Rhawn Gabriel Joseph, Ph.D.
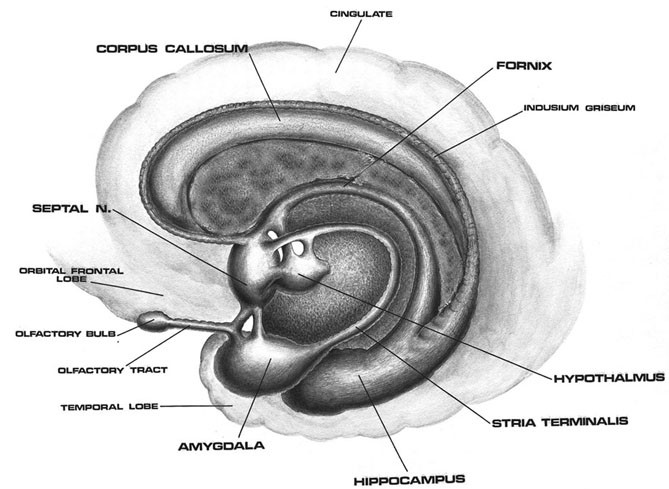
Cingulate Gyrus
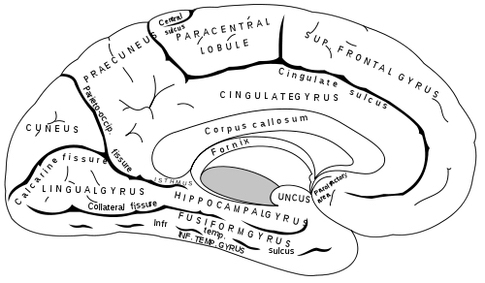
The five layered cingulate gyrus sits atop the corpus callosum and can be broadly divided into two segments: the anterior cingulate (areas 24, 25, and 33) which is concerned with vocalizing and emotional and motoric functioning involving the hands, and regulating autonomic and endocrine activities; and the posterior (area 23) cingulate which is involved in visual-spatial and tactile analysis as well as motor output and memory.
THE POSTERIOR CINGULATE
The posterior cingulate gyrus is richly interconnected with the superior parietal lobe (area 7) the parahippocampal (inferior) temporal and superior temporal lobe (area 22), frontal lobe, caudate, putamen, substantia nigra, pulvinar of the thalamus, and dorsal hypothalamus (Beleydier and Mauguiere 1980; recently reviewed in Devinksy et al. 2005). In addition, the posterior cingulate projects to the red nucleus in the midbrain (which also receives frontal motor fibers) and to the spinal cord. Presumably the posterior cingulate acts to integrate visual input with motoric output and is not concerned with emotional stimuli per se, with the possible exception of nocioceptive functions (Devinksy et al. 2005).
However, the posterior cingulate may also be involved in visual-spatial and memory-cognitive activities, particularly as relates to the body and movement -hence the interconnections with the superior parietal lobe and the parahippocampal gyrus. The posterior cingulate may have, at least in part, evolved from the dorsal hippocampus (e.g. Sanides, 1964).
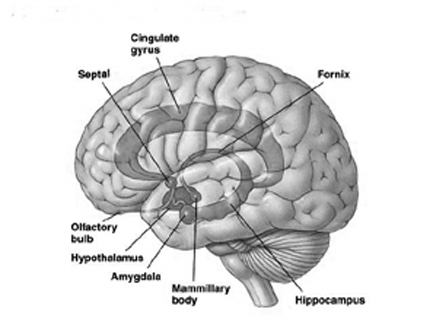
ANTERIOR CINGULATE GYRUS
The anterior cingulate (areas 24, 25, 33) is associated with processing and modulating the expression of emotional nuances, emotional learning and vocalization, the formation of long-term attachments and maternal behavior, including the initiation of motivationally significant goal directed behavior, as well as influencing and in part regulating endocrine and autonomic activities (reviewed in Devinsky et al., 2005; MacLean 2003).
The anterior cingulate maintains rich interconnections with the septal nuclei, amygdala, hypothalamus, mammilary bodies, hippocampus, dorsal medial nucleus of the thalamus and the periqueductal gray (Beleydier and Mauguiere 1980; Powell, 2008; Powell et al. 1974; Muller-Preuss and Jurgens 2007), as well as with the limbic striatum, caudate and putamen and the frontal motor areas.
The anterior cingulate thus appears to be a supra-modal area that is involved in the integration of motor, tactile, autonomic, and emotional stimuli, as well as with the production of emotional sounds (see below) and the capacity to experience psychological "pain and misery." In fact, the cingulate has long been associated with the experience of psychic and even physical pain (e.g. identifying the affective attributes of noxious and psychic stimuli).
Hence, during the 1930's and 1940's, bilateral cingulotomies were frequently performed to eliminate severe depressive and psychotic states as well as obsessive compulsive tendencies involving the hands (Le Beau, 1954; Whitty and Lewin 1957). However, following surgery, patients tended to become apathetic, emotionally blunted and/or socially and emotionally inappropriate or unresponsive.
Nevertheless, more recently it has been reported that 25% to 30% of patients with obsessive-compulsive disorder who were unresponsive to medication and behavioral treatment, significantly improve following cingulotomy (Baer et al. 2005), though these authors stress surgery is "a last resort treatment."
THE CINGULATE GYRUS AND EMOTIONAL FREE WILL
Electrical stimulation of the anterior cingulate can induce feelings of anxiety, pleasure and fear (Meyer et al. 1973) as well as changes in heart and respiratory rate and blood pressure accompanied by pupil dilation, gonadal and adrenal cortical hormone secretion, penile erection, and aggression (Buchanan and Powell 1982; Devinsky et al. 2005; MacLean 2003). Stimulation also induces a wide range of divergent vocalizations including growling, crying, high pitched cackling, and sounds similar to an infant's separation cry.
