial frontal lobes have been compromised as well.
In fact, due perhaps to it's extensive interconnections with the medial frontal lobes and SMA -a region which when destroyed can give rise to catatonia (Joseph 2011a), massive bilateral lesions to the anterior caudate and anterior putamen and surrounding tissue has been shown to produce catatonic or "frozen" states, where animals show a tendency to maintain a single posture or simply stand unmoving for weeks at a time (Denny-Brown, 1962).
Similarly, chemically lesions of the caudate can produce complete catatonia, posturing and a cessation of all movement (Spiegel & Szekely, 1961). However, if the amygdala-striatal pathway and/or the amygdala is destroyed prior to lesioning the caudate, these frozen catatonic states can no longer be induced (Spiegel & Szekely, 1961).
CATATONIA & THE FRONTAL-CAUDATE-AMYGDALA NEURAL NETWORK
As noted, the head of the caudate is extensively interconnected with the frontal lobes. Thus, some of the symptoms associated with caudate destruction (e.g. mania, apathy, catatonia) parallels those associated with frontal lobe injury (chapter 19). For example, whereas lesions to the frontal lobe may produce perseverative abnormalities, caudate lesions and/or increased metabolism or regional blood flow may induce obsessive-compulsive behaviors (Baxter et al. 1992; Rauch et al. 1994). In part this may represent a loss of frontal lobe control over the caudate, and/or the loss of striatal inhibitory influences over the frontal lobe.


However, the caudate and frontal lobes are also intimately linked to the amygdala (Ammaral et al. 1992); a stucture that under conditions of extreme fear and arousal, can induce a complete cessation of movement; i.e. catatonic-like frozen panic states (chapter 30). The amygdala is able to accomplish this via interconnections with the basal ganglia, brainstem, as well as the medial frontal lobes.
THE SUPPLEMENTARY MOTOR AREAS
If the medial frontal lobes are damaged or experimentally electrically stimulated, there sometimes results in a inability to initiate a voluntary movement, and the "Will" to move or speak may be completely attenuated and abolished (Hasslet, 1980; Laplane et al. 1977; Luria, 1980; Penfield & Jasper, 1954; Penfield & Welch, 1951). Similarly, lesions of the medial frontal lobes can cause subjects to simply sit or stand motionless, as if frozen, and display waxy flexibility, or "gegenhalten" or counterpull -- i.e. involuntary resistance to movement of the extemities (Brutkowski, 1965; Hasslet, 1980; Laplane et al., 1977; Luria, 1980; Mishkin, 1964). Posturing is also noted and patients might remain in odd and uncomfortable positions for exceedingly long time periods and make no effort to correct the situation (Freeman & Watts, 1942; Rose, 1950); as if they were dead and in the first stages of rigor mortise.
Similar reactions (see below) occur after disasters and in instances of extreme fear and terror. Affected individuals may display all the classical symptoms of catatonia, which, in many respects, could be likened to death feigning or "playing possum".

Presumably, in response to extreme fear the amygdala acts on the striatum and SMA (as well as the brainstem), thereby inducing not only rigidity and a frozen panic state, but a classic catatonic state accompanied by waxy flexibility, and a complete paralysis of the will. The SMA, in fact, is very susceptible to the disruptive influences of stress (Finlay & Abercrombie 2001).
THE AMYGDALA, STRIATUM, SMA, & LIFE THREATENING FEAR & AROUSAL
In situations involving exceedingly high levels of arousal coupled with extreme fear, the individual may simply freeze and attentional functioning may become so exceedingly narrow that little or nothing is perceived and cognitive activity may be almost completely (albeit temporarily) abolished (see chapter 30). These behaviors are apparently under the control of the amygdala which can trigger a "freezing" reaction and a complete arrest of ongoing behavior (Gloor, 1960; Kapp et al. 1992; Ursin & Kaada, 1960) via these brainstem/striatal interconnections. This is part of the amygdala attention response, which at lower levels of excitation may be followed by anxious glancing about, an increase in respiration and heart rate, pupil dilation, and perhaps cringing and cowering or flight (Gloor, 1960; Ursin & Kaada, 1960).

Among humans, the fear response is one of the most common manifestations of amygdaloid stimulation (Gloor, 2010; Halgren, 1992; Williams, 1956). However, if arousal levels continue to increase, subjects do not merely freeze in response to increased fear, they may become catatonic; a condition which may be secondary to dopamine and serotonin depletion and amygdaloid influences on the SMA as well as the striatum; nuclei which are intimately interconnected.
For example, in response to extreme fear, "one tendency is to remain motionless, which reaches its extreme form in death-feigning in certain animals and sometimes produces the waxy flexibility of catatonics" (Miller, 1951). The affected individual becomes psychologically and emotionally numb and unresponsive which is coupled with a complete blocking off of cognition. Moreover, the individual may resist and fail to respond to attempts at assistance (Krystal, 2004; Miller, 1951; Stern, 1951).
The airline industry has referred to this as "frozen panic states" (Krystal, 2004), a condition sometimes seen in air and sea disasters. For example, in mass disasters, 10-25% of the victims will become frozen, stunned, and immobile, and will fail to take any action to save their lives, such as attempting to evacuate a burning or sinking craft even though they have been uninjured (see Krystal, 2004).
According to Krystal (2004) with increasing fear "there is also a progressive loss of the ability to adjust, to take the initiative or defensive action, or act on one's own behalf... that starts with a virtual complete blocking of the ability to feel emotions and pain, and progresses to inhibition of other mental functions" (Krystal, 2004, p. 151).
Evolutionary Significance of Rigidity.
Catatonic panic states are prevalent in the animal kingdom, and constitute a life preserving reaction that is apparently mediated by the amygdala and striatum. That is, by freezing and not moving, predators may fail to take note of their presence, or conversely, refuse to eat them, believing them already dead; also known as "playing possum."

Catatonia, coupled with emotional and "psychological" numbing, also represents a total surrender reaction, usually as a prelude (and hopeful guarantee) of a painless death when attacked by predators or invaders. That is, the prey may cease to run or fight and simply stand still or lie down and allow predators to literally eat them alive. Or, in the case of humans as sometimes occurs during war and genocidal mass murders, passively allow themselves to be marched into a ditch and shot.
According to Krystal (2004, p. 144), "thousands of European Jews obeyed orders in an automatonlike fashion, took off their clothes, and together with their children decended into a pit, lay down on top of the last layers of corpses, and waited to be machine-gunned," all the while seemingly almost petrified with fear and/or completely numb as to what was going on around them.



