Rhawn Gabriel Joseph, Ph.D.
Structural Overview
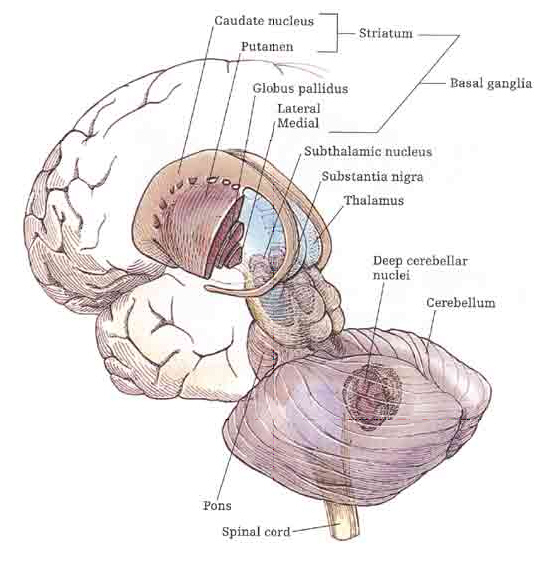
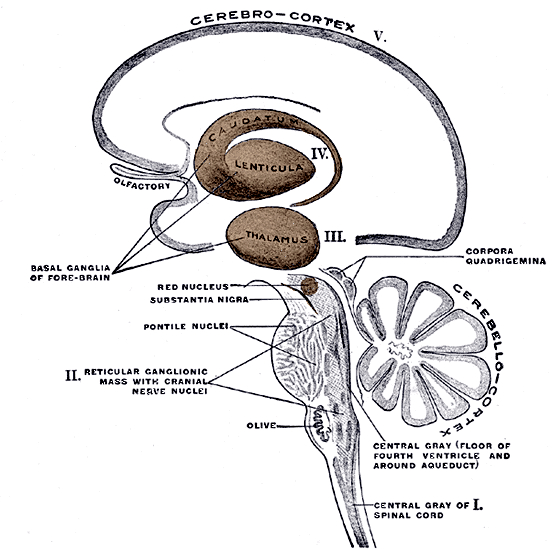
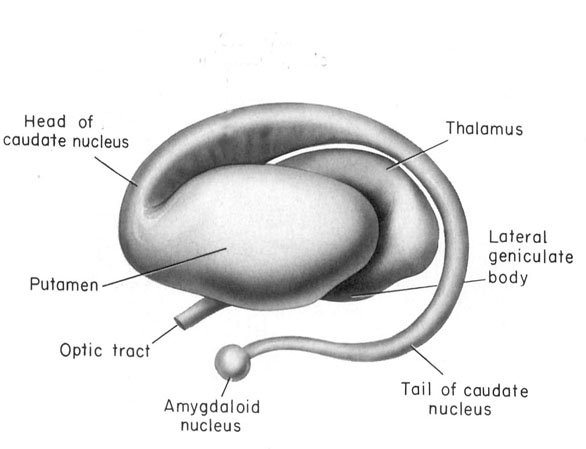
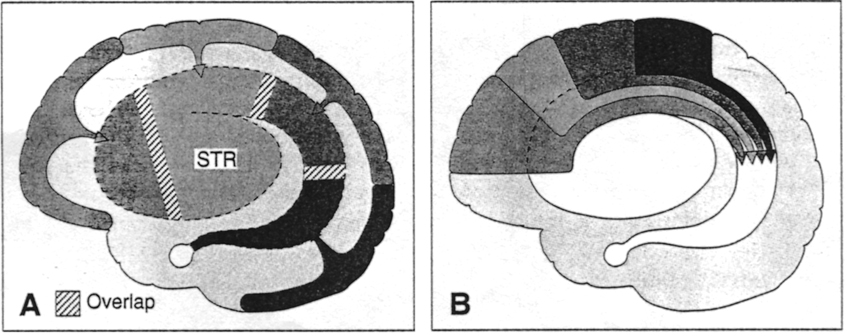
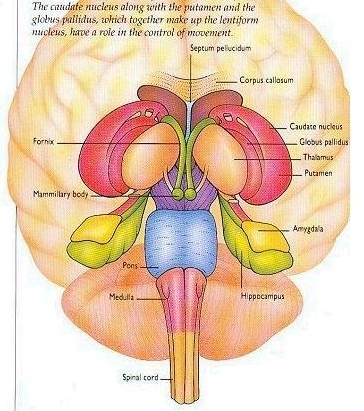
The basal ganglia is composed of several major nuclei which subserve different functions. These structures include the corpus (or dorsal) striatum ("striped bodies") i.e., the caudate and putamen which are extensively interconnected and which project to a variety of brain areas including the immediately adjacent globus pallidus ("pale globe").
OVERVIEW
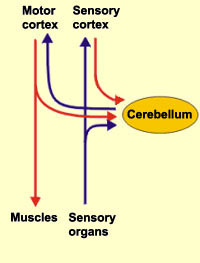
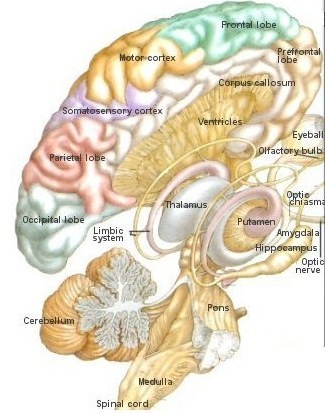
The "basal ganglia" (striatum, subthalamic nucleus) receives much of its input from the neocortex (Jones & Powell, 2010; Pandya & Vignolo, 2011), and the amygdala, and to a minimal extent the hippocampus. The majority of these incoming fibers are excitatory and terminate in the corpus striatum and subthalamic nucleus. Specifically, the anterior portion of the frontal lobes projects to the head of the caudate whereas the more posterior putamen receives converging and overlapping input from the primary and secondary motor and somesthetic cortices (Jones & Powell, 2010; Pandya & Vignolo, 2011). This structure does not receive any direct input from the peripheral sensory or motor systems.