THE ANTERIOR CINGULATE GYRUS AND EMOTIONAL SPEECH
The amygdala is able to produce complex social-emotional vocalizations via the stria terminals and amygdalafugal pathways to the hypothalamus and periaqueductal gray which acts on the oral-laryngeal musculature. The amygdala also increasingly interacts with the rapidly maturing cingulate gyrus which is one of the most vocal structures of the brain (Jurgens, 2003, 1992; MacLean, 2003; Ploog, 1992; Robinson, 1967, 1972) and which becomes activated in response to and when producing human speech (Passingham, 2013; Paulesu et al., 2013; Peterson et al., 1988). For example, the anterior cingulate (as well as the left frontal lobe) become highly active when generating as many words as possible for a given category, e.g. words beginning with "F" (Frith and Dolan, 2013).
The anterior cingulate gyrus is also directly linked to the neocortical expressive speech areas located in the left and right frontal lobe, as well as with the hypothalamus and periaqueductal gray (Powell, 2008; Powell, Akagi, and Hatton, 1974; Jurgens, 2003, 2011)--which explains why the anterior cingulate and left frontal lobe become active simultaneously during language tasks (Frith and Dolan, 2013; Peterson et al., 1988). Indeed, Broca's expressive motor-speech (and oral-facial/hand) area in the left frontal lobe, and the emotional-melodic speech (and oral-facial/hand) area in the right lateral frontal lobe, appear to have evolved from the anterior cingulate gyrus/medial frontal lobe (Joseph, 1999e). Therefore, the right and left frontal lobes responds to cingulate (and posterior neocortical) impulses by vocalizing. If the anterior cingulate/medial frontal lobe were destroyed, the patient would become mute (Barris and Schuman, 1953; Devinksy et al. 2005; Joseph, 1999a; Laplane et al., 2003; Tow and Whitty, 1953).
Among its many functions, the anterior cingulate (Brodmann's areas 24, 25, 33) is associated with processing and modulating the vocal expression of emotional, melodic, and prosodic nuances, emotional learning, identifying the affective attributes of noxious psychic stimuli, maternal behavior, separation anxiety, and the formation of long-term attachments (Devinsky, Morrell, and Vogt, 2005; Joseph, 1999b; MacLean 2003; Powell, 2008). As based on functional imaging, the anterior cingulate also becomes activated by hot, painful, and noxious stimuli (Casey et al., 2011; Coghill et al., 2011), and, as noted, has been considered by some to be the seat of pain and misery.
Depth electrode stimulation of the anterior cingulate can induce feelings of anxiety, pleasure and fear as well as a wide range of divergent vocalizations including growling, crying, high pitched cackling, laughing, and sounds similar to an infant's separation cry (Devinsky et al. 2005; Jurgens, 2003; MacLean 2003; Meyer et al. 1973; Robinson, 1967). The anterior cingulate also assists in setting thresholds for vocalization (Jurgens and Muller-Preuss, 2001; Robinson, 1967), including modulating some of the prosodic and melodic features which characterize different speech patterns, e.g. happiness vs sadness, and thus laughing vs crying. The vocalizing capabilities of the cingulate are made possible via subcortical connections with the periaqueductal gray (Jurgens 2003, 2011), and its axonal projections to the right and left frontal speech areas.
Whereas vocalizations triggered by excitation of the amygdala, hypothalamus, or septal nuclei are usually accompanied by mood-congruent behaviors (Gloor, 1960; Jurgens, 2003; Robinson, 1967; Ursin and Kaada, 1960) the cingulate is capable of producing exceedingly complex social emotional vocalizations which sometimes have no bearing on the organism's mood or true emotional state (Jurgens, 2003; Jurgens and Muller-Preuss, 2001; Meyer et al. 1973). In addition, completely different emotional calls can be elicited from electrodes which are immediately adjacent (Jurgens, 2003).
Thus the cingulate is capable of considerable vocal flexibility and apparently enables an individual to modulate the emotional-prosodic-melodic components of speech so that one's true feelings can be disguised or emphasized in order to produce sounds suggestive of, for example, sarcasm, incredulity, or hilarity. The anterior cingulate may well contribute to the "deceptive" vocalizations and behaviors demonstrated by innumerable mammalian and avian species (e.g., Hauser, 2013), such as when attempting to lure a predator away from one's helpless infants. Conversely, however, the anterior cingulate, coupled with the right frontal lobe, may also be responsible for the failure to hide one's true feelings, thus generating the complaint: "Its not what you said, but the way you said it!"
As noted, if the anterior cingulate were destroyed, of if the pathways linking this structure with the brainstem periaqueductal gray were severed, the individual would become mute (Barris and Schuman, 1953; Devinksy et al. 2005; Jurgens, 2003; Laplane et al. 2003; Tow and Whitty, 1953). However, if the cingulate and surrounding medial tissue were mildly injured, or became abnormally active, emotional-prosodic speech would become exceedingly abnormal and patients may stutter and repeat words such that, in the extreme, they may uncontrollably babble (Devinksy et al. 2005; Dimmer and Luders, 2005; Penfield and Welch, 1951).
