Rhawn Gabriel Joseph, Ph.D.
BrainMind.com
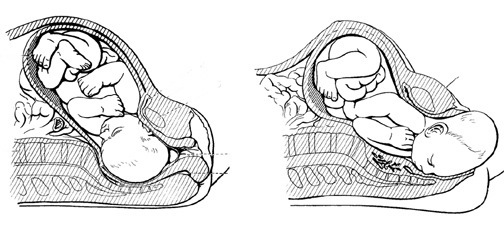
Over the course of human evolution the brain tripled in size; a development which required that infants remain relatively immature and dependent on their mothers for increasingly long time periods. A bigger more complex brain requires a number of years to reach maturity. This is because the relatively small size of the female-pelvic opening requires that the infant be born with a brain that is reduced in size. Moreover, because of its prolonged immature neurological status, the human infant requires considerable maternal and emotional stimulation throughout much of early life in order for the "experience expectant" nervous system to develop normally.
Fortunately, the human female is also neurologically predisposed to provide the infant with considerable social emotional and loving stimulation, which is why women experience a biological desire to become pregnant and to hold and care for infants. Be it chimpanzee, baboon, rhesus macaque, or human, females demonstrate an extraordinary interest in babies and will engage in play-mothering during even the earliest phases of their own childhood (Berman, 2003; Berman and Goodman, 2014; Blakemore, 2011; Devore, 1964; Fedigan, 2002; Frodi and Lamb, 1978; Goodall, 1971, 2011; Jolly 1972; Kummer, 1971; Melson and Fogel, 2012; Mitchell, 2009; Strum 2007; Zahn-Waxler et al., 2003). Female humans (Ainsworth and Wittig, 1969; Berman, 2003; Blakemore, 2011) and non-human (group living) female primates will eagerly cuddle, groom, and hold babies that are not their own (Jolly, 1972; Devore, 1964; Kummer, 1971, Strum, 2007; Suomi, 1972; Mitchell, 2009; Goodall, 1971).
Hence, whereas the nervous system of the infant requires considerable maternal input in order to thrive and to develop normally, the nervous system of the mother predisposes her to provide this input to her infant; which is a most adaptive relationship. However, because of the tremendous need for this stimulation, even subtle differences in the degree and quality of maternal contact can differentially effect the brain and cognition. For example, infants who are held and breast fed display superior neurological and cognitive capabilities as compared to those who are given a bottle or formula and allowed to lie alone in their beds (Lanting et al., 2005). These latter children are twice as likely to develop neurological abnormalities and cognitive-motor disturbances (Lanting et al., 2005). Similarly, it has been reported that infants who had been held and breast fed had a higher level of polyunsaturated fatty acids in their brains (Farquharson, 2003), which in turn effects and improves nerve cell conduction and cell membrane fluidity. Of course, in part, these latter effects may also be due to inferior quality of "baby formula" vs mother's milk.
Nevertheless, because the infant requires loving maternal input, the greater the degree of deprivation, the more serious might be the consequences. Children who are severely deprived of maternal contact may even die--even if all physical needs are being taken care of. Indeed, the mortality rates for infants placed in foundling homes during the 19th and early 20th century was found to be over 70% (Langmeier & Matejcek, 2005)--a consequence not of poor nutrition, but lack of maternal care from the biological mother. Likewise, it has been found that the risk of infant motorality increases by a factor of five if the mother dies even if care is provided by female relatives (Hill & Hurtado 1996). The tremendous need for prolonged maternal care may explain why women undergo menopause around the late 30's; that is, to insure that there will be a healthy mother to care for her infant. Until the 20th century life expectancy, on average, was not beyond the late 30 or 40s.
The infant and young child requires considerable loving and maternal contact, especially from its own mother. However, if that maternal input is abnormal, neglectful, or abusive, the child's developing brain, and all aspects of social and emotional functioning can become profoundly abnormal (Joseph, 1999b).