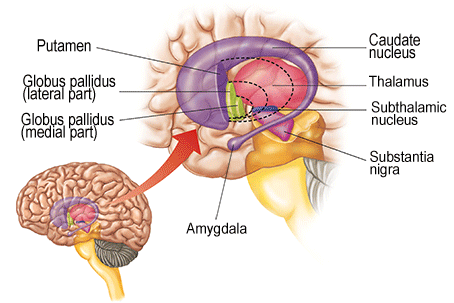
The corpus striatum represents the dorsal aspect of the "basal ganglia." Ventral to and intimately associated with the dorsal striatum is the limbic striatum, portions of which have also been referred to as the "extended amygdala." Indeed, the amygdala is a major component of the "basal ganglia," and these structures function as a cohesive interacting unit for the purposes of defensive and other affective behaviors regarding gross body movements, such as kicking, flailing, running, and kicking.
The corpus striatum responds to amygdala impulses, as they receive extensive amygdala projections (Gloor, 1955; Klingler & Gloor, 1960; Krettek & Price, 2008)--projections which are not reciprocated. The the right and left amygdala povides ipsilateral connections.
The amygdala (as well as the anterior cingulate, lateral hypothalamus, and hippocampus), therefore is able to exert considerable influence on the basal ganglia which appears to have evolved out of the amygdala in order to serve as an emotional-motor interface so that amygdala needs and impulses may be acted on in a flexible manner These are functions the basal ganglia (the limbic striatum in particular) continues to perform in humans as well as other creatures (e.g., MacLean, 1990; Mogenson 2011).
For example, the basal ganglia is exceedingly important in the stereotyped and species specific motoric expression of social and emotional states such as running away in fear, biting defensively, or via ballistic movements (hitting, kicking) or as manifested through facial expression, posture, muscle tone, or gesture (Mogenson & Yang, 2012; MacLean, 1990; Rapoport 2012). Because humans possess basically the same basal ganglia and limbic system, when happy, sad, angry, and so on, the facial and body musculature assumes the same readily identifiable emotional postures and expression regardless of culture or racial origins (Ekman 2003; Eibl-Ebesfedlt, 1990; However, see Russell 2009).

Because of this similarity in basal ganglia functional architecture, regardless of culture or race (and in many respects, mammalian species) if frightened, angry, or in the process of being assaulted, animals or humans may similarly engage in (ballistic) hitting and kicking, or biting, and/or all of the above, depending on context, mood and situational variables (including play). However, in contrast to the brainstem and cerebellum which provides reflexive and stereotyped motor programs that can be performed without thinking, the basal ganglia is capable of considerable flexibility in regard to motor-emotional expression, and is exceedingly responsive to the organisms motivational and emotional state -via its extensive interconnections with the limbic system.
Thus, the striatum contains cells which selectively respond to motivationally significant stimuli, including novel and familiar variables that are rewarding or punishing (Rolls & Williams, 2006; Schneider & Lidsky, 1981). Striatal neurons can also react differently to familiar stimuli depending on their reinforcement properties, and many neurons will in fact increase their responsiveness as stimuli approach the mouth, and/or touch the face and mouth (Rolls & Williams, 2006; Schneider & Lidsky, 1981). Some striatal neurons also respond when making tongue and lip movements (e.g. licking) and during arm movements toward a food item (Rolls & Williams, 2006).
These findings suggest the striatum is involved in orienting and guiding movements toward the mouth presumably so that a desired object can be licked, sucked on, chewed, and consumed. In this regard the striatum could be considered a primary motor center which enables various limbic desires, needs, and impulses such as hunger, to be satisfied.
The limbic striatum (e.g. the nucleus accumbens) and the mesolimbic DA system which projects to these nuclei also appear to be highly involved in mediating feelings of pleasure including the rewarding effects of amphetamine, cocaine and opiates (see Ellison, 2009; Hakan et al. 2009; Koob et al. 2012). These effects (including the aversiveness of opiate withdrawal) are probably due not only to the presence of opiate, DA, and related receptors, but the rich interconnections maintained with the amygdala as well as the lateral hypothalamus (Kelsey & Arnold 2009; Olton et al. 2012; Zaborszky et al. 2012) which constitute part of the "pleasure circuit" maintained by the medial forebrain bundle (Olds & Forbes, 1981; see also chapter 13).
However, animals will also work in order to self-administer opiates directly into the accumbens (Koob et al. 2012; Olds & Forbes 1981). Conversely, lesions to the nucleus accumbens may disrupt the capacity to experience pleasure or the rewarding effects of opiates and cocaine (Koob et al. 2012); or to engage in complex coordinated defensive acts (see below).
Catatonia, Parkinson's Disease, & Psychosis.