THE CINGULATE, THE SEPARATION CRY AND "MOTHERESE"
The medially located cingulate gyrus begins to myelinate around the second postnatal month and achieves an advanced stage of myelination by the end of the first year (Debakan, 1970; Gibson, 1991; Yakovlev and Lecours, 1967); around the same time the amygdala increasingly vocalizes feelings of fear. However, in addition to fear, the anterior cingulate contributes to the experience of unpleasant feelings (Casey et al., 2011; Coghill et al., 2011) including separation anxiety and vocalizes a separation cry which is similar if not identical to that produced by a frightened infant (MacLean 2003; Robinson, 1967). In fact, abnormal activity in the anterior cingulate has in some cases induced not just anxious vocalizations, but infantile behavior, such as assuming the fetal position (Devinksy et al. 2005).
The anterior cingulate is thus responsible for producing complex emotional-prosodic vocalizations, including, perhaps, the prosodic variations which mothers employ when speaking to their babies and vice versa; i.e. "motherese." As is well known, considerable vocalizing typically occurs between mothers and their infants; and the infants of many species will often sing along or produce sounds in accompaniment to those produced by their mothers (Bayart et al., 2003; Hauser, 2013; Jurgens, 2003; Wiener, Bayart, Faull, and Levine, 2003). These interactions appear to be limbically mediated and reinforce and promote mutual vocalization, attachment behaviors, and may contribute to the development of language. Among animal and human mothers, much of this initial mutual sound production consists of exaggerated emotional prosody (Cooper and Aslin, 2003; Fernald, 1991, 1992; Fernald et al., 1989; Hauser, 2013; Jurgens, 2003); i.e. "limbic language."
The cingulate is also sexually differentiated (MacLusky, Clark, Naftolin and Goldman-Rakic 2007; MacLusky et al.,2011). Thus there is a "male" vs a "female" cingulate which in turn likely contributes to sex differences in melodic speech patterns as well as in "maternal" vs "paternal" behaviors. For example, regardless of culture, mothers not only produce emotional-prosodic-melodic vocalizations but emphasize and even exaggerate social-emotional, and melodic-prosodic vocal features when interacting with their infants (Fernald, 1992; Fernald, et al. 1989; Nakazima, 1975). Presumably, it is these limbic foundations which explain why the acoustics of these nuances are basically identical regardless of culture (Nakazima, 1975), and why even mothers or infants who are born deaf produce these same prosodic vocalizations when speaking to their deaf babies (Oller et al., 2005; Woll and Kyle, 1989).
These emotional-melodic vocalization greatly influence infant-emotional behavior and attention as infants not only produce but prefer and are more responsive to these exaggerated prosodic vocalizations (Cooper and Aslin, 2003; Fernald, 1991). In fact, by 5 months of age infants become quite adept at perceiving and distinguishing between different emotional vocalizations so as to determine the mood state and intentions of the speaker (Fernald, 1993; Haviland and Lelwica, 2007). Likewise, mothers are generally able to determine the mood and desires of her 5-month old offspring when it produces similar emotional vocalizations (D'Odorico, 1984; Wolff, 1969).
In many respects these mutual mother-infant social-emotional interactions appear to be a reflection of the limbic system of the mother communicating with the limbic system of her infant. The infant-neocortex is much too immature to comprehend non-emotional words and sentences.
The female limbic system (and the right frontal-temporal speech areas) are in fact adapted and organized so as to promote social-emotional communication with her young. That is, the cingulate gyrus, amygdala and hypothalamus are sexually differentiated such that there is a "male" and a "female" limbic system (see chapter 13). Being in possession of a "female" limbic system presumably confers a superior ability to perceive and express social-emotional nuances and vocalizations (Joseph, 2000a); capacities at which females excell (Burton and Levy, 1989; Brody, 2005; Buck, 2001, 1984; Buck, Miller, and Caul, 1974; Fuchs and Thelan, 1988; Heller and Levy, 1992; Soloman and Ali, 1972; Strayer, 1980). Indeed, in contrast to males, females are not only more emotionally perceptive and expressive, but tend to employ 5-6 different prosodic variations and utilize the higher and fluctuating registers when conversing (Joseph, 1993, 1999e), especially with their infants (Fernald, 1992; Fernald, et al. 1989). Human (as well as non-human) infants are not only more responsive to the emotional-prosody conveyed by a female voice, but are most responsive to the higher as well as fluctuating registers (Fernald, 2005; Hauser, 2013).
However, in contrast to the infant, adult females (and males) also rely on the neocortices of the right frontal-temporal lobe to produce and comprehend emotional-melodic-prosodic vocalization. As noted, over the course of evolution the anterior cingulate appears to have contributed to the evolution of the frontal motor-speech areas, whereas the auditory areas in the superior temporal lobe appear to be evolutionary derivatives of as well as richly interconnected with the amygdala (Joseph, 1999e). The frontal and temporal auditory areas, however, do not begin to significantly mature until around the first year after birth; a process which can take 7 to over 20 years to complete (Blinkov and Glezer, 1968; Brody, et al. 2007; Conel, 1939, 1941; Flechsig, 1901; Huttenlocher, 2003; Yakovlev and Lecours, 1967).
MATERNAL BEHAVIOR & THE EVOLUTION OF INFANT SEPARATION CRIES
Sharks, teleosts, amphibians, and reptiles possess a limbic system, consisting of an amygdala, hippocampus, hypothalamus, and septal nuclei (see chapter 5). It is these limbic nuclei which enable a group of fish to congregate and "school", and which makes it possible for reptiles to form territories which include an alpha female, several sub-females, and a few juveniles. These nuclei promote social attachment and interaction.
However, sharks, fish, and the first amphibians and reptiles, like their modern counterparts lacked the four to five layered cingulate gyrus. Moreover, these creatures do not possess an inner ear or true middle ear, though amphibians and reptiles are attuned to hear low level vibrations and sounds, such as croaking, tails thumped on the ground, and a few distress calls and those of contentedness. Limbic language capabilities are not well developed in these creatures.