OVERVIEW: LIMBIC SYSTEM "EXPERIENCE-EXPECTANT" DEVELOPMENT
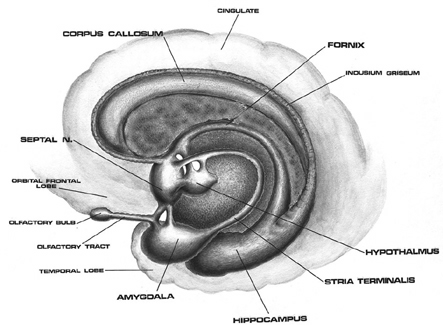
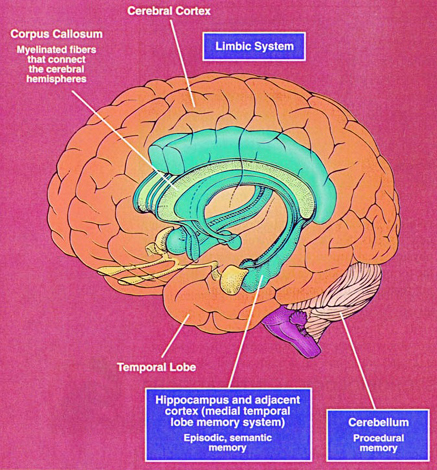
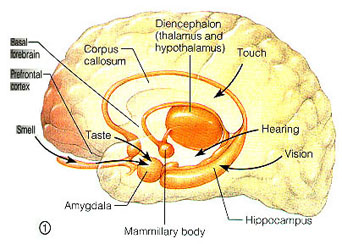
The human brainstem is almost fully functional at birth whereas the limbic forebrain including the neocortex are more plastic, "experience-expectant," and much slower to mature, taking years even decades to develop and mature (Barkovich et al., 1988; Benes, et al., 1994; Brody et al., 2007; Gibson, 2011; Harbord, et al., 2011; Holland, et al., 2006; Jernigan, et al., 2011; Kinney et al., 1988; Lee et al., 2006; Paus et al., 1999; Pfefferbaum, et al., 1994; Reiss, et al., 1996). Whereas the brainstem mediates reflexive motor and vital functions, the limbic forebrain (e.g. the hypothalamus, amygdala, septal nucleus, cingulate, and hippocampus) monitors and attempts to satisfy hunger and thirst and is responsible for the experience and expression of emotions including pleasure, rage, fear, sex, and joy and the desire for social-emotional contact (chapter 13). By contrast, the neocortex mediates higher level cognitive and intellectual functions including human language and speech, and remains relative immature for 10 or more years (chapter 26).
Because these latter structures are so immature and relatively plastic, they also require considerable, age-appropriate, "experience-expectant" social emotional and perceptual stimulation to develop normally, including considerable face-to-face and eye-to-eye contact. If sufficient normal stimulation is not provided, or if exposed to an abnormal, abusive, or neglectful environment, developing neurons and dendrites will establish or maintain aberrant, abnormal interconnections, or whither, die, and drop out at an accelerated rate (Dimond, 2011; Greenough & Black, 1993; Joseph, 1999b; Siegel et al., 1993).
As will be detailed below, these brain structures, including the neocortex, are not only "experience-expectant" but require different forms of emotional and perceptual input at different ages, in order to develop normally. This is because these different structures subserve different emotional and cognitive activities which in turn begin to emerge and become manifest at different ages as these structures begin to mature. As these brain areas mature, therefore, they require "experience expectant" stimulation in order to develop normally.
As detailed in chapter 25, the development of wariness, fear, separation anxiety, and the establishment of long term emotional attachments, is mediated by, and parallels the differential rates of growth and maturation of the hypothalamus, amygdala, septal nuclei, cingulate gyrus, as well as the hippocampus (Joseph, 2002a, 1998b, 1999b). Consider for example, fear, an emotion associated with the amygdala (Davis, Walker, & Lee, 1997; Gloor, 1997; Halgren, 2002; Rosen & Schulkin, 1998). The human medial amygdala is exceedingly immature at birth and doesn't become well myelinated until around the 8th postnatal month (Gilles, Leviton, & Dooling, 2003; Langworthy, 1937; Yakovlev & Lecours, 1967).
As is well known, the development of axonal myelin insulation improves neural transmission and is an indicator of functional maturation (see Grafstein, 1963; Lamantia & Rakic, 2011; Pujol et al., 1993). Myelinated axons do not normally undergo programmed cell death (Lamantia & Rakic, 2011).
Hence, paralleling the maturation of the medial amygdala, at 8-months, the infant first begins to experience and express feelings of fear, and then becomes progressively more fearful, such as in response to an approaching stranger (Bronson, 1972; Emde, Gaensbauer, & Harmon, 1976; Sroufe & Waters, 1976), adult male strangers in particular with female infants becoming more fearful than males infants. This initial fearlessness (and amygdala immaturity) is exceedingly adaptive, for the generation of fear would interfere with the initial establishment of intimate emotional attachments, and the infant's need for considerable social-emotional and physical stimulation, which is initially and eagerly welcomed even from strangers. The later development of fear ensures that an infant that can crawl, will not indiscriminately crawl into the arms of a stranger, or dangerous animals, only to be whisked away or eaten.