Lesions to the corpus striatum and lenticular nucleus (putamen and globus pallidus) can attenuate one's capacity to motorically express their emotions via the musculature; e.g. the face may become frozen and mask-like. These latter motor disturbances are well known symptoms associated with Parkinson's disease, a disturbance directly linked to dopamine deficiency (Fahn, 1999) and neuronal degeneration not only in the putamen (Goto, et al. 1990; Kish, et al. 1988; see also Hauser et al., 1999), but within the limbic striatum, i.e. the nucleus accumbens (see Rolls & Williams, 2006), as well as in the supplementary motor areas and medial frontal lobe -which maintains rich interconnections with the striatum.
However, when chemical or structural lesions extend beyond the basal ganglia and come to include the medial frontal lobe, not only might an individual suffer motor rigidity, they may become catatonic and experience extreme difficulty responding to external or internally mediated impulses (Joseph, 1999a). Various aspects of this symptom complex also characterize those with Parkinson's disease (see below).
Other disturbances associated with striatal abnormalities include Huntington's chorea, ballismus, restless leg syndrome, sensory neglect and apathy, obsessive compulsive disorders, mania, depression, "schizophrenia" and related psychotic states (Aylward et al. 2009; Baxter et al. 1992; Caplan, et al. 1990; Castellanos et al. 2009; Chakos et al. 2009; Davis, 1958; Deicken et al. 2010; Ellison, 2009; Rauch et al. 2009; Richfield, et al. 2006; Turjanski, et al., 1999). Severe memory loss and social-emotional agnosia and an inability to recongize friends or loved ones is also characteristic of striatal abnormalities, particularly disturbances involving the limbic striatum.
Thus although the basal ganglia is often viewed and described as a major motor center (detailed below), the functional capacities and symptoms associated with this group of nuclei are quite diverse, and vary depending on the nuclei and chemical neurotransmitters involved as well as the laterality, location, and extent of any lesion.

THE CORPUS STRIATUM
PATCHES & MATRIX
The caudate and putamen are tightly interlinked and in some respects are indistinguishable and possess a similar internal compartmental structure of patches and matrix (Graybiel 1986; Gerfen, 2004). This is why a gross analysis of the the mammalian caudate and putamen reveals a striated (patchlike) appearance.
The patches and matrix are biochemically distinct and receive projections from different regions of the neuroaxis (Gerfen, 2004, 2006; Graybiel 1986). For example, the patches contain dense concentration of opiate receptors (Graybiel, 1986) and receive projections from the amygdala, hippocampus, and other limbic tissue and maintain interconnections with the DA neurons in the substantia nigra. The patches in fact form a continuous labyrinth which snakes throughout the striatum.
The surrounding matrix also receives projections from the cingulate gyrus, the motor thalamus, and from throughout the neocortex and maintains interconnections with GABA and DA neurons in the substantia nigra (Gerfen, 2006). The matrix also contains large amounts of acetylcholinesterase.
MOTOR FUNCTIONS
Although tightly linked and similar in structural organization, the caudate appears to exert more influence and provide more input to the putamen, than vice versa. However, like the caudate, the putamen also receives considerable input from the medial frontal, supplementary, secondary and primary motor cortex, as well as areas 5 and 7 of the parietal lobe (Jones & Powell, 2010; Pandya & Vignolo, 2011).
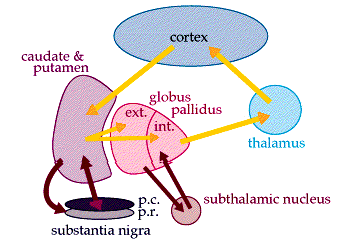
As per motor functioning, presumably the putamen, in conjunction with the caudate, transmits this information to the globus pallidus which in turn projects to the motor thalamus, brainstem reticular formation, as well as to the motor neocortex, thus creating a very elaborate feedback loop (see Mink & Thach, 2012; Parent & Hazrati 2010) whose origin may begin in the medial frontal lobes (Alexander & Crutcher, 1990; Crutcher & Alexander, 1990) or perhaps the limbic system, e.g. anterior cingulate, amygdala.
For example, when anticipating or preparing to make a movement, but prior to the actual movement, neuronal activity will first begin and then dramatically increase in the medial supplementary motor areas (SMA), as well as in the striatum (Montgomery & Buchholz, 2012; Schultz & Romo, 1992) followed by activity in the secondary and then the primary motor area (see chapter 19), and then again in the caudate, putamen and globus pallidus which become increasingly active prior to and then during movement and ceasing their activity once the movement is completed (Alexander & Crutcher, 1990; Mink & Thach, 2012; Schultz & Romo, 1992).
Hence, these areas, including the "motor" thalamus, in many respects act in a coordinated fashion so as to mediate purposeful movement. As noted in chapter 12, this was a principle role of the basal ganglia long before the evolution of the neocortex and frontal motor areas.
It is perhaps important to point out that some investigators have argued there are at least five different motor circuits involving the basal ganglia which are segregated to varying degrees (Alexander et al. 1990). The dorsal striatal motor circuit also consists of at least two separate systems involving the putamen and medial (internal) globus pallidus, and the putamen and lateral (external) globus pallidus (reviewed in Marsden & Obeso, 2009; Parent & Hazrati 2010).
FUNCTIONAL DIFFERENTIATION OF THE CAUDATE & PUTAMEN
Over the course of evolutionary metamorphosis, the corpus striatum and the motor thalamus began to develop in tandem and became increasingly interlinked in order to subserve motoric and related activities, including the processing and analysis of sensory information and the expression of feeling states via specific motor activities. That is, the corpus striatum initially served not only the motor functions and related information requirements of the limbic system but in many respects performed (at a rudimentary levels) some of the same "analytical" and perceptual functions that would later be subsumed by the neocortex.