As they also lack a cingulate gyrus, amphibians and most but not all reptiles show little or no maternal care, and rarely vocalize. They will also greedily cannibalize their infants who in turn must hide from their parents, and other reptiles, in order to avoid being eaten (MacLean, 2003).
When reptiles began to differentiate and evolve into the repto-mammals some 250 million years ago, and then, twenty-five million years later, when the first tiny dinosaurs (who diverged from a different line of reptiles, the theocondants) began to roam the Earth, major biological alterations occurred involving cranial and post-cranial skeletal structure, mammillary development, thermo-regulation, sexual reproduction, and limbic system function and structure (Bakker, 1971; Brink, 1956; Broom, 1932, Crompton & Jenkins, 1973; Duvall, 1986; Paul, 1988; Quiroga, 1980; Romer, 1966) --all of which coincided with tremendous advances in the ability to engage in audio-vocal communication and the capacity to nurse the young (chapter 5).
It was not until the appearance of the the therapsids, around 200 to 150 million years ago, that mammilary glands, and thus the capacity to nurse, came into being (Duvall, 1986). It was at this time that the middle ear began to undergo tremendous modification and the first rudiments of an inner ear developed (Broom, 1932; Brink, 1956; Crompton & Jenkins, 1973; Romer, 1966). Although the hypothalamus, septal nuclei, and amygdala continued to evolve, it was also around this time that the cingulate gyrus began to appear and increasingly enshroud the dorsal surface of the limbic forebrain -an event which corresponded with the appearance of nursing nipples (Duvall 1986) and the inner ear. When this began to occur, sounds came to serve as a means of purposeful and complex communication, not only between potential mates or predator and prey, but between a mother and her infant (Joseph, 1993, 2011; Maclean, 2003). This ability in turn was probably made possible by the amygdala and in particular, the evolution of the four to five-layered transitional neocortex, the cingulate gyrus.
As noted, it is the limbic system and the interactions of limbic nuclei such as the amygdala and the cingulate gyrus which not only stimulates the desire to communicate, but to form attachments, social groups, and eventually, the formation of the family. In fact, many of the late repto-mammals, as well as some dinosaurs and the later appearing therapsids, lived in packs or social groups, and presumably cared and guarded their young for extended time periods lasting until the juvenile stage (Bakker, 1971; Brink, 1956; Crompton & Jenkins, 1973; Duvall, 1986; Paul, 1988; Romer, 1966). Presumably long term attachments were made possible via the evolution of the anterior cingulate.
As also noted, the first appearance of rudimentary nipples coincided with therapsid development. Hence, one of the hallmarks of this evolutionary transitional stage, some 200 million years ago, was the cingulate gyrus and the first evidence of nursing, maternal feeling, and the creation of large social groups and hunting packs, and what would become the family.
MOTHER INFANT VOCALIZATION
Among mammals and primates the production of sound is very important in regard to infant care, for if an infant becomes lost, separated, or in danger, a mother could not quickly detect this by olfactory-pheromonal cues alone. These conditions would have to be conveyed via a cry of distress or a sound indicative of separation fear and anxiety; which would cause a mother to come running to the rescue. Conversely, vocalizations produced by the mother would enable an infant to continually orient and find its way back if perchance it got lost or separated. Hence, the first forms of complex limbic social-emotional communication may well have been first produced in a maternal context.
As noted, considerable vocalizing typically occurs between mothers and their infants (be they human, primate, or mammal); and the infants of many species will often sing along or produce sounds in accompaniment to those produced by their mothers. These mutual interactions reinforce and promote mutual vocalization which is often initiated by the mother.
In fact, primate females are more likely to vocalize when they are near their infants versus non-kin, and infants are more likely to vocalize when their mother is in view or nearby (Bayart et al. 2003; Jurgens, 2003; Wiener et al. 2003). Similarly, infant primates will loudly protest when separated from their mother so long as she is in view and will quickly cease to vocalize when isolated (Bayart et al. 2003; Wiener, et al. 2003). However, adult males are also more likely to call or cry when in the presence of their mother or an adult female vs an adult male (see Jurgens, 2003). It thus appears that the purpose of these vocalizations are to elicit a vocal response from mother, or an adult female, who in turn is likely to respond with soothing limbic language.
Hence, ontogentically and phylogenetically, the initial production of emotional sounds is limbically based, and increasingly, over the course of evolution, and as evident during early development, the production of these sounds is associated with maternal-infant care, and/or interactions with an adult female. As noted, the cingulate is sexually differentiated (MacLusky et al., 2007, 2011), and regardless of culture, human mothers tend to emphasize and even exaggerate social-emotional, and melodic-prosodic vocal features when interacting with their infants (Fernald, 1992; Fernald et al., 1989), which in turn appears to greatly influence infant emotional behavior and attention (Fernald, 1991). Similarly, human infants prefer listening to and are more responsive to these exaggerated limbic vocalizations, as compared to "normal" adult speech patterns (Cooper and Aslin, 2003) particularly when produced by a female; i.e. by a female cingulate gyrus.