Presumably this numbing is made possible via the massive secretion of opiates within the amygdala and basal ganglia, whereas the rigidity and loss of the will to resist is a consequence of overwhelming fear and hyper-amygdala influences on the medial frontal lobe and corpus and limbic striatum.
Once prey have sighted a predator, some animals, however, instead of running in fear, will simply "freeze," fall to the ground, and lie stiff, rigid, and motionless as if dead. Unless exceedingly hungry, many predators will avoid eating creatures which appear to be already dead (i.e. unresponsive).
As sometimes occurs to potential victims during mass killings (e.g. the 1994 Rwanda civil war between the Hutus and the Tutus) humans too, will sometimes fall down as if dead and may remain frozen, stiff and and unmoving for long time periods even though they may not have been harmed (Krystal 2004). Indeed, sometimes these individuals are believed to be dead even by rescuers or those who are clearing away and burying bodies.
Presumably it is via connections with the basal ganglia and medial frontal lobes that the amygdala is able to induce these catatonic states, which in part is also dependent on dopamine. For example, it has been demonstrated that under extremely stressful conditions the striatal and frontal lobe DA system is adversely affected (see Le Moal & Simon 2001).
IMPLICATIONS REGARDING PARKINSON'S DISEASE
As noted, many of the functions originally associated with the basal ganglia have been subsumed by the neocortex, and damage to the frontal lobes can produce essentially similar symptoms as seen following caudate destruction; including stiffness, rigidity, and difficulty initiating movement (see chapter 19).
Given that the medial frontal lobes, corpus and limbic striatum, and amygdala are extensively interconnected, and given the powerful influences of the limbic system on all aspects of behavior, it thus appears that when exceedingly aroused or emotionally stressed, the amygdala is able to inhibit (or overactivate) the frontal-striatal motor centers which (in addition to the amygdala) are simultaneously undergoing DA depletion (which in turn results in hyperactivation of these nuclei including the amygdala; see Le Moal & Simon 2001). When this occurs, the organism may fall and cease to move, blink or even breathe (or breathe only shallowly and slowly). The creature therefore appears to be in a state of rigor mortis and thus dead (i.e. catatonic).
Overall, these amygdala-basal ganglia-medial frontal lobe-fear induced frozen and catatonic states are exceedingly adaptive; that is, unless the hapless victim is in a burning airplane or sinking ship.
Although not as dramatic or severe, similar states occur with biochemical abnormalities involving the dopamine pathways from the substantia nigra which not only feed the caudate, but the medial frontal lobes and the amygdala. Specifically, loss of DA (or excessive amygdaloid arousal) results in motor neuron hyperactivity and tonic EMG activity and thus limb and facial rigidity; conditions which also afflict those with Parkinson's disease.
In that some of these same exact "semi-frozen" and akinetic states are present in many of those with Parkinson's disease, and given that affected individuals are sometimes described as excessively aroused and/or unable to relax, and to suffer from heightened autonomic nervous system activity (Stacy & Jankovic, 1992) this raises the possibility that the amygdala and related limbic nuclei may significantly contribute to the development of this disorder. Indeed, as noted, destruction of the amygdala prior to chemically lesioning the corpus striatum prevents the development of Parkinsonian symptoms.
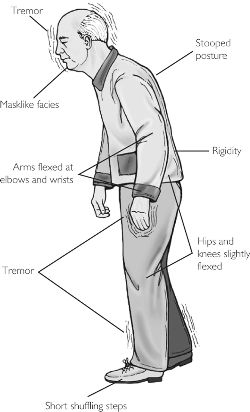
Parkinson's disease is a progressive degenerative disorder characterized by rigidity and shuffling gait, stooped posture, generalized slowness and stiffness of movement, and a loss of facial emotional expression, as well as a loss of spontaneity and flexibility in making postural adjustments when eating, going to the toilet, or having sex. Moreover, many Parkinson's patients experience not only rigidity and akinesia, but suffer from episodic freezing of movement (Dietz, et al. 2010; Pascual-Leone, et al. 1994), a tendency to easily fall (Stacy & Jankovic 1992) an impairment of "righting reflexes" (Calne 1994), and a reduced capacity to blink (Freedman 1992) and even to breathe (Stacy & Jankovic, 1992); similar to the frozen panic states and induced catatonia.
Hypophonia (reduced voice volume) dysarthria and a tendency to speak in a monotone, micrographia (small handwriting), and a 4-8 c/sec. ("pill rolling") resting tremor (exacerbated by stress) involving antagonistic muscles are common (Freedman, 1992; Stacy & Jankovic, 1992). Depression, changes in personality and slowed thought processes are also not unusual among Parkinson's patients (Walters, 2011). Parkinsonism can result following neurological trauma or vascular abnormalities (Koller, 2002; Murrow et al. 2010) or isolated lesions to the substantia nigra (Stern, 1966) and can therefore arise from a number of different causes including infection and toxic exposure. Parkinson's disease may also overlap with other striatal disturbances such as Alzheimers disease (Calne 1994). Indeed, significant cognitive deficits are not unusual among those with Parkinson's disease (Freedman, 1992; Rajput 1992); i.e. subcortical dementia.
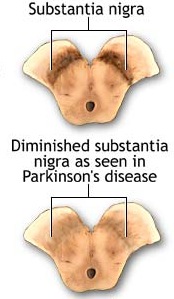
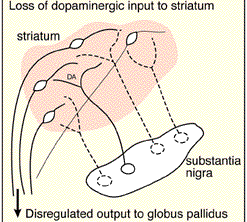
Parkinson's disease usually begins after age 60 (though the first signs may appear during the early 40's), effects about 2% of the population (Koller, 2002) and is usually characterized by a massive loss of up to 80-85% of the dopamine neurons in the substantia nigra and an 80% decrease in striatal DA, with DA depletion greatest in the putamen (Goto, et al. 2010; Kish, et al. 2004). In fact, transplanting fetal nigral tissue into the putamen, can result in increased dlurodopa intake and some long-term clinical improvement (Hauser et al., 2011).
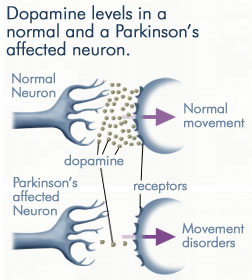
Hence, whereas the substantia nigra DA system is directly implicated, the mesolimbic pathways and limbic striatum are only a mild factor and are only mildly effected in this disorder.
STRIATAL IMBALANCE & PARKINSON'S DISEASE
The differential involvement of the nigrostriatal vs mesolimbic DA system raises the possibility that whereas the corpus striatum and medial frontal lobes are negatively impacted, the amygdala and limbic striatum may continue to function normally -due to preservation of the mesolimbic DA system. However, because the normal balance between these various nuclei is disrupted, amygdala influences received within the SMA and corpus striatum may in fact overwhelm and massively inhibit (or over activate) these nuclei thereby giving rise to Parkinsonian symptoms (see Le Moal & Simon 2001 for related discussion); i.e. rigidity, a tendency to fall coupled with reduced blinking and disturbed righting reflexes, etc.
In addition to DA in the genesis of Parkinsonian symptoms, significant reductions in opiate receptors within the putamen as well as the globus pallidus have also been reported (Goto et al. 2010), such that neurons involved in the experience of "reward" are effected. This selective loss of "reward" neurons suggests a a reduction in the capacity of the basal ganglia to receive pleasurable or positive emotional input as might be provided not only by opiates, but via the lateral amygdala and lateral hypothalamus.
Possibly, because striatal neurons associated with negative feelings states may be selectively preserved in Parkinson's disease, the basal ganglia (but not the amygdala, hypothalamus, or neocortex) may respond as if in a highly aroused negative state (e.g. fearful, stressed) even when that is not the case. Adding to this imbalance would be the relative preservation of the mesolimbic DA system, and the continued input from the medial amygdala into the corpus and limbic striatum -the medial amygdala being more involved in the generation of unpleasant including fearful mood states (chapter 13)
Indeed, loss of corpus striatal DA and/or excessive amygdaloid arousal is directly associated with the development of tonic EMG activity, motor neuron hyperactivity and thus excessive tonic excitation of the musculature and limb rigidity; i.e. Parkinson's symptoms. Conversely, excess striatal DA can result in chorea and excessive movement as well as psychosis.
THE SMA & PARKINSON'S DISEASE
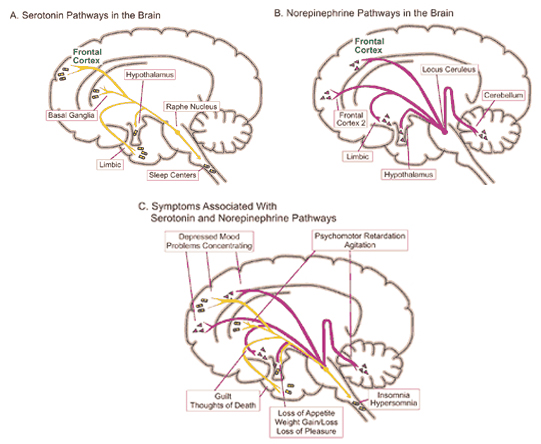
It has been reported that many patients with Parkinson's disease display reductions in norepinephrine (NE) as well as DA throughout the upper layers of the motor, premotor, and medial frontal lobes (Gasper et al. 2001; Hornykieciz, 1982). NE is produced by the Locus coeruleus in the brainstem and NE pathways innervate the cerebellum, limbic system, striatum, and the neocortex.
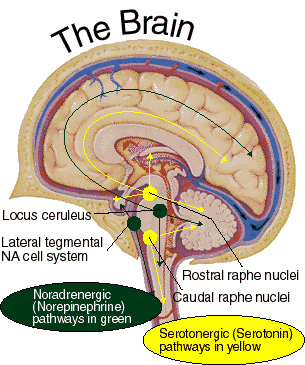
NE (and 5HT) depletion also occurs following heightened emotional states, including extreme fear, prolonged stress and heightened arousal, and with amygdala hyperactivation. As noted, individuals with Parkinson's disease are also sometimes described as excessively aroused and/or unable to relax, and to suffer from heightened autonomic nervous system activity and gastrointestinal disturbances (Stacy & Jankovic, 1992) --disturbances which also implicate the amygdala and excessive limbic system arousal. Conversely, the autonomic nervous sytem of those who suffer from Alzheimer's disease have been characterized has hypoactive (Borson et al. 1992); the limbic striatum being implicated in Alzheimer's disease.
The SMA typically becomes functionally activated prior to movement (chapter 19) and well before the caudate or putamen with which it is interconnected. When Parkinson's patients attempt to make repetitive movements, SMA functional activity is abnormally reduced as is putamen activity (Playford et al. 1992). This suggests that in addition to the corpus striatum one of the major regions of the neuroaxis involved in the "will" to move and the capacity to prepare to move; i.e. the SMA, is dysfunctional in those suffering from Parkinson's disease. Thus the SMA and medial frontal lobes as well as the corpus striatum, DA system, and amygdala, are implicated in the genesis of this disorder (e.g. Playford et al. 1992).
Consider, for example, that Parkinson's patient's have difficulty using both hands to perform two different movements (e.g. squeeze a ball with one hand, draw a triangle with the other). Rather, they tend to perform one action, then switch to the other (Caligiuri et al. 1992). SMA dysfunction can result in similar disturbances (see chapter 19). Similarly, the ability of Parkinson's patients to engage in sequential and repetitive movements (Playford et al. 1992), or to simultaneously engage in separate movements such as walking, turning, and talking (Stacy & Jankovic 1992) is severely effected; a disorder which is also associated with frontal lobe abnormalities.
THE HIPPOCAMPUS & SMA
As noted, the striatum is also innervated by the hippocampus which is involved not only in memory, but spatial and cognitive mapping of the environment (chapters 13, 14). Thus the corpus striatum presumably relies on hippocampal (as well as parietal lobe) input in order to coordinate movement in visual-space.
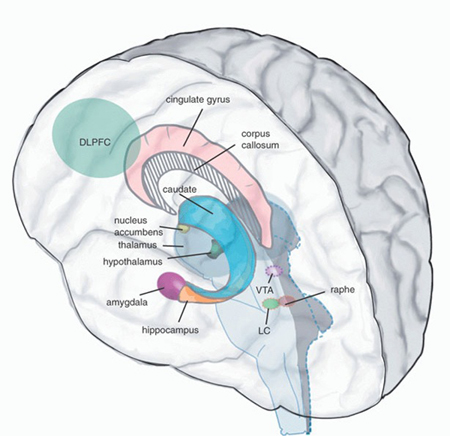
It is noteworthy that Parkinson's patients find it easier to perform movements that are cued or guided by external visual stimuli, whereas those which are internally generated are more severely effected (Dietz, et al. 2010). Hence, perhaps those striatal neurons which normally receive and integrate hippocampal input may also be dysfunctional.
However, the role of the hippocampus appears to be minimal in the development of Parkinson's disease, at least in those cases without memory loss or dementia. Instead, the beneficial effect of external cues again raises the specter of significant medial frontal lobe and SMA involvement.
For example, lesions to the medial frontal lobes can result in an inability to internally generate movements whereas external environmental and visual cues can exert an almost "magnetic" effect on limb activation and patients may involuntarily respond and react to external stimuli by reaching, grasping, or utilizing whatever objects may be near (see chapter 19). In contrast, the lateral frontal lobes are involved in externally driven movements (chapter 19).
Hence, Parkinson's patients are possibly better able to "move" in response to visual stimuli presumably because of preserved lateral frontal lobe functioning and the loss of inhibitory SMA control -which (in part) accounts for their difficulty internally "willing" purposeful and fluid movements.
DA, ACh, & STRIATAL IMBALANCE.
The DA and cholinergic, ACh system appear to exert counterbalancing influences. For example, loss of nigrostriatal DA results in increased ACh, neuron hyperactivity (see Aghanjanian & Bunney, 1977; Bloom et al. 1965) and tonic EMG activity and thus limb rigidity which can be reversed by anti-cholinergic drugs (Klockgether, et al., 2002). That is, drugs which decrease ACh and those which increase DA, can ameliorate Parkinsons symptoms (Fahn, 2011), which suggests these two transmitters play oppositional and balancing roles (see Stoof et al. 1992). Moreover, ACh can be inceased by drugs that decrease DA, and can be decreased by drugs which increase DA (reviewed by Stoof et al. 1992).