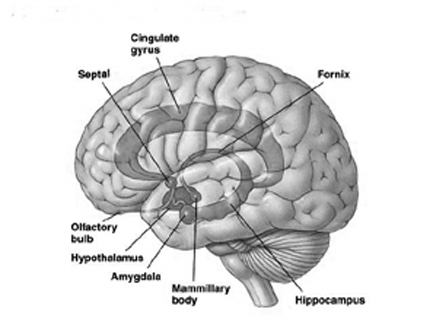
The hypothalamus, amygdala, as well as the septal nuclei, hippocampus, and anterior cingulate, all begin to mature at different, as well as at overlapping rates, and each subserves a variety of unique as well as overlapping functions. For example, in addition to fear, the amygdala subserves the ability to form emotional attachments, whereas the hippocampus subserves memory and the cingulate is associated with separation anxiety (chapters 13, 14,15). As these structures develop, they thus require input which may enhance memory or social emotional functioning.
Again, if denied sufficient social, maternal, and emotional "experience-expectant" (e.g. Greenough & Black, 2002) stimulation early in development, or if reared in an abnormal, neglectful, or abusive environment, these nuclei, and other forebrain structures (including the neocortex), may atrophy, establish aberrant interconnections, and cease to function normally (Cain, 2002; Henriksen, Bloom, & McCoy, 1978; Joseph, 1998b, 1999b,d; Kraemer, 2002; Lupien & McEwen, 1997; Sapolsky, 1996), as demonstrated in humans (Bremner et al., 2005; Heath, 1954; Stein et al., 2005) and nonhuman subjects (Cain, 2002; Diamond, 1985, 2011; Kraemer, 2002; Siegel, et al., 1993; Walsh, Budtz-Olsen, Penny, & Cummins, 1969). In consequence, various or all aspects of social emotional and various aspects of cognitive and perceptual functioning may become abnormal.
THE NEOCORTEX: DEPRIVATION, NEURAL ATROPHY & PERCEPTUAL BLINDNESS
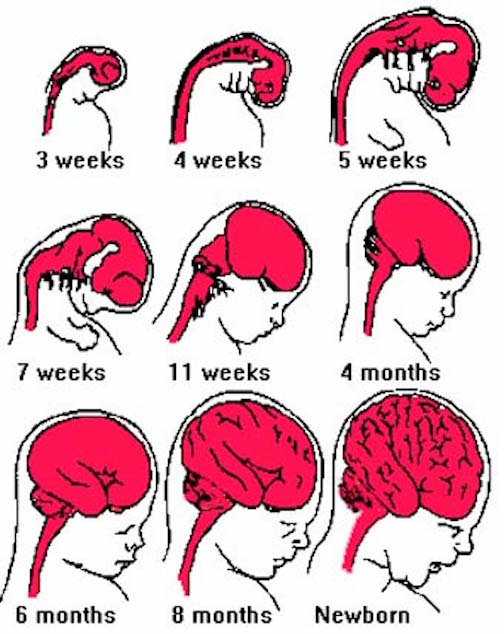
Although the seven layered neocortex develops well before birth, with the exception of a few pyramidal cells in the primary motor cortex of the frontal lobe, there is no evidence of myelinization of metabolic activity within this region of the brain at birth (Chugani, 1994; Fleshig, 1901; Langworthy, 1937). Even at one year of age, the frontal, temporal, occipital, and parietal lobes are only about half the size of the lobes of the adult brain (Blinkov & Glezer, 1968). In fact, the neocortex of the frontal, parietal, occipital, and temporal lobes can take well over 7, 10, or more years to fully mature (Benes 1994; Huttenlocher, 2011, 1994; Paus et al., 1999; Pfefferbaum, et al., 1994; Reiss, et al., 1996).