FEMALE SUPERIORITIES IN LIMBIC LANGUAGE
In addition to the cingulate, the amygdala and hypothalamus are also sexually differentiated (Allen and Gorski, 1992; Allen et al., 1989; Blier et al., 1982; Gorski et al. 2008; Goy and McEwin, 1980; Raisman and Field, 1971; Swabb and Fliers, 2005; Swabb and Hoffman, 1988). In addition, the rope of nerve fibers which interconnect the right and left amygdala and inferior temporal lobes (the anterior commissure) is 18% larger in females than males (Allen and Gorski, 1992), which in turn likely contributes to sex differences in language, emotion, and maternal vs paternal behavior. Thus, females tend to produce a greater range of limbic (social-emotional) vocalizations than males (Glass 1992; Joseph, 1993, 2000a; Tannen 1991) and they tend to employ 5-6 different prosodic variations and to utilize the higher registers when conversing. They are also more likely to employ glissando or sliding effects between stressed syllables (Brend, 1975; Coleman, 1971; Edelsky, 1979).
Men tend to be more monotone, employing 2-3 variations on average, most of which hovers around the lower registers (Brend, 1975; Coleman, 1971; Edelsky, 1979). Even when trying to emphasize a point males are less likely to employ melodic extremes but instead tend to speak louder. Perhaps this is why men are perceived as more likely to bellow, roar, or growl, whereas females are perceived as more likely to shriek, squeal, coo, and purr. Nevertheless, although influenced by sex differences in the oral-laryngeal structures, these differential capacities are also reflected in the greater capacity of the female brain to express and perceive these nuances (chapter 7), which also appears to be the case in female primates. Thus female monkeys and apes are more vocal and engage in more social vocalizations, and in fact vocalize more often that males who in turn are more likely to vocalize when threatening or engaged in dominance displays (Cross and Harlow, 1965; Erwin, 1980; Fedigan, 1992; Goodall, 1986, 2003; Mori, 1975; Mitchell, 1979).
It has been repeatedly demonstrated that human females are also more emotionally expressive, and are more perceptive in regard to comprehending emotional verbal nuances (Burton and Levy, 1989; Hall, 2008; Soloman and Ali, 1972). This superior sensitivity includes the ability to feel and express empathy (Burton and Levy, 1989; Safer, 2003). From childhood to adulthood women appear to be much more emotionally expressive than males in general (Brody, 2005; Burton and Levy, 1989; Gilbert, 1969); abilities which confer upon her a greater emotional sensitivity to the needs and feelings of others, especially her babies. These superiorities assist her in being a good mother.
MATERNAL BEHAVIOR, ATTACHMENT, AND THE FEMALE LIMBIC SYSTEM
It has been proposed that these limbic system sex differences are responsible for and are an evolutionary consequence of woman's role in bearing and rearing children and the female desire to form long term attachments, and engage in maternal care and verbal communication (Joseph, 1993). Female humans, primates and mammals apparently find these activities rewarding in-themselves, due to these same limbic system sex differences.
That is, given that the sexually differentiated anterior cingulate, at least in part, evolved in a maternal context and promoted the development of maternal feelings and long term mother-infant attachment, whereas the amygdala and hypothalamus are also sexually differentiated, it appears that these structures may account for why human and non-human female primates differentially respond and desire to nurture, hold, cuddle, and stare at infants. Indeed, female humans, chimps, baboons and rhesus macaques cuddle more and more closely, and are cuddled more by their sisters, mothers and other females (Jensen et al., 1968a, Hansen, 1966; Mitchell, 1968; Goodall 1971, 2003), whereas males are much more resistant to being held, and will kick and fuss, and actively attempt to escape their mothers much more so than females (Elia, 1988; Fedigan, 1992; Freedman, 1974, 1980; Mitchell, 1968, 1979; Goodall 1971, 2003; Kummer, 1971). In part this sex differences also reflects a struggle against potential physical domination which most males find aversive (Joseph, 1993). Hence, be it a male dog, chimpanzee, baboon, or child, they are far more likely than females to struggle, squirm, or resist attempts to hold or pick them up, and may even respond as if they find it aversive.
Mothers are therefore more willing to hold female babies and for longer time periods as they are also easier to calm and are more fun to hold as they seem to enjoy it more than males. Since females demonstrate greater social responsiveness and are more likely to employ facial, vocal and social signals, mothers are more likely to physically, socially and vocally interact with their infant daughters and vice versa (Moss, 1974).
Being similarly socially inclined, mothers find it more socially rewarding and enjoyable to interact with their daughters who are also in possession of a "maternal" (albeit immature) limbic system and cingulate gyrus.
Be it a female chimpanzee, baboon, rhesus macaque, or human, females also begin to demonstrate an extraordinary interest in babies and in play-mothering during even the earliest phases of their own childhood (Devore, 1964; Elia, 1988; Fedigan 1992; Goodall 1971, 2003; Jolly 1972; Kummer, 1971, Mitchell, 1979; Strum, 2007; Suomi, 1972). When girls play together, much of their fantasy and conversation concerns fashion and making out and revolves around adult relationships, including the raising of a family and the behavior and misbehavior of children (their dolls). Babies are of enormous interest to females, be they human, ape or monkey, and social primates and female humans who have babies usually become tremendously popular and the center of attention (Fedigan 1992; Jolly 1972, Mitchell 1979, Strum ,2007). Even among women enslaved in a harem, once she becomes pregnant and has a child, her status is quickly and permanently elevated.
Mothers, grandmothers, young and adolescent females, and even women who describe themselves as "feminists" show much more interest in babies than do men, even when the baby is not their own (Azhn-Waxler et al., 1983; Berman, 2003; Berman and Goodman, 1984; Blakemore, 2003, 2005, 2003; Frodi & Lamb, 2008; Melson and Fogel, 1982; Nash and Fledman, 2003). Adolescent girls spend significantly more time talking about new baby's than boys (Berman, 2003), and mothers spend more time talking about the baby with their daughters than their sons (Berman, 2003).