With the continued expansion of the the neocortex and the exponential increase in the capacity to analyze and respond to divergent sensory information, the corpus striatum essentially was split in two by the tremendous proliferation of thalamic axons (i.e. the internal capsule, or rather, the thalamic radiations) that not only terminated on striatal dendrites, but which swept forward and radiated outward to innervate the frontal lobes (Kemp & Powell, 2010). Thus the putamen and caudate nucleus were formed and began to receive differential input from the thalamus as well as from the neocortex and therefore began to subserve somewhat different functions.
For example, although both nuclei contain significant amounts of DA, 5HT, ACH, and GABA (reviewed in Ellison, 2009; Parent & Hazrati 2010; Stoof et al. 1992) and receive input from the amygdala, hippocampus, and motor and somatosensory perceptual data (Haber et al. 2004; Van Hoesen, et al. 1981; Whitlock & Nauta, 1956), the putamen is the recipient of considerable bilateral and topographical input, such that a motor and sensory map of the body (particularly the face, mouth, leg, and arm) is maintained in this region (Delong et al. 1983; Parent & Hazrati 2010). Axonal projections from the motor and sensory neocortex which are concerned with the arm converge in one area of the putamen, whereas those concerned with the leg converge in another.
When coupled with the symptoms and experiments describes below, it is suspected that the putamen is concerned with integrating sensory with intended motor actions and coordinating the movement of the limbs and body in visual space via projections maintained with the medial and lateral globus pallidus as well as the parietal lobe.
In contrast, the caudate nucleus is dominated by axons from association cortices including the inferior temporal lobe and anterior cingulate (Percheron, et al. 2006), the amygdala (Ammaral et al. 1992; Heimer & Aheid, 2012) and the frontal motor areas. The caudate appears to be more involved in multi-modal motor, emotional and sensory integration, analysis and inhibitory functions. Consequently lesions to the caudate can produce sensory neglect and unresponsiveness, or conversely, loss of inhibitory control over the musculature; depending on the extent and laterality of the lesion.
THE CAUDATE: MANIA, APATHY, CATATONIA
The head of the caudate nucleus is concerned with multi-modal information processing and inhibition. Via inhibition the caudate is able to exert modulatory effects on motor activity and facial-gestural posture and expression, and aids in the maintenance of selective motoric attention, e.g. standing still and observing. In consequence, in primates and humans, lesions, destruction, or shrinkage of the head of the caudate can result in sensory neglect, agitation, hyperactivity, distractibility and in some cases what appears to be a manic or "schizo-affective" psychosis (Aylward et al. 2009; Caplan, et al. 1990; Castellanos et al. 2009; Chakos et al. 2009; Davis, 1958; Richfield, et al. 2006) depending on the extent and laterality of the destruction.
For example, Richfield et al. (2006, p. 768 ) report a 25 year old female honor student (soon to be married) who after complaining of headaches and nausea disappeared for 3 days. "When found, she had undergone a dramatic personality change manifested by alterations in affect, motivation, cognition, and self-care." These changes were largely permanent. "Her abnormal behaviors included vulgarity, impulsiveness, violent outbursts, enuresis, indifference, hypersexuality, shoplifting and exposing herself. She was inattentive and uninterested in her surroundings but could be encouraged to concentrate for short periods of time. She would frequently lie down to sleep. Her affect was flat." CT-scan indicated bilateral damage to the head of the caudate nuclei.
Some patients will alternate between hyperactivity and apathy- a function perhaps, of the laterality and extent of the lesion as well as associated biochemical alterations. For example, injuries or abnormalities restricted to the right caudate are more likely to result in a manic-like psychosis (Castellanos et al. 2009) --quite similar to what occurs after right frontal lobe injury (chapter 19). Similarly, right caudate hypermetabolism and blood flow has also been associated with obsessive compulsive disorders (Baxter et al. 1992; Rauch et al. 2009) whereas frontal abnormalities may induce perseverative disturbances (chapter 19) as well as obsessive- compulsions (Rauch et al. 2009).
Conversely, left caudate injuries (particularly those which extend to the mesial and left frontal lobe) may induce severe apathy as well as speech disturbances. As noted, left frontal injuries are associated with similar abnormalties (chapter 19).
With extensive left or bilateral caudate injuries it is not uncommon for patients to appear agitated, apathetic, with decreased spontaneous activity and slowed and delayed, dysarthric (or stuttering) and emotionally flat speech, with some patients responding to questions only after a 20-30 second delay (Caplan et al. 1990). This condition is particularly likely if the medial frontal lobes have been compromised as well.
In fact, due perhaps to it's extensive interconnections with the medial frontal lobes and SMA -a region which when destroyed can give rise to catatonia (Joseph 1999a), massive bilateral lesions to the anterior caudate and anterior putamen and surrounding tissue has been shown to produce catatonic or "frozen" states, where animals show a tendency to maintain a single posture or simply stand unmoving for weeks at a time (Denny-Brown, 1962).
Similarly, chemically lesions of the caudate can produce complete catatonia, posturing and a cessation of all movement (Spiegel & Szekely, 1961). However, if the amygdala-striatal pathway and/or the amygdala is destroyed prior to lesioning the caudate, these frozen catatonic states can no longer be induced (Spiegel & Szekely, 1961).