DA normally inhibits ACh release and increases GABA activity. The DA system appears to exert an controlling influences not only on GABA but ACh neurons in the striatum (Le Moal & Simon 2001). Hence, reductions in DA result in decreased GABA activity and increased limbic striatal arousal and ACh activity, and reduced corpus striatal arousal and reduced GABA activity (see Stoof et al. 1992). Under these latter conditions the subject becomes rigid.
However, under conditions where striatal DA is depleted, the excessive release of ACh creates excessive neural activity (Aghanjanian & Bunney, 1977; Bloom, et al. 1965) which in turn may be manifested in the form of excessive motor actions; i.e. ballismus, chorea, if the medial and ventral globus pallidus-subthalamic circuit is effected, or hypomovement and Parkinson's symptoms if the corpus striatum and dorsal globus pallidus is effected.
For example, fluctuations in DA levels can either act to excite or inhibit motor and related cognitive-memory activity within the limbic striatum (Le Moal & Simon 2001; Mogenson & Yang 2001). However, with high levels of limbic striatal DA (and/or amygdala) activity, coupled with reduced GABA, animals may appear hyperactive and engage in running, "galloping," and biting, and related movements.
Presumably these latter actions are due to inhibitory release (a consequence of striatal dysfunction or hyperactivation), and thus the triggering of brainstem motor programs that subserve these specific behavioral acts. These same stimulus (predator) released actions, across evolution, and across most animal species, are directly related to the functional integrity of the limbic system and striatum, and the capacity to survive when living in close proximity to predators, the elements, and cospecies. Actions without thought are in fact the province not of the basal ganglia, but the brainstem, which is why these and related motor programs are stored within brainstem nuclei (see chapter 17).