Girls not only talk more but play and care for their infant sisters and brothers significantly more and show consider amounts of nurturant interest in the babies well being (Blakemore, 2003) even when there has been no request or pressure to do so. Indeed, girls often demonstrate an intrusive interest in babies (Berman, 1983), and will give infants much more care than they require (Ainsworth & Wittig, 1969), as if often the case with mothers (Stewart, 2003). These behaviors also appear to be limbically mediated, as they are demonstrated by females of other species.
Non-human female primates, be it gorilla, chimpanzee, baboon, rhesus macaques, lemur, and so on, will eagerly seek to groom, cuddle, and carry not only their own infants but those of other females (Jolly, 2005; Devore, 1964; Kummer 1971, Strum 2007; Suomi, 1972; Mitchell, 1979; Goodall, 1971). These primates may also spend all day passing them back and forth. Like human females, some will even steal these infants. Those female primates who show the greatest interest, however, are young females who had not yet had babies.
Moreover, among almost all social primates, the birth of a new baby has an extremely excitatory effect on all the other females of the troop who will gather around and touch, stare, hold, and cuddle it. This female interest, of course, is certainly quite adaptive, at least for those living in the dangerous condition of the wild for it insures that if a mother dies another will adopt her baby.
Such behavior is obviously not the result of sexist training for it is typical of almost all social female primates, whereas males, including young males show relatively little interest in babies. For example, boy chimpanzees show little interest in their younger infant siblings, whereas girl chimps become increasingly fascinated and will hold and cuddle them and will attempt to model their mother's interactions with the infant (Goodall, 1971). If a new mother dies but her baby has older male siblings, less than 25% will adopt the little orphan whereas females siblings are quite anxious and happy to take this role.
THE MALE LIMBIC SYSTEM AND INFANT CARE
With the exception of the baboon (Rowell et al., 1968; Kummer, 1968, 1971; Mitchell, 1968, 1979, Fedigan, 1992), lack of interest in infants is characteristic of most social male primates and almost all male mammals, reptiles, amphibians and fish, as well as human fathers and men and boys in general who generally have little or no interest in babies and generally provide little or nurturant care for their own or the children of others (Rossi, 2005; Gordon and Draper, 1982). Of course, there are always exceptions; particularly among males who may possess a "female" limbic system. Rather, like other social primates, boys seek boys for playmates and engage in considerable amounts of rough housing, wrestling, and hitting; behavior that is completely inappropriate in regard to infant interactions. When boys or male primates begin to separate from their mothers, they show no interest in younger siblings but seek out adolescent and adult males to play with. Although they may on occasion seek nurturance they seldom provide it in return.
Human males and fathers rarely behave in any manner that approximates normal female maternal behavior (Belsky et al., 1984; Clarke-Stewart, 2008; Frodi et al., 1982) as this is simply not an activity they find interesting, pleasurable or rewarding. This is why, for example, child care professions and those jobs involving high levels of child interactions, such as elementary school teacher, are overwhelmingly made up of women (Gordon and Draper, 1982); a function not of pay but lack of heterosexual-male interest (Blakemore et al., 1988). Rather, fathers and adult heterosexual males tend to express interest in younger males (and females) only when they reach adolescence, and this is also true of most male primates.
Given that these sex differences are obviously innate, it could therefore be argued that in contrast to male humans, primates, and mammals who have little or no interest in child care, that the female limbic system is designed to promote these interests. Just as the male limbic system rewards males for engaging in competitive and aggressive actions (see chapter 13), the female limbic system probably generates rewarding feelings, coupled with appropriate emotional vocalizations, when females look at, hold, care for, and form attachments to their babies, infants, young children. Although this has yet to be determined, the female limbic system probably contains nuclei, neural networks, and individual neurons which respond selectively to infant visual and auditory related stimuli; e.g. baby faces, infant cries.
Again, consider that the anterior cingulate, in part, evolved in a "maternal" context and acts to promote the development of maternal behavior and mother-infant communication. Indeed, sex specific structural differences in the limbic system probably account in large part for most all sex differences in emotionality and related behavior, including childcare, the desire to have and nurture babies, and the greater female propensity for developing affective and mood disorders. However, these sex differences also make her a more communicative mother.
CINGULATE MATERNAL INFANT COMMUNICATION
The anterior cingulate gyrus, in conjunction with the amygdala and right frontal lobe, appears to provide the foundation for mother-infant communication, the generation of separation anxiety, as well as the desire to provide as well as receive prolonged maternal care (Davidson and Fox, 1989; Joseph, 1993, MacLean, 2003). Long-term mother-infant communication and prolonged maternal care is unique to human and non-human primates, as well as some mammals (e.g. Hauser, 2013), and appears to be directly associated with the rather recent evolution of the five-layered neo-limbic mammalian cingulate gyrus (Joseph, 1993, MacLean, 2003). Again, animals lacking the more recently evolved cingulate gyrus, but who possess a hypothalamus, amygdala, and brainstem (e.g. such as reptiles, amphibians, teleosts, and sharks) fail to provide even short-term maternal care and sometimes cannibalize their young.
As noted, with the evolution of the cingulate and mammal-like therapsids, it appears that vocalization came to serve as a means of complex communication, not only between potential mates or predator and prey, but between mother and infant (for related discussion see MacLean, 2003; Ploog, 1992). Hence, in humans, whereas the anterior cingulate is one of the most vocal structures of the brain and becomes highly active when speaking (Frith and Dolan, 2013; Passingham, 2013; Paulesu et al., 2013; Peterson et al., 1988), destruction of the anterior cingulate abolishes emotional speech production, and results in severe abnormalities in social and emotional behavior and a loss of maternal responsiveness (Barris and Schuman, 1953; Laplane et al. 2003; Maclean, 2003; Tow and Whitty, 1953). Behavior, in fact, becomes reptilian, and human and non-human primates become mute, cease to groom or show acts of affection, and treat their infants as if they were inanimate objects that might be walked on and discarded. In non-human primates, the majority of infants whose mothers have suffered anterior cingulate destruction, die from lack of care (MacLean, 2003).