CATATONIA & THE FRONTAL-CAUDATE-AMYGDALA NEURAL NETWORK
As noted, the head of the caudate is extensively interconnected with the frontal lobes. Thus, some of the symptoms associated with caudate destruction (e.g. mania, apathy, catatonia) parallels those associated with frontal lobe injury (chapter 19). For example, whereas lesions to the frontal lobe may produce perseverative abnormalities, caudate lesions and/or increased metabolism or regional blood flow may induce obsessive-compulsive behaviors (Baxter et al. 1992; Rauch et al. 2009). In part this may represent a loss of frontal lobe control over the caudate, and/or the loss of striatal inhibitory influences over the frontal lobe.
However, the caudate and frontal lobes are also intimately linked to the amygdala (Ammaral et al. 1992); a structure that under conditions of extreme fear and arousal, can induce a complete cessation of movement; i.e. catatonic-like frozen panic states (chapter 30). The amygdala is able to accomplish this via interconnections with the basal ganglia, brainstem, as well as the medial frontal lobes.
THE AMYGDALA, STRIATUM, SMA, & LIFE THREATENING FEAR & AROUSAL
In situations involving exceedingly high levels of arousal coupled with extreme fear, the individual may simply freeze and attentional functioning may become so exceedingly narrow that little or nothing is perceived and cognitive activity may be almost completely (albeit temporarily) abolished (see chapter 30). These behaviors are apparently under the control of the amygdala which can trigger a "freezing" reaction and a complete arrest of ongoing behavior (Gloor, 1960; Kapp et al. 1992; Ursin & Kaada, 1960) via these brainstem/striatal interconnections. This is part of the amygdala attention response, which at lower levels of excitation may be followed by anxious glancing about, an increase in respiration and heart rate, pupil dilation, and perhaps cringing and cowering or flight (Gloor, 1960; Ursin & Kaada, 1960).
Among humans, the fear response is one of the most common manifestations of amygdaloid stimulation (Gloor, 1990; Halgren, 1992; Williams, 1956). However, if arousal levels continue to increase, subjects do not merely freeze in response to increased fear, they may become catatonic; a condition which may be secondary to dopamine and serotonin depletion and amygdaloid influences on the SMA as well as the striatum; nuclei which are intimately interconnected.
For example, in response to extreme fear, "one tendency is to remain motionless, which reaches its extreme form in death-feigning in certain animals and sometimes produces the waxy flexibility of catatonics" (Miller, 1951). The affected individual becomes psychologically and emotionally numb and unresponsive which is coupled with a complete blocking off of cognition. Moreover, the individual may resist and fail to respond to attempts at assistance (Krystal, 1988; Miller, 1951; Stern, 1951).
The airline industry has referred to this as "frozen panic states" (Krystal, 1988), a condition sometimes seen in air and sea disasters. For example, in mass disasters, 10-25% of the victims will become frozen, stunned, and immobile, and will fail to take any action to save their lives, such as attempting to evacuate a burning or sinking craft even though they have been uninjured (see Krystal, 1988).
According to Krystal (1988) with increasing fear "there is also a progressive loss of the ability to adjust, to take the initiative or defensive action, or act on one's own behalf... that starts with a virtual complete blocking of the ability to feel emotions and pain, and progresses to inhibition of other mental functions" (Krystal, 1988, p. 151).
Evolutionary Significance of Rigidity.
Catatonic panic states are prevalent in the animal kingdom, and constitute a life preserving reaction that is apparently mediated by the amygdala and striatum. That is, by freezing and not moving, predators may fail to take note of their presence.
Catatonia, coupled with emotional and "psychological" numbing, also represents a total surrender reaction, usually as a prelude (and hopeful guarantee) of a painless death when attacked by predators or invaders. That is, the prey may cease to run or fight and simply stand still or lie down and allow predators to literally eat them alive. Or, in the case of humans as sometimes occurs during war and genocidal mass murders, passively allow themselves to be marched into a ditch and shot.
According to Krystal (1988, p. 144), "thousands of European Jews obeyed orders in an automaton-like fashion, took off their clothes, and together with their children descended into a pit, lay down on top of the last layers of corpses, and waited to be machine-gunned," all the while seemingly almost petrified with fear and/or completely numb as to what was going on around them.
Presumably this numbing is made possible via the massive secretion of opiates within the amygdala and basal ganglia, whereas the rigidity and loss of the will to resist is a consequence of overwhelming fear and hyper-amygdala influences on the medial frontal lobe and corpus and limbic striatum.
Once prey have sighted a predator, some animals, however, instead of running in fear, will simply "freeze," fall to the ground, and lie stiff, rigid, and motionless as if dead. Unless exceedingly hungry, many predators will avoid eating creatures which appear to be already dead (i.e. unresponsive).
As sometimes occurs to potential victims during mass killings (e.g. the 2009 Rwanda civil war between the Hutus and the Tutus) humans too, will sometimes fall down as if dead and may remain frozen, stiff and and unmoving for long time periods even though they may not have been harmed (Krystal 1988). Indeed, sometimes these individuals are believed to be dead even by rescuers or those who are clearing away and burying bodies.
Presumably it is via connections with the basal ganglia and medial frontal lobes that the amygdala is able to induce these catatonic states, which in part is also dependent on dopamine. For example, it has been demonstrated that under extremely stressful conditions the striatal and frontal lobe DA system is adversely affected (see Le Moal & Simon 2012).
IMPLICATIONS REGARDING PARKINSON'S DISEASE
As noted, many of the functions originally associated with the basal ganglia have been subsumed by the neocortex, and damage to the frontal lobes can produce essentially similar symptoms as seen following caudate destruction; including stiffness, rigidity, and difficulty initiating movement (see chapter 19).