That is, abnormal levels of corpus striatal DA activity can interfere with selective attention and the reception of neocortical and limbic impulses within the striatum -as can increases in mesolimbic DA activity (Le Moal & Simon 2001). However, with increases in striatal DA, cognitive, social, and emotional integrative functions may become abnormal and distorted and the individual may become psychotic (see Castellanos et al. 1994; Chakos et al. 1994; Ring et al. 1994; Snyder, 1972).
As is well known, individuals diagnosed as paranoid and schizophrenic with psychomotor retardation have been found to have elevated DA levels and increased DA receptor binding (Crow, 1979; Ellison, 1994; Matthyse, 1981; Ring et al. 1994). Presumably this is why dopamine blockers such as the phenothiazines are effective in reducing psychotic behavior; i.e. they reduce excessive DA binding and excessive striatal activity which in turn restores the capability of the striatum (and related nuclei) to engage in cognitive-emotional integrative activities.
The Amygdala, Temporal Lobe & DA.
Psychotic disturbances, including "schizophrenia" need not involve the basal ganglia or DA dysfunction, as disorders of thought and mood may also appear following frontal or temporal lobe damage (chapter 19, 21). However, with frontal lobe damage the basal ganglia and SMA are often effected, whereas the amygdala is implicated in temporal lobe related psychotic states.
Indeed, abnormal DA as well as norepinephrine (NE) and serotonin (5HT) have been reported in the amygdala of those diagnosed as psychotic (Spoont 2003; Stevens 1992). In addition, a gross asymmetry in the post-mortem levels of mesolimbic DA has been noted in the brains of those diagnosed as schizophrenic (reviewed in Le Moal & Simon 2001). Specifically, an increase in DA has been noted in the central amygdala and in the striatum of the left hemisphere, whereas DA levels within the right cerebral are similar to normals.
In fact, the left amygdala and left temporal lobe (Flor-Henry, 1969; Perez, et al. 2001; Stevens, 1992) have long been thought to be a major component in the pathophysiology of psychosis and schizophrenia (Heath, 1954; Stevens, 1973; Torey & Peterson, 1974). For example, abnormal activity as well as size decrements have been noted in the left amygdala (Flor-Henry, 1969; Perez, et al. 2001) as well as the left inferior temporal lobe in schizophrenic patients (see Stevens, 1992). Spiking has also been observed in the amygdala of psychotic individuals who are experiencing emotional and psychological stress (see Halgren, 1992).
In this regard it has been suggested that stress, particularly when experienced prenatally may be responsible, in some cases, for the development of DA abnormalities (see Le Moal & Simon 2001), whereas adverse early environmental experiences and trauma may contribute to the development of kindling, spiking and abnormal amygdala functioning (see chapters 28, 30). As noted, Parkinson's symptoms in some respects also resemble the effects of chronic stress and an inability to relax.
Obsessive-compulsive disorders are characterized by the repetitive experience of unwanted and recurrent, perseverative thoughts (obsessions) and/or a compulsion to repetitively perform certain acts. The world wide prevalance rate is about 2.3% (see Okasha et al. 1994). However, some patients tend to suffer from compulsions whereas others experience obsessions, with the majority experiencing both (DSM IV).
Obsessions not uncommonly tend to be religious or concerned with contamination coupled with cleaning and washing rituals. The experience of intrusive and unpleasant (e.g. violent and/or sexual) images is not uncommon (Lipinsk & Pope 1994).
In some instances obsessive compulsions serve as a means of coping with unwanted or troubling impulses; i.e. by perseverating in thinking certain thoughts or engaging in certain actions, the individual is able to avoid focusing on and recognizing thoughts, actions, or impulses which he or she finds disturbing.
For example, consider "Bob" a 30 year old socially inept, single male with a BA degree in English, who for several years voiced "homophobic" and "right wing" extremist views and who experienced and engaged in a compulsion to work on his car and/or wash and dust the engine late in the evening and even after midnight. However, after neighbors repeatedly complained about the noise, he was forced to cease these acts, underwent a noticeable personality change and was soon frequenting bars late at night, taking midnight drives and having sex with men he met while intoxicated.
As noted, the basal ganglia is intimately associated with the hypothalamus, amygdala, and orbital frontal lobes; nuclei which are also associated with sexual activity and the mediation of emotional functioning, with the orbital areas acting to gate and inhibit limbic system activity (Joseph, 2011a). Hence, the orbital frontal lobes are also implicated in the development of obsessive compulsive disorders (Baxter et al. 2004; Rappoport 2001; Rauch et al. 1994).
In that obsessive-compulsive disorders are also associated with hyper-metabolism within the caudate nucleus (Baxter et al. 1992; Rappoport 2001; Rauch et al. 1994) -as well as reduced 5HT (serotonin) activity (Goodman et al. 2010; Zohar & Insel 2002) which in turn results in disinhibition- this raises the possibility that the inhibitory circuit maintained by the orbital frontal lobes and basal ganglia, in some instances, may be deactivated or overwhelmed.
Given that it is via the frontal lobes that thoughts and emotions come to be integrated and infused with emotion, and/or inhibited and suppressed, this also suggests that caudate/orbital dysfunction may result in the inability to extinguish certain thoughts and actions, even in the absence of an underlying emotional problem (see Baxter et al 1992, and Rauch et al. 1994, for a different albeit related explanation). The individual may perseverate on a single thought or action, which in turn may interfere with their capabilty to engage in alternative modes of response due to neurological dysfunction involving this circuit.
Conversely, as is suggested by the actions of "Bob" referred to above, it is possible that this same frontal-inhibitory circuit may be employed so as to prevent an individual from engaging in certain behavioral acts which the "conscious" mind finds objectionable or "dirty." They perseverate on a certain theme, thought, or action to the exclusion of others. Therefore, when "Bob" was feeling homosexually aroused, rather than "cruising" for sex partners, a different (albeit related) motor program was initiated and he would wash and dust his car and even its engine (which simultaneously and symbolically cleansed him of his dirty thoughts and/or at least prevented him from thinking and acting on them). As noted, the frontal lobes are instrumental in memory search and activation as well as impulse inhibition and memory avoidance (e.g. repression).
THE LIMBIC STRIATUM, DA, 5HT, ACH, NE, & MEMORY
The limbic striatum consists of the nucleus accumbens, olfactory tubericle, the extended (centro-medial) amygdala, as well as the ventral aspects of the caudate, putamen and globus pallidus (-substantia innomminata).
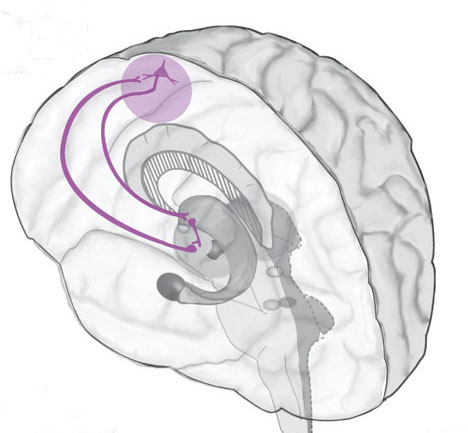
The nucleus accumbens is located immediately beneath the anterior portion of the caudate and from a microscopic level appears to be part of the ventral caudate (Olton et al. 2001; Zaborszky et al. 2001). However, it maintains extensive interconnections with the amygdala as well as the hippocampus via the fimbria-fornix fiber bundle (DeFrance et al. 2001), which implicates this nuclei in memory functioning and probably the learning of visual-spatial and perhaps social-affective relationships.
Similarly, the dorsal portion of the substantia innominata ("great unknown") merges (dorsally) with the ventral globus pallidus (and ventrally with the centro-medial amygdala), and maintains interconnections with the accumbens, hippocampus, lateral amygdala, and dorsal medial (DM) nucleus of the thalamus (Young et al. 2004; Zaborszky et al. 2001) -the DM being involved in regulating neocortical arousal and information reception as well as memory (see chapter 19).
Like the accumbens, the substantia innominata (SI) is also important in memory functioning and has been implicated as one of the principle sites (along with the nucleus basallis) for the initial development of Alzheimers disease (see Olton et al. 2001; Zaborszky et al. 2001, for review of related details).
The nuclei of the limbic striatum are also interconnected with the lateral and medial hypothalamus, brainstem reticular formation, and the frontal and inferior temporal lobes (Everitt & Robbins, 1992; Groenewegen, et al. 2001; Heimer & Alheid, 2001; Kelly, et al. 1982; Mogenson & Yang 2001; Olton et al. 2001; Parent & Hazrati 2005; Van Hoesen, et al. 1981; Zaborszky et al. 2001). The limbic striatum is able, therefore, to exert widespread effects on neocortical and subcortical structures.