BABBLING, LIMBIC LANGUAGE, AND NEUROANATOMICAL MATURATIONAL EVENTS
The capacity to vocalize is initially the province of the brainstem and midbrain periaqueductal gray which responds reflexively to the immature hypothalamus. Thus, for the first 30-days following birth, infants tend to cry, cough, belch, grunt, and express displeasure and distress. These initial sounds are likely produced reflexively by the periaqueductal gray, perhaps in reaction to the hypothalamus which may trigger crying when experiencing hunger or thirst. However, as the brainstem, hypothalamus, and then the amygdala and cingulate continue to mature the infant begins to babble and increasingly expresses feelings of pleasure and other social-emotional nuances.
For the first six months of life, with the exception of the somatomotor areas, much of the neocortex is so immature that its influences are negligible. However, the somatomotor areas begin to mature quite early; reflected in dendritic and pyramidal neocortical development (Flechsig, 1901; Joseph, 1982; Gibson, 1991; Gilles et al. 1983; Scheibel, 1991) and the growth and myelination of the corticospinal tracts which invades the brainstem several months before birth (Kertesz and Geschwind, 1971; Yakovlev and Rakic, 1966). However, the corticospinal tracts (which project from the motor areas to the brainstem and spinal cord), take well over a year to reach advanced levels of myelination (Yakovlev and Lecours, 1967).
The myelination of the corticospinal tract coincides with the descent of the larynx, the myelinization and development of the amygdala and the amygdalafugal brainstem pathway, and later, the maturation of the cingulate gyrus and its pyramidal brainstem pathways (Debakan, 1970; Langworthy, 1937; Yakovlev and Lecours, 1967; Yakovlev, and Rakic, 1966). These overlapping maturational and physical events also coincide with vocal development and the onset of early and late babbling followed by canonical and jargon babbling.
Early Babbling, Probable Meanings, and Prosody
By 2-3 months of age amygdala-brainstem pyramidal fibers as well as corticospinal axons have already begun to myelinate. These maturational events coincide with an initial shift in the emotional utterances of the infant which become progressively complex and prosodic and increasingly subject to sequencing and segmentation. The infant begins to "coo," "goo," and babble.
Specifically, as the amygdala (followed by the anterior cingulate) matures and begins establishing hierarchical control over the hypothalamus, midbrain periaqueductal gray, and the brainstem masticatory centers with which it maintains a massive fiber pathway (Takeuchi et al., 1988) the infant will laugh, becomes increasingly oral, displays genuine pleasure, stares and smiles at the human face, and while so doing, will phonate and babble. This early babbling stage generally involves the repetition of pleasant friction and voicing sounds which tend to be produced while making face-to-face and eye-to-eye contact and while engaged in social interaction (Kent and Miolo, 2005). Moreover, whereas the expression of pleasant sounds are in the ascendant, crying tends to become less frequent but more variable in tone, and can be differentiated into requests, calls, and sounds of discomfort (D'Odorico, 1984; Wolff, 1969).
As the amygdala, corticospinal tracts, and cingulate continue to mature, and the larynx continues to assume an adult pattern of orientation, the infant not only babbles but vocalizes a variety of sounds which increasingly convey probable meanings which may signify to the listener a variety of diffuse feelings and needs (D'Odorico, 1984; Wolff, 1969). Infants produce different noncry vocalizations depending on context and in reaction to people vs objects. The 3-4 month old infant can in fact produce at least four different pitch contours differening in fundamental frequency, each of which conveys probable meanings regarding affective state (Fernald, 1992; Hauser, 2013).
For example, if the 4 month old infant coos and babbles "mama," to the primary caretake (and depending on context, facial expression, and prosody/fundamental frequency) this may be interpreted to mean: "mama come here," "mama I hurt," "mama I thirst," etc. (e.g., D'Odorico, 1984; Fernald, 1992; Joseph 1982, Piaget, 1952; Vygotsky, 1962; Wolff, 1969). Hence, although the infant's utterances are not referential and may at times represent little more than the random universal babbling produced by all infants, they can also convey meaning and serve as a means of communicating with the primary caretaker (see Fernald, 1992; Hauser, 2013, for related discussion).
MATURATION OF THE AMYGDALA, CINGULATE, AND EARLY AND LATE BABBLING
As detailed above, the increased complexity of the infant's utterances likely reflect the maturational influences of the amygdala, and later, the cingulate, coupled with physiological/anatomical changes in the position and orientation of the larynx. These same maturational events are also correlated with different babbling stages.
Activation of the amygdala can trigger lip smacking, rhythmic jaw movement, babbling, and manidibular-teeth "chattering" (Gloor, 2013), which, when coupled with sound production may induce babbling. Infant's display similar behaviors, which is presumably a direct consequence of the immaturity of the amygdala and its projections to the masticatory centers in the brainstem. Indeed, early babbling appears to be a direct function of reflexive and spontaneous jaw movement (e.g, MacNeilage and Davis, 2003; Moore and Ruark, 1996; Weiss, 1951) and lip smacking. Hence, early babbling may reflect immature amygdala (as well as amygdala-striatal and motor neocortical) influences on the brainstem masticatory centers and the periaqueductal gray which reflexively triggers the oral musculature thereby inducing rhythmic movement of the jaw.