Given that the medial frontal lobes, corpus and limbic striatum, and amygdala are extensively interconnected, and given the powerful influences of the limbic system on all aspects of behavior, it thus appears that when exceedingly aroused or emotionally stressed, the amygdala is able to inhibit (or overactivate) the frontal-striatal motor centers which (in addition to the amygdala) are simultaneously undergoing DA depletion (which in turn results in hyperactivation of these nuclei including the amygdala; see Le Moal & Simon 2012). When this occurs, the organism may fall and cease to move, blink or even breathe (or breathe only shallowly and slowly). The creature therefore appears to be in a state of rigor mortis and thus dead (i.e. catatonic).
Overall, these amygdala-basal ganglia-medial frontal lobe-fear induced frozen and catatonic states are exceedingly adaptive; that is, unless the hapless victim is in a burning airplane or sinking ship.
Although not as dramatic or severe, similar states occur with biochemical abnormalities involving the dopamine pathways from the substantia nigra which not only feed the caudate, but the medial frontal lobes and the amygdala. Specifically, loss of DA (or excessive amygdaloid arousal) results in motor neuron hyperactivity and tonic EMG activity and thus limb and facial rigidity; conditions which also afflict those with Parkinson's disease.
In that some of these same exact "semi-frozen" and akinetic states are present in many of those with Parkinson's disease, and given that affected individuals are sometimes described as excessively aroused and/or unable to relax, and to suffer from heightened autonomic nervous system activity (Stacy & Jankovic, 1992) this raises the possibility that the amygdala and related limbic nuclei may significantly contribute to the development of this disorder. Indeed, as noted, destruction of the amygdala prior to chemically lesioning the corpus striatum prevents the development of Parkinsonian symptoms.
PARKINSON'S DISEASE
The basal ganglia plays a major role in controlling and facilitating specific movements, as well as inhibiting unwanted movements (Marsden & Obesco 2009; Turjanski, et al., 1999). The basal ganglia is also directly implicated in the generation of a variety of movement disorders, including chorea, ballismus, restless leg syndrome, rigidity, stiffness, and Parkinson's disease.
Parkinson's disease is a progressive degenerative disorder characterized by rigidity and shuffling gait, stooped posture, generalized slowness and stiffness of movement, and a loss of facial emotional expression, as well as a loss of spontaneity and flexibility in making postural adjustments when eating, going to the toilet, or having sex. Moreover, many Parkinson's patients experience not only rigidity and akinesia, but suffer from episodic freezing of movement (Dietz, et al. 1990; Pascual-Leone, et al. 2009), a tendency to easily fall (Stacy & Jankovic 1992) an impairment of "righting reflexes" (Calne 2009), and a reduced capacity to blink (Freedman 1992) and even to breathe (Stacy & Jankovic, 1992); similar to the frozen panic states and induced catatonia.
Hypophonia (reduced voice volume) dysarthria and a tendency to speak in a monotone, micrographia (small handwriting), and a 4-8 c/sec. ("pill rolling") resting tremor (exacerbated by stress) involving antagonistic muscles are common (Freedman, 1992; Stacy & Jankovic, 1992). Depression, changes in personality and slowed thought processes are also not unusual among Parkinson's patients (Walters, 1999). Parkinsonism can result following neurological trauma or vascular abnormalities (Koller, 2006; Murrow et al. 1990) or isolated lesions to the substantia nigra (Stern, 1966) and can therefore arise from a number of different causes including infection and toxic exposure. Parkinson's disease may also overlap with other striatal disturbances such as Alzheimers disease (Calne 2009). Indeed, sigificant cognitive deficits are not unusual among those with Parkinson's disease (Freedman, 1992; Rajput 1992); i.e. subcortical dementia.
Parkinson's disease usually begins after age 60 (though the first signs may appear during the early 40's), effects about 2% of the population (Koller, 2006) and is usually characterized by a massive loss of up to 80-85% of the dopamine neurons in the substantia nigra and an 80% decrease in striatal DA, with DA depletion greatest in the putamen (Goto, et al. 1990; Kish, et al. 1988). In fact, transplanting fetal nigral tissue into the putamen, can result in increased dlurodopa intake and some long-term clinical improvement (Hauser et al., 1999).
Hence, whereas the substantia nigra DA system is directly implicated, the mesolimbic pathways and limbic striatum are only a mild factor and are only mildly effected in this disorder.
STRIATAL IMBALANCE & PARKINSON'S DISEASE
The differential involvement of the nigrostriatal vs mesolimbic DA system raises the possibility that whereas the corpus striatum and medial frontal lobes are negatively impacted, the amygdala and limbic striatum may continue to function normally -due to preservation of the mesolimbic DA system. However, because the normal balance between these various nuclei is disrupted, amygdala influences received within the SMA and corpus striatum may in fact overwhelm and massively inhibit (or over activate) these nuclei thereby giving rise to Parkinsonian symptoms (see Le Moal & Simon 2012 for related discussion); i.e. rigidity, a tendency to fall coupled with reduced blinking and disturbed righting reflexes, etc.
In addition to DA in the genesis of Parkinsonian symptoms, significant reductions in opiate receptors within the putamen as well as the globus pallidus have also been reported (Goto et al. 1990), such that neurons involved in the experience of "reward" are effected. This selective loss of "reward" neurons suggests a a reduction in the capacity of the basal ganglia to receive pleasurable or positive emotional input as might be provided not only by opiates, but via the lateral amygdala and lateral hypothalamus.