As noted, the limbic striatum receives DA from the mesolimbic system as does the amygdala. The amygdala also sends axons that terminate in striatal neurons immediately adjacent to those innervated by mesolimbic DA neurons (Kelly et al. 1982; Yim & Mogenson, 1983, 2005). Thus, a complex interactional loop is formed between these nuclei, the integrity of which, in part is dependent on the mesolimbic DA system which can act on the amygdala, hippocampus and limbic striatum simultaneously so as to modulate striatal reception of amygdala (Maslowski-Cobuzzi & Napier, 1994) and hippocampal excitatory signals, and regulate the transmission of accumbens input into the SI. Alterations in mesolimbic DA activity, therefore, can significantly influence limbic striatal, amygdala and hippocampal activity as well as the motoric expression of limbic impulses.
For example, depletions in mesolimbic DA can disrupt motor and social-emotional memory functioning; a condition compounded by DA influences on acetylcholine (ACh) neurons, and the effects of striatal DA on the reception of hippocampal, amygala, and neocortical input.
Indeed, the limbic striatum (and limbic system) contain high densities of ACh neurons which are involved in memory as well as motor functioning (Olton et al. 2001; McGaugh et al. 1992; Zaborszky et al. 2001). In fact, of all striatal nuclei, densities of DA (and ACh) neurons are highest within the (SI) and nucleus accumbens (Meredith et al. 2005) which in turn greatly influences the SI (which is a major source of neocortical ACh), as does the amygdala (Yim & Mogenson 1983) and hippocampus; i.e. these signals converge on the SI.
Like the striatum, the hippocampus (and the amygdala) also receives meso-limbic DA input and contains D1 and D2 receptors (Camps et al. 2010) as well as ACh. ACh influences on neuronal activity can be excitory or inhibitory depending on the resting membrain potential of the receiving neuron (Ajima et al. 2010) as well as concurrent DA activity. Specifically, mesolimbic DA mediated signals are relayed from the accumbens to ACh neurons in the SI which in turn project to DM and the neocortex, the frontal lobes in particular (Mogenson & Yang 2001; Olton et al. 2001; Zaborszky et al. 2001). However, the DA and cholinergic, ACh system appear to exert counterbalancing influences.
For example, ACh is usually inhibited by DA. By contrast, DA depletion results increased ACh and neuron hyperactivity (see Aghanjanian & Bunney, 1977; Bloom et al. 1965; Klockgether, et al., 2002) which can greatly disrupt cognitive and memory functioning as well as motor activities, unless reversed by anti-cholinergic drugs. In fact, damage to this DA/cholinergic system can result in Alzheimers disease (Olton et al. 2001; Zaborszky et al. 2001).
As per memory functioning, it appears that mesolimbic DA modulates the reception of hippocampal (motor-spatial) input into the accumbens which projects to the SI (which in turn distributes these influences, perhaps via ACh) to the neocortex). DA accomplishes this via inhibitory influences on ACh and facilitation of the GABA system which appears to exert inhibitory influences within the striatum and the transmission of impulses from the nucleus accumbens (and amygdala/hippocampus) to the SI. Therefore, if the inhibitory influences of GABA and ACh on the SI are dampened, the SI becomes activated and memory functioning may be enhanced or disrupted at the neocortical level as this nucleus exerts widespread ACh influences on the cerebrum. Thus fluctuations in DA levels can either act to excite or inhibit cognitive-memory activity within the limbic striatum (see Mogenson & Yang 2001) as well as the corpus striatum (Packard & White 2001) and the neocortex.
However, also of importance in this memory-striatal neural network is (5HT) serotonin (McLoughlin et al. 1994; Mogenson & Yang 2001) and (NE) norepinephrine (Roozendaal & Cools, 1994). For example, NE levels have been shown to fluctuate within the amygdala and nucleus accumbens when presented with novel stimuli (Cools et al. 2001) and during information acquisition. Presumably NE levels within the accumbens can regulate or influence the reception of amygdala and hippocampal impulses within the accumbens and SI which in turn projects to the neocortex and, in this regard, may act to shunt amygdala-hippocampal impulses to discrete or wide areas of the cerebrum.
Specifically, high alpha-NE activity is associated with reduced amygdala input, whereas low beta NE may reduce hippocampal input into the striatum. Conditions such as these, however, are most likely to result when stressed or traumatized in which case it is possible for amygdala (and thus emotional) input to be received in, learned, and expressed by the striatum in the absence of hippocampal participation (see Rozzendaal & Cools 1994). It is conditions such as these that can give rise to amnesia with preserved learning (see chapters 14, 30).
However, in some cases, it is also possible for both the alpha and beta NE system to be effected simultaneously such that amygdala and hippocampal input to the limbic striatum are inhibited in which case profound memory loss may result (see Rozzendaal & Cools 1994). Similar disturbances are associated with the serotonin (5HT) system (McLoughlin et al. 1994).
Depletion of 5HT can significantly effect the capacity to inhibit irrelevant sensory input (at the level of the brainstem, amygdala, basal ganglia, and neocortex). Hence, 5HT abnormalities or depletion typically results in confusion and sensory overload and in some instances the production of hallucinations (see chapter 30).
Thus disruption of the mesolimbic DA system and/or severe disruptions in the 5HT system in turn interferes with the hippocampal-limbic striatal memory system (Packard & White 2001) and can create neuronal hyperactivity -a condition which interferes with perceptual and hippocampal- memory-SI-amygdala functioning. In this regard it is noteworthy that the central 5HT system is severely disrupted among those with severe memory loss and Alzheimer's disease (McLoughlin et al. 1994) and that calcification of the globus pallidus/SI can induce visual and auditory hallucinations as well as cognitive deterioration (Lauterbach et al. 1994).
OVERVIEW: ALZHEIMER'S DISEASE
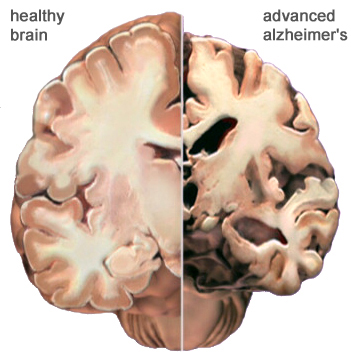
Alzhiemer's disease is associated with a profound loss of memory and cognitive and intellectual functioning, including, at its later stages, an inability to recognize friends, loved one's or their own personal identity. Alzhiemer's disease is estimated to afflict approximately 10% of those over age 65, and 50% of those over 85.
In its later stages, Alzhiemer's disease is associated with profound loss of cerebral functional capacity, coupled with neural degeneration and a loss of neurons and the development of amyloid (senile) plaque and neurofibrillary tangles. Because structures such as the substantia innominata, amygdala, and entorhinal cortex have been injured (Morrison et al. 2010; Gomez-Isla, et al., 2000; Rapoport 2010) it is thought that the destruction of this tissue and adjacent tissue accounts for the loss of memory, and social-emotional, facial recognition, and related visual abnormalities, including visual agnosia (Giannakoulos, et al., 2011).
However, because there is no single factor that has been implicated, and due to the number of associated disturbances, it has been argued that Alzheimer's disease and associated cognitive and memory disturbances are due to a "global cortico-cortical disconnection" syndrome (see Morrison et al. 2010; Rapoport 2010). Nevertheless, for the purposes of this chapter, the striatal contribution to this disorder will be emphasized.
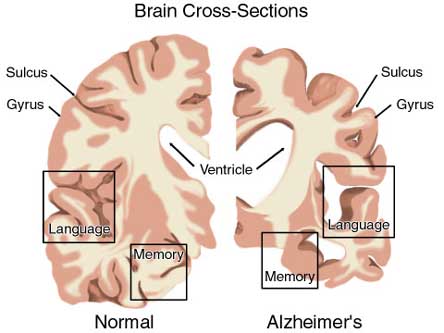
MEMORY & ALZHEIMER'S DISEASE
As detailed in chapter 15, the amygdala and hippocampus provide massive input to the limbic striatum as well as the dorsal medial nucleus of the thalamus (DM) , the frontal lobes and reticular activating system, as does the accumbens and SI (see Heimer & Alheid, 2001; Koob et al. 2001; Mogenson & Yang 2001; Zaborszky et al. 2001). As noted, these nuclei, including the overlying entorhinal cortex, play a significant role in memory and the gating of information destined for the neocortex and appear to be part of a massive neural network designed to control information processing and to establish memory related neural networks (see also chapter 14). Presumably the nucleus accumbens apparently acts to integrate hippocampal and amygdala input, which is then transmitted to the SI which is also the recipient of limbic impulses concerned with cognitive, memory, and motoric activities (see Mogenson & Yang 2001).
In part, it appears that the accumbens and SI play an inhibitory (filtering) role on information processing, and may exert inhibitory (and counterbalancing) influences on the the medial frontal lobes, the DM, and corpus striatum (see Mogenson & Yang 2001). The amygdala exerts similar influences on these nuclei (chapter 15) and is also able to inhibit the SI and accumbens.
However, the role of the limbic striatum in cognitive and memory related activity also includes the learning of reward-related and aversion processes, the facilitation of approach and withdrawal responses and the memorization of where a reward or aversive stimulus was previously received (Everitt et al. 2001; Kelsey & Arnold 1994). In this regard the nucleus accumbens and SI are dependent on the medial and lateral amgydala, especially in the learning of negative experiences (Kelsey & Arnold 1994).
As noted, the SI is the primary source of neocortical cholinergic innervation (Carpenter 2001) and appears to be concerned with integrating social-emotional and cognitive input with motor memories and then storing them perhaps within the SI as well as within the neocortex. Hence, if the SI is lesioned the cholinergic projection system is disrupted, and cognitive as well as social-emotional memory functioning is negatively impacted.
Since the SI merges with and becomes coextensive with the centromedial amygdala (Heimer & Alheid 2001),and is an amygdala derivative, not suprisingly, a significant loss of neurons, neurofibrillary changes and senile plaques have been found in the amygdala (and hippocampus, Rapoport 2010), as well as in the SI, in patients with Alzheimer's disease and those suffering from degenerative disorders and memory loss (Herzog & Kemper, 1980; Mann, 1992; Sarter & Markowitsch, 2001). Degeneration in the amygdala would also account for the social-emotional agnosia and prosopagnosia that is common in the advanced states of Alzhiemer's disease; i.e. failure to recognize or remember loved ones (chapter 13).
WIDESPREAD NEURONAL DEATH & ALZHEIMER'S