"Early" babbling is soon replaced by "late" babbling which has its onset around 4 months of age (de Boysson-Bardies et al.,2003; Oller, 1980; Oller and Lunch, 1992). Late babbling is sometimes described as "repetitive babbling" (Mitchell and Kent, 2003), and at later stages of development may include the repetitive production of CV syllables in which the same consonant is repeated, such as "dadada." As noted, electrical stimulation in the cingulate and surrounding medial frontal tissues can trigger the repetitive babbling of certain words and sounds, such as "dadadada" (Dimmer and Luders, 2005; Penfield and Welch, 1951).
The development of late babbling also occurs in conjunction with the infant's increased ability to produce sophisticated social-emotional nuances, and appears to be associated with increasing cingulate (as well as amygdala) influences. For example, around 4-months, the infant's intonational-melodic vocal repertoire becomes more elaborate and tied to a variety of specific feeling states (Piaget, 1952); which may reflect increasing amygdala maturational dominance.
However, over the ensuing months vocalizations also begin to assume an imitative quality (Nakazima, 1980) which are often context specific but which do not necessarily reflect the infant's internal state--a characteristic also of cingulate vocalizations. Some infant vocalizations are produced in mimicry and in play (Piaget, 1952), and the cingulate is also associated with mimicry and play behavior (MacLean, 2003). The late babbling stage has also been repeatedly described as a form of "sound play;" an activity which increasingly contributes to phonetic development (de Boysson-Bardies et al. 2003; Ferguson and Macken, 1983). As the cingulate is associated with mimicry and the onset of play behavior (MacLean, 2003) and since the production of these sounds do not necessarily reflect the infant's true emotional state, the cingulate, therefore, is implicated in all aspects of the late babbling stage.
As noted, late, repetitive babbling consists of sequences of CV syllables in which the same consonant is repeated, e.g. dadadada. However, infants will also produce what has been referred to as non-duplicated, variegated, and concatenated babbling (see Oller, 1980; Mitchell and Kent, 2003). That is, they will increasingly vocalize phonetically-varied-multisyllables which are periodically inserted into what is otherwise a repetitive sequences of CV syllables. These latter tendencies in fact, appear to be present even at the onset of the late babbling stage (Mitchell and Kent, 2003).
Repetitive, late babbling increases in frequency until around the seventh to tenth month of postnatal development (de Boysson-Bardies et al. 2003; Ferguson and Macken, 1983; Nakazima, 1980; Oller, 1980; Oller and Lunch, 1992), at which point the tendency to produce phonetically varied multisyllables becomes dominant. Thus the late babbling stage comes to be largely replaced by what has been termed "variagated" or "canonical" babbling (Oller, 1980; Oller and Lunch, 1992) which in turn is followed by "jargon" babbling (around 12 months).
Likewise, during these latter babbling periods the infant is also able to express fear, separation anxiety, and a variety of subtle social-emotional nuances. Although it appears that there is considerable overlap between these so called stages (e.g. Mitchell and Kent, 2003), the onset and increased frequency of late babbling followed by the increased production of variagated/canonical and then jargon babbling (Nakazima, 1980; Oller, 1980; Oller and Lunch, 1992), coincides with and appears to reflect the (overlapping) maturational influences of the amygdala followed by the anterior cingulate and then the frontal motor neocortice (which produce true speech).
BABBLING AND AMYGDALA, CINGULATE, NEOCORTICAL MATURATION
The maturation of the amygdala is associated with the onset of early babbling, whereas the anterior cingulate likely contributes to the development of late (repetitive) babbling, and the increased production of canonical/variagated babbling. These latter maturational events, however, also correspond to those taking place in the motor areas of the neocortex and may represent increasing pyramidal influences on the brainstem and oral-laryngeal musculature. In fact, jargon babbling appears to be a function of the immature neocortical somatomotor areas slowly gaining control over the limbic system, midbrain inferior-colliculus, and periaqueductal gray (see also Herschkowitz et al. 2013).
For example, pyramidal fibers from the somatomotor neocortex to the brainstem become increasingly well myelinated from 4 to 12 months of age (Debakan, 1970; Yakovlev and Lecours, 1967). Likewise, the somatomotor areas of the neocortex begin to rapidly mature around the first postnatal year (Brody et al. 2007; Chi, Dooling, and Gilles, 2001; Gilles et al. 1983; Scheibel, 1991, 1993). Hence, the neocortex likely increasingly contributes to the development of jargon babbling, especially around one year of age.
Moreover, just as the pyramidal/corticospinal tracts as well as the somatomotor areas continue to mature and myelinate over the first and second years (Conel, 1937, 1941; Debakan, 1970; Yakovlev and Lecours, 1967) and beyond (Paus et al., 1999), babbling continues throughout the first and second years. It is during these same time periods in which the child gradually acquires and develops the phonetic structure which underlies speech production (de Boysson-Bardies et al. 2003; Oller, 1980; Oller and Lunch, 1992). This implies considerable forebrain as well as right and left neocortical influences over vocal behavior (see below).
With increasingly neocortical control, what appears to be a "new and unique motor skill" slowly emerges (Moore and Ruark, 1996) which directly contributes to the development of speech. That is, once the neocortical speech area establish hierarchical control, and begin to program the oral-laryngeal motor areas, a new form of (neural-muscular) vocalization emerges which appears somewhat distinct from its precursors (e.g. Moore and Ruark, 1996). The infant begins to speak their first words.