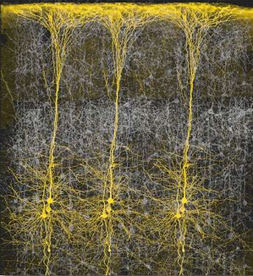
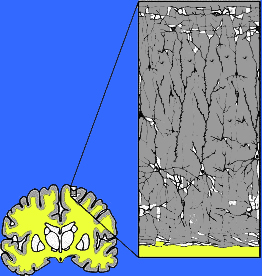
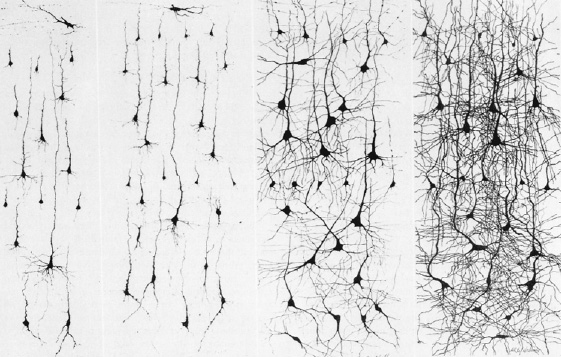
Abnormal (deprived-left) vs Normal (right) neural development
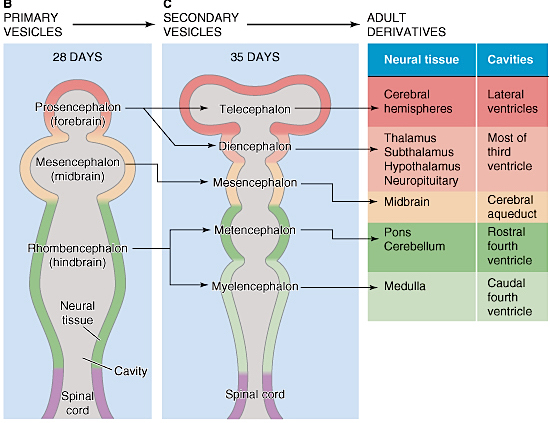
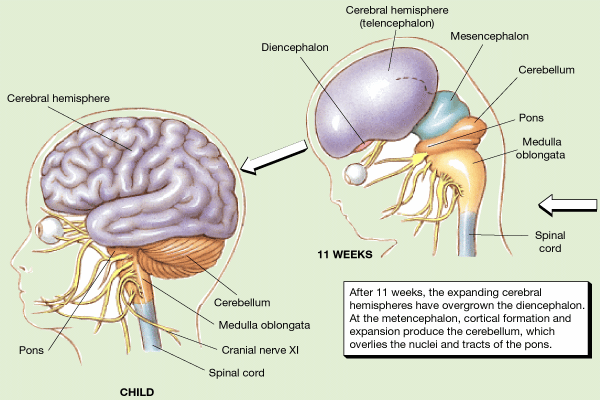
As detailed in earlier chapters, the brainstem and cerebellum begin to develop in advance of the forebrain, which in turn matures in a rostral arc, i.e. diencephalon, limbic system, striatum, neocortex (Barkovich et al., 1988; Brody et al., 2007; Gibson, 2011; Harbord, et al., 2011; Holland, et al., 2006; Lee et al., 2006). And these maturational events continue well into late childhood, adolescence and adulthood (e.g., Pfefferebaum, et al., 1994; Jernigan, et al., 2011; Kinney et al., 1988).
Because of neocortical (and subcortical/limbic) immaturity, and as these tissues are also "experience-expectant" they require considerable stimulation early in life, in order for neurons to grow, mature, divide, and establish and maintain synaptic connections. Indeed, it is well established that early environmental influences exert significant organizing effects on neural growth throughout the neocortex and limbic system, determining neocortical thickness, the density, size, shape and growth of dendrites and synapses, and the die off and drop out rate of glia, neurons, and axons (Diamond, 2011; Greenough & Black, 2002; Siegel et al., 1993). An enriched rearing environment can increase neural growth and establish billions if not trillions of synapses throughout the brain and neocortex and increase a thousand fold the number of synapses per axon (Greenough & Black, 2002; Greer, Diamond, & Tang, 2012). In the occipital cortex, for example, the dendritic fields are increased by about 20% among those reared in an enriched visual environment.
Moreover, different regions of the brain may be differentially influenced, depending on the age of the brain and the nature of the experience. For example, when young animals were trained to use either the right or left paw for a reaching task that was rewarded by cookie crumbs, it was found that pyramidal cell apical dendritic growth and branching was more extensive in the trained vs non-trained hemisphere (reviewed in Greenough & Chang 1988). Given that pyramidal neurons give rise to the cortico-spinal tract, presumably training involving just the right or left paw differentially acted and stimulated neuronal growth in those neurons.
By contrast, abnormal and deprived early experience during specific critical developmental periods can result in the elimination of millions of neurons, axons, dendrites and synapses, and may even induce functional invasion by competing neuronal cell assemblies which establish functional dominance over those neurons that have been insufficiently or adversely stimulated (e.g., Casagrande & Joseph, 1978, 1980; Hirsch & Spinelli, 1971; Joseph, 2006b; Spinelli et al., 1972).
As demonstrated in animal (Geyer et al., 1993; Hirsch & Spinelli,1971; Spinelli et al., 1972) and in human studies (Joseph, 2006b; Novelly & Joseph, 2003), neocortical and limbic neurons deprived of normal experience sometimes come to subserve wholly different functions. In consequence, even when normal experience is later provided, deprived neurons are unable to function normally.