Neuronal loss has also been reported in the entorhinal and parahippocampal areas of the inferior temporal lobe (see Morrison et al. 2010; Rapoport 2010) within which is buried the hippocampus and amygdala. Specifically, in addition to senile plaques, neurofibrillary tangles, a 40% to 60% loss of neurons during the early stages of this disease have been found in layers 4 and 2 of the entorhinal cortex (Gomez-Isla, et al., 2000)--the gateway to the hippocampus. Destruction of this tissue and adjacent tissue would also result in memory, social-emotional, facial recognition, and related visual abnormalities, including visual agnosia, as recently demonstrated among those with Alzheimer's disease (Giannakoulos, et al., 2011).
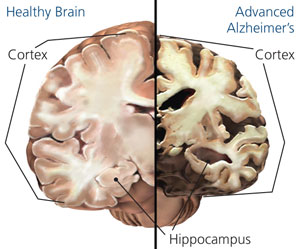
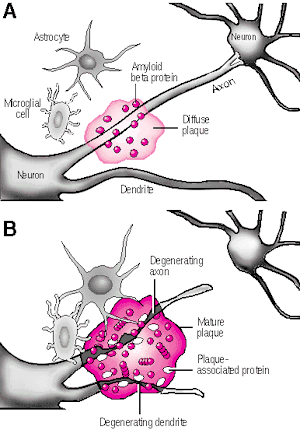
Indeed, among those with Alzheimer's disease, widespread atrophy, amyloid plaques, tangles, and metabolic disturbances have been noted in a variety of cortical areas with relative preservation of the motor and primary receiving areas (Rapoport 2010). Based on these findings it has been argued that Alzheimer's disease and associated cognitive and memory disturbances are due to a "global cortico-cortical disconnection" syndrome (see Morrison et al. 2010; Rapoport 2010); i.e., a loss of neurons in and interconnections with association neocortex, which in part would account for the cognitive deterioration.
The initiation of this deteriorative process may well be in the SI, and it may be due to an olfactory borne infection due to viral or bacterial invasion. That is, these bacterial or viral agents may enter the olfactory system and invade those structures directly innervated by the olfactory nerves, i.e. the amygdala, entorhinal cortex, and SI (Joseph, 2008d). On the other hand, or perhaps related to this scenario, are findings suggesting a genetic foundation for this disorder, such that defects in at least four specific genes (located on chromosomes 1, 14, and 21) may be responsible (Levy-Lehad et al., 2005; Saunders et al., 2003).
Given the likelihood that the limbic striatum and olfactory-limbic structures may be selectively involved in the early stages of Alzheimers, and given the fact that the SI provides cholinergic input to the neocortex, then the subsequent and progressive loss of SI (and amygdala) neurons might result in a progressive deterioration and cell death within the neocortex such that otherwise healthy neurons are killed. That is, since the SI contains high concentrations of cholinergic neurons which in turn project to widespread areas throughout the neocortex (reviewed in Carpenter 2001), perhaps the initial cell loss within the SI (also referred to as the nucleus basalis), may trigger further cell death in healthy neurons (which project to or receive fibers from the affected cells) which essentially become "pruned" and drop out form disuse.
Defective Axonal Transport.
There is evidence which indicates that perhaps due to head injury, drug or toxic exposure, or perhaps the loss of synaptic junctions (due to the death of unhealthy cells and dendritic retraction), axonal transport becomes defective due to the death of its target neuron. However, if a healthy cell cannot discharge and exchange information it too may die, thus leading to a domino effect and thus widespread cell death (see Burke et al. 1992). That is, due to the loss of terminal synaptic junctions (due to cell death) or to other chemical abnormalities including defects in microtubule assembly (which participates in neuronal transmission), axonal transport becomes dysfunctional as the receiving cell and it's dendrite have died. Hence, there is a buildup of toxic oxidatative metabolites and naturally occurring neurotoxins in the healthy cell body that project to the dead cell, which causes the normal cell to die as well. This would result in a progressive loss of neurons such that widespread areas of the cerebrum soon become effected.
Presumably this what may occur if the limbic striatum becomes abnormal and cells within the SI and accumbens begin to die. As the disturbance and deterioration spreads, cognitive, emotional, memory and related abnormalities including Parkinsonian symptoms begin to appear and become progressively worse as neocortical, striatal, and limbic neurons die and drop out.
The limbic and corpus striatum are richly interconnected and send projections to many of the same brain areas. However, due to differential input from the amygdala and DA systems, these nuclei exert tremendous counterbalancing influences on each other and their associated neural networks. For example, the centro-medial as well as the basolateral amygdala projects to and exerts excitatory influences on the the limbic striatum (Maslowski-Cobuzzi & Napier, 1994; Yim & Mogenson 1982), whereas the medial and posterior-lateral amygdala projects to the corpus striatum and the ventral and dorsal globus pallidus. Via these dual interconnections, the amygdala can exert simultaneous and even oppositional influences on these nuclei.
In addition, the corpus striatum receives the bulk of its DA from the nigrostriatal systems, whereas the limbic striatum receives DA from the mesolimbic DA system (see Fibiger & Phillips, 1986 for review) -transmitter systems which interact at the level of the brainstem, limbic system, striatum, and neocortex (Maslowski-Cobuzzi & Napier, 1994). For example, it has been shown mesolimbic DA can act on the amygdala and limbic striatum simultaneously so as to modulate striatal reception of amygdala excitatory signals (Maslowski-Cobuzzi & Napier, 1994).
However, because the corpus and limbic striatum are largely (but not completely) innervated by different clusters of midbrain DA neurons, reductions or increases in one DA system can exert profound influences on those neurons innervated by the others -for example, by eliminating inhibitory or counterbalancing influences. Thus depletion of DA in the nigrostriatal (but not the mesolimbic) pathways can result in increased activity within the limbic striatum (see Yim & Mogenson, 1983, 2005) and medial amygdala, but deceased activity within the corpus striatum. If this occurs, movement programming may be disrupted resulting in rigidity or tremors -a consequence, in part, of an imbalance in amygdala-DA-striatal activation.
Conversely, mesolimbic DA depletion can result in enhanced corpus striatal activity and decreased limbic striatal and lateral amygdala and hippocampal activity, such that motor and social-emotional memory functioning may be disrupted; a condition compounded by DA influences on acetylcholine (ACh) neurons and the reception of hippocampal, amygala, and neocortical input within the striatum. Hence, the functioning of the limbic or corpus striatum can be severely disrupted even when the functional integrity of its own neurotransmitter systems are otherwise intact; i.e. due to a loss of counterbalancing influences.
THE SMA, PUTAMEN, AND GLOBUS PALLIDUS
As noted, some studies have indicated that those with Parkinson's disease demonstrate the greatest amounts of DA depletion and related DA neuronal degeneration within the putamen. Hence, many authors have argued that Parkinson's symptoms are a consequence of damage to this nuclei (e.g. Goto et al.2010), which in turn can result in the production of unwanted movements such as tremor. Presumably, in these instances, the medial (internal) globus pallidus (which receives putamen input) ceases to inhibit irrelevant motor activity.
The putamen receives much of it's input from the caudate and in particular the SMA, the secondary and primary motor cortex, as well as areas 5 and 7 of the parietal lobe (Jones & Powell, 1970; Pandya & Vignolo, 1971). Presumably the putamen, in conjunction with the caudate, transmits this information to the medial and lateral GP which in turn projects to the motor thalamus, and brainstem. However, like the caudate, the putamen and GP also project back to the motor neocortex, thus creating a very elaborate feedback loop (see Mink & Thach, 2001; Parent & Hazrati 2005) whose origin may begin in the SMA (Alexander & Crutcher, 2010; Crutcher & Alexander, 2010) or perhaps the limbic system (chapter 13).
For example, when anticipating or preparing to make a movement, but prior to the actual movement, neuronal activity will first begin and then dramatically increase in the SMA, followed by activity in the secondary and then the primary motor area (see chapter 19), and then the caudate and last of all the putamen-GP (Alexander & Crutcher, 2010; see also Mink & Thach, 2001).
Ignoring for the moment the role of the limbic system, this indicates that impulses to move first appear in the SMA and that other motor regions are temporally-sequentially recruited in a step-wise fashion; i.e. SMA - premotor - primary motor - caudate - putamen - GP - motor thalamus/frontal motor areas - brainstem...
However, the caudate nucleus also contains neurons which become active prior to, and in anticipation of making body movements, and in response to associated auditory and visual environmental cues (Rolls, et al. 1983; Rolls & Williams, 2002). This caudate neuronal activity precedes, or occurs simultaneously with excitation in the lenticular nucleus, which also results (via feedback) in SMA activation. This is because the GP and the putamen not only receive caudate and neocortical afferents, but they project back to the frontal motor areas (as well as to the motor thalamus). Hence, parallel processing also occurs.
That is, activity in these regions quickly begins to overlap such that neurons in the motor neocortex and basal ganglia often remain activated simultaneously (Alexander & Crutcher, 2010). Moreover, the motor areas all independently send axons to the brainstem, with axons from different areas converging on the same motor neuron.
Therefore, activity in the motor areas is characterized by both temporal-sequential and parallel processing that in turn is made possible via feedback neural circuitry. Because these different networks are intimately linked and mutually interactive they make coordinated and goal directed movements possible.
Indeed, multiple feedback loops are probably also necessitated by the numerous variables and body- spatial- visual- kinesthetic- motor references, etc., that need to be computed in order to make a planned movement. Thus both temporal-sequential and parallel processing is necessitated as each area is functionally specialized to analyze specific types of information and to perform certain actions in semi-isolation as well performing other functions in parallel with yet other motor areas.
THE PUTAMEN & MEDIAL & LATERAL GLOBUS PALLIDUS
Just as different regions of the caudate appear to subserve different functions, a variety of neuronal types also characterize the lenticular nucleus. For example, preparatory and movement related neurons are segregated within the putamen (Alexander & Crutcher, 2010). This suggests that the putamen employs two different neural networks, one which executes movements, the other which prepares to make the movement; information which is normally transmitted to the GP as well as back to the motor neocortex.