Possibly, because striatal neurons associated with negative feelings states may be selectively preserved in Parkinson's disease, the basal ganglia (but not the amygdala, hypothalamus, or neocortex) may respond as if in a highly aroused negative state (e.g. fearful, stressed) even when that is not the case. Adding to this imbalance would be the relative preservation of the mesolimbic DA system, and the continued input from the medial amygdala into the corpus and limbic striatum -the medial amygdala being more involved in the generation of unpleasant including fearful mood states (chapter 13)
Indeed, loss of corpus striatal DA and/or excessive amygdaloid arousal is directly associated with the development of tonic EMG activity, motor neuron hyperactivity and thus excessive tonic excitation of the musculature and limb rigidity; i.e. Parkinson's symptoms. Conversely, excess striatal DA can result in chorea and excessive movement as well as psychosis.
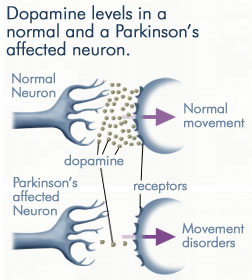
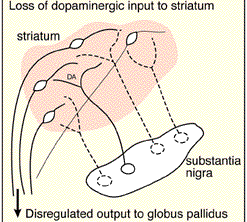
DA, ACh, & STRIATAL IMBALANCE.
The DA and cholinergic, ACh system appear to exert counterbalancing influences. For example, loss of nigrostriatal DA results in increased ACh, neuron hyperactivity (see Aghanjanian & Bunney, 1977; Bloom et al. 1965) and tonic EMG activity and thus limb rigidity which can be reversed by anti-cholinergic drugs (Klockgether, et al., 2006). That is, drugs which decrease ACh and those which increase DA, can ameliorate Parkinsons symptoms (Fahn, 1999), which suggests these two transmitters play oppositional and balancing roles (see Stoof et al. 1992). Moreover, ACh can be inceased by drugs that decrease DA, and can be decreased by drugs which increase DA (reviewed by Stoof et al. 1992).
DA normally inhibits ACh release and increases GABA activity. The DA system appears to exert an controlling influences not only on GABA but ACh neurons in the striatum (Le Moal & Simon 2012). Hence, reductions in DA result in decreased GABA activity and increased limbic striatal arousal and ACh activity, and reduced corpus striatal arousal and reduced GABA activity (see Stoof et al. 1992). Under these latter conditions the subject becomes rigid.
However, under conditions where striatal DA is depleted, the excessive release of ACh creates excessive neural activity (Aghanjanian & Bunney, 1977; Bloom, et al. 1965) which in turn may be manifested in the form of excessive motor actions; i.e. ballismus, chorea, if the medial and ventral globus pallidus-subthalamic circuit is effected, or hypomovement and Parkinson's symptoms if the corpus striatum and dorsal globus pallidus is effected.
For example, fluctuations in DA levels can either act to excite or inhibit motor and related cognitive-memory activity within the limbic striatum (Le Moal & Simon 2012; Mogenson & Yang 2012). However, with high levels of limbic striatal DA (and/or amygdala) activity, coupled with reduced GABA, animals may appear hyperactive and engage in running, "galloping," and biting, and related movements.
Presumably these latter actions are due to inhibitory release (a consequence of striatal dysfunction or hyperactivation), and thus the triggering of brainstem motor programs that subserve these specific behavioral acts. These same stimulus (predator) released actions, across evolution, and across most animal species, are directly related to the functional integrity of the limbic system and striatum, and the capacity to survive when living in close proximity to predators, the elements, and co-species. Actions without thought are in fact the province not of the basal ganglia, but the brainstem, which is why these and related motor programs are stored within brainstem nuclei (see chapter 17).
PSYCHOSIS.
A major function of the head of the caudate (particularly the caudate nucleus of the right hemisphere) appears to be inhibition. Similarly, the principle effect of striatal DA is inhibition (Ellison, 2009; Le Moal & Simon 2012; Mercuri, et al. 2004). Hence, excessive and abnormal amounts of corpus striatal DA can result in increased levels of caudate-putamen activity such that limbic striatal, and the reception of amygdaloid/emotional input may be severely diminished and/or abnormally processed. Thus, excessive corpus striatal activity might also result in emotional blunting or distortion as well as cognitive abnormalities. Conversely, because benzodiazipine receptors are located throughout the striatum, anti-psychotic agents, due to their dopamine properties, can reduce this abnormal activation.
That is, abnormal levels of corpus striatal DA activity can interfere with selective attention and the reception of neocortical and limbic impulses within the striatum -as can increases in mesolimbic DA activity (Le Moal & Simon 2012). However, with increases in striatal DA, cognitive, social, and emotional integrative functions may become abnormal and distorted and the individual may become psychotic (see Castellanos et al. 2009; Chakos et al. 2009; Ring et al. 2009; Snyder, 1972).
As is well known, individuals diagnosed as paranoid and schizophrenic with psychomotor retardation have been found to have elevated DA levels and increased DA receptor binding (Crow, 1979; Ellison, 2009; Matthyse, 1981; Ring et al. 2009). Presumably this is why dopamine blockers such as the phenothiazines are effective in reducing psychotic behavior; i.e. they reduce excessive DA binding and excessive striatal activity which in turn restores the capability of the striatum (and related nuclei) to engage in cognitive-emotional integrative activities.