The putamen appears to act at the behest of impulses arising in the neocortical motor areas (as well as the limbic system) and then only secondarily acts to prepare for and then to participate in the guidance of movement (Mink & Thach, 2001). In conjunction with the caudate, the putamen accomplishes this via signals transmitted to the medial and lateral GP, the motor thalamus, and brainstem reticular formation (Marsden & Obeso, 1994) and then back to the motor neocortex (see also Parent & Hazrati 2005, for a related review).
As noted, the putamen and globus pallidus are also coextensive (the lenticular nucleus) and in many respects function as a prepatory motor unit involved in the guidance, and perhaps even the learning of various motor activities (Crutcher & DeLong, 2004; Kimura, 2002). For example, alterations in neuronal activity have been demonstrated in the globus pallidus and the putamen during tasks involving learned body movement (Crutcher & DeLong, 2004). Lenticular neurons also become highly active when learned facial or limb movements are triggered in response to a particular auditory or visual stimulus associated with that movement (Kimura, 2002).

Some putamen and GP neurons also fire in response to reward but not to movement and vice versa (Kimura, et al. 2004). However, these same neurons do not respond when the same learned and rewarded movements are made spontaneously and in the absence of associated cues, or when they were no longer rewarded. These findings raise the possibility that specific neurons within these nuclei are responsive to motivational cues associated with movement and that these neurons utilizes these cues in preparation to make a movement (Kimura, 2002).
Neurons in the GP also selectively respond and change their activity when making ballistic movements, particularly those which are visually guided (Mink & Thach, 2001). However, activation occurs too late for these neurons to be involved in the initiation or planning of the movement (Mink & Thach, 2001). Conversely, destruction or massive inhibition of the GP can result in difficulty turning off a movement (see Mink & Thach, 2001) -thus giving each movement a ballistic quality. Similarly, destruction or massive inhibition of the GP can make it exceedingly difficulty to rapidly alternate between movements and thus switch from an ongoing to a different motor program (Hore & Vilis, 1980). This loss of control over motor programming with GP impairment extends even to attempts to make purposeful ballistic reaching or stepping movements (Horak & Anderson, 2004; Hore & Vilis, 1980), and the velocity and amplitude of these movements may in fact be significantly slowed and reduced (Mink & Thach, 2001).
MEDIAL/Internal & LATERAL/External GLOBUS PALLIDUS
The medial/internal and lateral/external GP appear to play different and counterbalancing roles in the execution, inhibition and excitation of different motor programs (Crossman, et al. 2002; Marsden & Obeso, 1994). For example, the medial/internal GP is believed to provide positive/excitatory feedback to the neocortical motor areas, whereas the lateral/external GP provides indirect "negative" and inhibitory feedback so that unwanted movements are prevented. Moreover, DA inhibits the medial GP, but excites the lateral GP (reviewed in Marsden & Obeso, 1994).
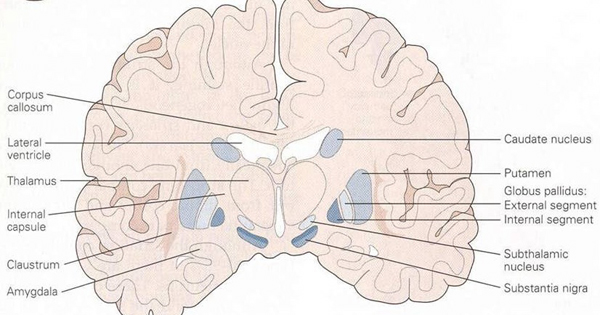
In consequence (at least in theory), reduced nigrostriatal DA levels can result in an overexcitation of the medial GP, which inhibits cortically mediated or initiated movements thereby producing akinesia (loss of movement), bradykinesia (reduction in movement) and hypokinesis (slowness of movement) coupled with tremor.
On the other hand excessive lateral GP (and limbic striatal) activity can also result in gross involuntary and excessive hyperkinetic and ballistic movements usually involving the limbs on the contralateral side of the body, along with choeriform movements and tremors. However, surgical destruction of the fibers tracts leading to and from the lateral and medial GP can significantly diminish such disturbances, including hemiballismus, tremor, dystonia, chorea and athetosis (see Carpenter & Sutin, 1983).
As noted above, lesions to the amygdala prior to the development of Parkinsonian and related symptoms, can also prevent the development of these motor abnormalities, which in part is due to the extensive interconnections maintained with these regions and the fact that the amygdala becomes coextensive with the ventral GP.


In addition, because the putamen projects to the GP, lesions restricted or localized to the putamen can also result in abnormal competition between antagonist and agonist muscles, due to the release of the GP (and associated neural circuitry) from putamen control. Patients or primates so effected suffer from severe dystonia as well as rigidity and increased muscle tone sometimes accompanied by chorea (Segawa, et al. 2002). Similarly, among those with Parkinson's disease, activity within the putamen is significantly reduced (Playford et al. 1992), and reduced functional activity is also seen in the caudate which merges with the putamen.


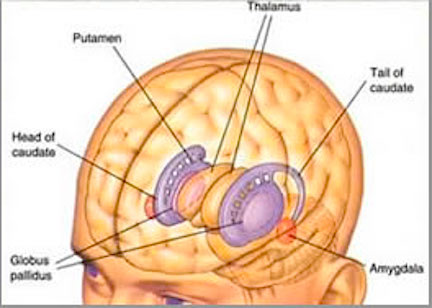
The motor thalamus consists of the ventromedial, ventrolateral, ventralis intermedius, centromedian and parafascicular (posterior) intralaminar thalamic nuclei, and receives input from the brainstem, cerebellum, and neocortex, and maintains reciprocal projections with the GP and SMA (Carpenter 2001; Brodal, 1981; Kemp & Powell, 1970; Narabayashi, 2002; Royce, 2002; Vitek et al. 1994). These thalamic "motor" nuclei also receive input from the facial, leg, and arm regions of the motor and somatosensory cortex (Kunzle, 1976; Vitek et al. 1994) and maintains reciprocal interconnections with the amygdala, cingulate gyrus, substantia nigra, and superior colliculus (Jones et al. 1979; Mesulam et al. 1977; Royce, 2002; Vogt et al. 1979).
Thus the motor thalamus is intimately associated with limbic and motor nuclei throughout the brain and is able to influence as well as receive multiple inputs from a variety of nuclei which in turn are interlinked. For example, some intralaminar thalamic nuclei send collateralizing axons which project to both the striatum and the neocortex, whereas some motor neocortical axons project to both the striatum and the intralaminar nuclei (Royce, 2002). Hence, a richly interconnected neural circuit is maintained by these nuclei so as to control and guide motor functions.
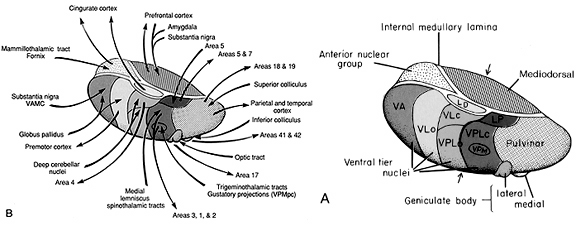
In some respects the motor thalamus appears to act as a nexus where multiple forms of input are integrated so as to regulate motor activity. Therefore, when the motor thalamus is abnormally inhibited or activated, significant motor abnormalities result; e.g., rigidity, tremor, ballismus, catatonia, and catalepsy. For example, unilateral infarcts and hemorrhages involving the thalamus can induce unilateral thalamic ataxia and apraxia (Nadeau et al. 1994; Solomon et al. 1994).
Conversely, injuries or surgical destruction of the specific thalamic nuclei can in some instances eliminate or reduce the influences of abnormal activity received from other regions within the motor circuit (Marsden & Obeso, 1994; Narabayashi, 2002). For example, patients suffering from Parkinsonian symptoms appear to derive the greatest benefit from thalamic lesions which abolish contralateral tremor and rigidity (see Marsden & Obeso, 1994) via disruption of the GP-motor thalamus-frontal motor area motor circuit.
Specifically, there is evidence which indicates that destruction of the ventrolateral (VL) motor thalamic nuclei can reduced rigidity (if the lesion is more anterior), or tremor (if the lesion is posterior). Neurosurgical destruction of the GP to motor thalamus projection fibers can also significantly decrease rigidity, whereas section of the thalamic to brainstem/cerebellum pathway also reduces tremor (see Narabayashi, 2002). Moreover, VL lesions can significantly reduced choreic and ballistic movements such as secondary to trauma or encephalitis.
In addition, neurosurgical destruction or electrical stimulation of these nuclei (e.g. ventralis intermedius) can eliminate tremors (see Narabayashi, 2002). Indeed, neurons in the motor thalamus not only fire in tandem with tremor, but electrical stimulation of this nuclei at a frequency similar to the tremor, increases the frequency and amplitude of the tremor. However, high levels of thalamic stimulation reduces or abolishes tremor (Narabayashi, 2002).
Due to its interconnections with so many motor related areas of the brain (as well as the limbic system), the subthalamic nucleus is able to exert significant, albeit indirect as well as direct influences on motor expression which is accomplished in conjunction with the nigro-striatal DA system and the GP which in turn modulates subthalamic activity and its reception of cortical input (Parent & Hazrati 2005; Wichman et al. 1994). Hence, the subthalamic nucleus appears to also exert modulating influences on movement (although it also serves non-movement functions), especially those involving the proximal limbs.
Specifically, subthalamic neurons are somatotopically organized, such that those representing the hand, arm, leg, or eyes, are clustered together, and these clusters change their activity during eye, or limb movements (Wichmann, et al., 1994). In fact, they increase their activity just prior to movement.
Abnormalities localized to or involving the subthalamic nucleus can therefore result in significant motor disturbances including chorea and hemiballismus (Crossman, et al. 2002) including sudden and involuntary flinging movements of the arm or leg--movements that might be appropriate if engaged in defensive maneuvers. However, patients can still make normal voluntary movements.
Chorea and hemiballismus are due presumably to interuption of the normal GABAinergic reciprocal relationship it maintains with the caudate, putamen, and in particular the GP (McGeer & McGeer, 2002; Parent & Hazrati 2005). That is, due to loss of inhibitory GP input, the patient makes sudden and involuntary movements, due to excessive activity directed, ultimately, the motor neocortex. Hence, again, these are normal movements which are abnormally produced if the subthalamic nucleus is injured or receives abnormal signals.
For example, Parent and Hazrati (2005) argue that inhibitory influences exerted on the motor thalamus, neocortex, or subthalamic nucleus by the GP can result in akinesia if GP activity is reduced, or hyperkinesia if enhanced. Similarly, Crossman et al (2002) argue that in hyperkinetic states the medial GP and subthalamic nucleus is underactive and the lateral GP is overactive. In Parkinsons and akinetic disorders, the medial GP and subthalamic nucleus is overactive whereas the lateral GP is underactive (Crossman, et al. 2002).
Conversely, lesions of the subthalamic nucleus can result in increased activity within the GP and motor thalamus, such that the motor thalamus, SMA and brainstem become hyperactivated, which results in excessive movement including ballismus. If the GP is subsequently destroyed, hemiballismus disappears. However, in one case it has been reported that a hemorrhage involving the subthalamic nucleus, resulted in the amelioration of a patients Parkinson's symptoms (Wichmann et al. 1994).
DA & GABA STRIATAL INTERACTIONS & MOVEMENT DISORDERS
The GP receives about two thirds of its input from the putamen, and one third from the caudate (as well as some fibers from the SMA). Presumably, activation of the SMA -primary motor -caudate -putamen loop results in differential activation of the lateral and medial GP, the subthalamus, and the motor thalamus and the brainstem -nuclei which project back to the SMA and primary motor areas from which arises a massive rope of nerve fibers (the corticospinal/pyramidal tract) which directly innervates the brainstem and spinal cord. Presumably these complex feedback loops insure that coordinated and smooth continuous movements are planned, coordinated, and finally carried out. As noted, these interactions are also dependent on DA activity.