LIMBIC & CORPUS STRIATAL COUNTERBALANCING INFLUENCES
The limbic and corpus striatum are richly interconnected and send projections to many of the same brain areas. However, due to differential input from the amygdala and DA systems, these nuclei exert tremendous counterbalancing influences on each other and their associated neural networks. For example, the centro-medial as well as the basolateral amygdala projects to and exerts excitatory influences on the the limbic striatum (Maslowski-Cobuzzi & Napier, 2009; Yim & Mogenson 1982), whereas the medial and posterior-lateral amygdala projects to the corpus striatum and the ventral and dorsal globus pallidus. Via these dual interconnections, the amygdala can exert simultaneous and even oppositional influences on these nuclei.
In addition, the corpus striatum receives the bulk of its DA from the nigrostriatal systems, whereas the limbic striatum receives DA from the mesolimbic DA system (see Fibiger & Phillips, 1986 for review) -transmitter systems which interact at the level of the brainstem, limbic system, striatum, and neocortex (Maslowski-Cobuzzi & Napier, 2009). For example, it has been shown mesolimbic DA can act on the amygdala and limbic striatum simultaneously so as to modulate striatal reception of amygdala excitatory signals (Maslowski-Cobuzzi & Napier, 2009).
However, because the corpus and limbic striatum are largely (but not completely) innervated by different clusters of midbrain DA neurons, reductions or increases in one DA system can exert profound influences on those neurons innervated by the others -for example, by eliminating inhibitory or counterbalancing influences. Thus depletion of DA in the nigrostriatal (but not the mesolimbic) pathways can result in increased activity within the limbic striatum (see Yim & Mogenson, 1983, 1989) and medial amygdala, but deceased activity within the corpus striatum. If this occurs, movement programming may be disrupted resulting in rigidity or tremors -a consequence, in part, of an imbalance in amygdala-DA-striatal activation.
Conversely, mesolimbic DA depletion can result in enhanced corpus striatal activity and decreased limbic striatal and lateral amygdala and hippocampal activity, such that motor and social-emotional memory functioning may be disrupted; a condition compounded by DA influences on acetylcholine (ACh) neurons and the reception of hippocampal, amygala, and neocortical input within the striatum. Hence, the functioning of the limbic or corpus striatum can be severely disrupted even when the functional integrity of its own neurotransmitter systems are otherwise intact; i.e. due to a loss of counterbalancing influences.
HUNTINGTON'S CHOREA
Chorea ("dance") is characterized by jerky, writhing, twisting, and unpredictable movements of the extremities. The two main types are Sydenham's Chorea (St. Vitus dance) and Huntington's Chorea. Sydenham's Chorea involves choreiform movements of the facial, tongue, and extremities, and is accompanied by loss of nerve cells in the caudate and putamen, as well as within the cerebral cortex, substantia nigra, and subthalamic nucleus.
Huntington's chorea is a progressive deteriorative inherited genetic disorder which is passed on by an autosomal dominant gene located on chromosome 4. It is characterized by an insidious onset that may begin during childhood or old age, with the illness beginning earlier in those who have an affected father (reviewed in Folstein et al. 1990; Young 2010). Cognitive decline, however, is gradual.
Affected individuals tend to suffer from memory and visual-spatial deficits, depression, and reduced fluent output although aphasia is not typical. Difficulty with motor coordination, planning skills, decision making, and a reduced capacity to consider alternate problem solving strategies or to shift form one mental set to another, is not uncommon (reviewed in Folstein et al. 1990). Hence, in some respects this disorder is suggestive of frontal lobe abnormalities (see chapter 19).


This syndrome is also associated with widespread neuronal loss in the caudate, putamen, brainstem, spinal cord, cerebellum, and atrophy in the GP (see Vonsattel, et al. 2006; Young 2010). Degeneration is predominantly of small striatal neurons whereas larger neurons remain intact. Opiate neurons located in the striatum are also significantly effected (Reiner et al. 1988). It is believed that the degeneration of these corpus striatal neurons, as well as the loss of GABA influences, results in reduced striatal control over the GP (see Narabayashi, 2006), thereby producing excessive movement.
It is noteworthy that some reports indicate that the posterior caudate and putamen are more severely effected than the anterior regions (Vonsattel, et al. 2006). Indeed, the posterior caudate and it's tail is usually the earliest and most severely effected part of the brain -which implicates the amygdala as a factor in the development of chorea. Indeed, in the early stages of Huntington's chorea, atrophy and degeneration begin in the tail and spreads dorsally and anterior thus effecting the striatum and lenticular nucleus (Young 2010).
In this regard it is noteworthy that affective disorders and personality and mood changes are prominent early signs suggesting amygdala involvement. Indeed, disturbances of emotion may precede any motor or cognitive decline by as much as 20 years, with some patients displaying mania, depression as well as antisocial tendencies (reviewed in Folstein et al. 1990). As noted, neural degeneration tends to begin in the amygdaloid tail of the corpus striatum (Young 2010).
As per disturbances of movement, those with Huntington's disease tend to suffer from either or both voluntary and involuntary abnormalities. The involuntary aspects include the jerking and unpredictable movements of the limbs, trunk and face which may occur when at rest, walking, or while actively engaged in some task such that they may appear to be intoxicated and/or attempting to dance about.
Voluntary disorders include rigidity, slowed, clumsy, or difficulty initiating movement. Those who suffer from voluntary movement disorders are the most likely to demonstrate cognitive decline (Folstein et al. 1990).