For example, Parkinson's disease, being characterized by reduced DA, is associated with a reduction in striatal inhibitory activity and thus increased GP output to the motor thalamus and brainstem which would produce tonic EMG activity and akinesia and rigidity (Kockgether, et al. 1986; Starr & Summerhayes, 1983); i.e. hypomovement due to excessive tonic excitation.
Conversely, excessive corpus stritatal DA would result in increased striatal GABA activity and thus inhibition of the GP which would lead to a disihibition of the motor thalamic and subthalamic nucleus and thus increased activation of brainstem and spinal motor neurons. Movements would become hyper, ballistic, and chorea may develop -as frequently occurs with prolonged neuroleptic treatment.

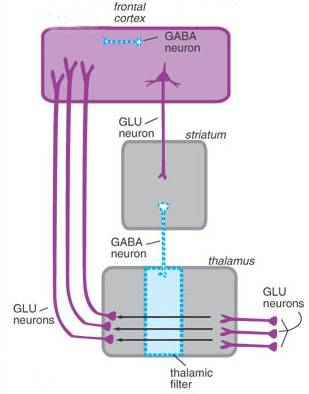
Specifically, GABA activity within the GP and subthalamus are significantly effected by DA activity within the corpus striatum. For example, deceases in striatal DA result in decreased striatal GABA neural activity (-which is normally inhibitory). As these GABA neurons project to the GP, the loss of this GABA inhibitory input would increase tonic activity within the GP. which in turn would result in increased inhibitory GP influences on the motor thalamus and brainstem thereby decreasing their activity. Hence, the modulatory influences of these latter nuclei on motor functioning would be eliminated, resulting in heightened activity levels and thus overactivation of this aspect of the feedback circuit. Brainstem and spinal motor neurons would become tonically activated thereby creating rigidity and stiffness of movement.
Conversely, the loss of GABA influences could induce choreic movements. In this regard it is noteworthy that significant GABA loss and abnormalities have been noted in the substantia nigra and basal ganglia of those with Huntington's chorea (Bird & Iversen, 1974), along with degeneration of the nigrostriatal DA projection pathway.


Huntington's chorea is a progressive deteriorative inherited genetic disorder which is passed on by an autosomal dominant gene located on chromosome 4. It is characterized by an insidious onset that may begin during childhood or old age, with the illness beginning earlier in those who have an affected father (reviewed in Folstein et al. 2010; Young 2005). Cognitive decline, however, is gradual.
Affected individuals tend to suffer from memory and visual-spatial deficits, depression, and reduced fluent output although aphasia is not typical. Difficulty with motor coordination, planning skills, decision making, and a reduced capacity to consider alternate problem solving strategies or to shift form one mental set to another, is not uncommon (reviewed in Folstein et al. 2010). Hence, in some respects this disorder is suggestive of frontal lobe abnormalities (see chapter 19).
This syndrome is also associated with widespread neuronal loss in the caudate, putamen, brainstem, spinal cord, cerebellum, and atrophy in the GP (see Vonsattel, et al. 2002; Young 2005). Degeneration is predominantly of small striatal neurons whereas larger neurons remain intact. Opiate neurons located in the striatum are also significantly effected (Reiner et al. 2004). It is believed that the degeneration of these corpus striatal neurons, as well as the loss of GABA influences, results in reduced striatal control over the GP (see Narabayashi, 2002), thereby producing excessive movement.
It is noteworthy that some reports indicate that the posterior caudate and putamen are more severely effected than the anterior regions (Vonsattel, et al. 2002). Indeed, the posterior caudate and it's tail is usually the earliest and most severely effected part of the brain -which implicates the amygdala as a factor in the development of chorea. Indeed, in the early stages of Huntingtons chorea, atrophy and degeneration begin in the tail and spreads dorsally and anteriorally thus effecting the striatum and lenticular nucleus (Young 2005).
In this regard it is noteworthy that affective disorders and personality and mood changes are prominent early signs suggesting amygdala involvement. Indeed, disturbances of emotion may precede any motor or cognitive decline by as much as 20 years, with some patients displaying mania, depression as well as antisocial tendencies (reviewed in Folstein et al. 2010). As noted, neural degeneration tends to begin in the amygdaloid tail of the corpus striatum (Young 2005).
As per disturbances of movement, those with Huntington's disease tend to suffer from either or both voluntary and involuntary abnormalities. The involuntary aspects include the jerking and unpredictable movements of the limbs, trunk and face which may occur when at rest, walking, or while actively engaged in some task such that they may appear to be intoxicated and/or attempting to dance about.
Voluntary disorders include rigidity, slowed, clumsy, or difficulty initiating movement. Those who suffer from voluntary movement disorders are the most likely to demonstrate cognitive decline (Folstein et al. 2010).
Unfortunately, the role of the amygdala in movement is often ignored by investigators (and almost all textbooks) although all aspects of the motor circuit are directly or indirectly innervated by this nuclei and respond to amygdala mediated impulses. Although the amygdala can be surgically destroyed bilaterally without significantly effecting normal motor functioning, in many instances the amygdala is implicated in the genesis of abnormal motor activities and may play a significant role in striatal dysfunction and the development of Parkinson's and related diseases.
Brain Mind Lecture 7: Frontal Lobes