For example, kittens reared in a visual environment consisting only of thick horizontal lines were later unable to see lines or objects oriented in a vertical direction. They would bump into table legs and furniture as if blind (Hirsch & Spinelli, 1971; Spinelli et al., 1972). In addition, neurons in the visual cortex became unresponsive to vertical stimuli, and instead could only react to objects oriented in a horizontal direction. Moreover, kittens that were provided considerable visual input, but were denied the opportunity to motorically interact with their environment (as they were strapped into a carriage and only allowed to move freely in the dark), were later found to be blind.
Likewise, as demonstrated in human (Freeman & Bradley, 1980) and non-human primates (Casagrande & Joseph, 1978, 1980; Joseph & Casagrande, 1978, 1980), abnormal and deprived experience during early critical developmental periods may even induce perceptual blindness. This blindness is secondary to functional invasion and occupation by competing neuronal cell assemblies which were provided normal input and then took over those neurons that have been insufficiently or adversely stimulated. For example, in non-human primates, if one eye is sutured shut soon after birth and patterned visual input is prevented from reaching target cells in the lateral geniculate nucleus of the thalamus and visual neocortex, target neurons and cellular layers and columns become smaller and functionally suppressed by adjacent cells receiving normal input (Casagrande & Joseph, 1978, 1980; Hubel &Wiesel, 1968). Cortical columns and layers innervated by the deprived eye shrink and those of the experienced eye grow larger, filling in the vacated space. Perceptual functioning becomes exceedingly abnormal and the deprived eye is unable to see objects, places, or smiling faces.
In primates, if lid sutures are reversed and the visually deprived eye is opened, the subject appears to be blind, although the eye is normal (Joseph & Casagrande, 1978, 1980). The deprived eye has become functionally disconnected from the thalamus and visual cortex.
Blindness following monocular visual deprivation is due to insufficient visual stimulation during this early critical period of development, and is directly attributable to the competitive influences of normal neurons (that receive normal input) which invade, conquer and take over vacant (or inactive) cortical space. These invading and retreating neurons prevent the transfer and neocortical reception of visual input transmitted from the inexperienced eye which is now functionally blind.
In monkeys, deprived of visual input, the superior parietal lobe suffers a 92% reduction in visually responsive neurons in the multi-modal somesthetic-visual-hand area 7. By contrast, the number of parietal neurons responsive to tactile somesthetic stimulation increases by up to 117% (Hyvarinen 2012). Similarly, in mice, the auditory cortex also exponentially increases by default, taking over abandoned cortical territory left behind by the retreating population of formerly visually responsive neurons whose cortical territory substantially shrinks in size (Gyllenstein, Malmfors, & Norrlin, 1966). Hence, early visual deprivation limited to one eye results in a massive abandonment of "visual" neurons throughout the brain, including the neocortex, and leads to their acquisition by cells receiving different forms of input.
Nevertheless, these deprived and/or functionally reorganized neurons still retain some plastic capabilities. For example, in primates who display loss of vision in the deprived eye, if the normal eye is subsequently surgically removed, and the deprived eye is provided considerable visual stimulation, some vision returns to the formerly deprived eye and some functional neuronal recovery is observed (Casagrande & Joseph, 1980; Joseph & Casagrande 1980). Visual recovery is due to the removal of competitive influences and functional sprouting in those axons and dendrites which are subsequently activated by the formerly deprived eye. However, although there may be some perceptual and neuronal recovery, this recovery is not complete or equivalent to what might normally be expected given normal rearing and perceptual experiences. This is because, although even the adult nervous system is capable of significant plastic changes and neural growth (Churchill et al., 1998; Feldman et al. 2002; Florence et al., 1998; Jenkins et al. 2011; Jones & Pons, 1998; Juliono et al. 1994; Ramachandran 1993; Strauss et al. 2002; Weiller et al. 1993), and although adult neurons (such as those in the hippocampus) retain the capacity to divide (Joseph, 1998c,d,e), certain neural assemblies require certain experiences at a specific time, in order for those circuits to stabilize or even form. That is, if appropriate stimulation is not provided these neurons will cease to function normally and will fail to completely regain their functional capacities even when later provided with appropriate stimulation.
On the other hand, if sufficient stimulation is provided and the neuron develops normally, it also retains some neuroplastic capability, including the ability to divide and generate new neurons (Joseph, 1998c,d,e), and/or the capacity to take on additional functions even in the brain of the adult (Churchill et al., 1998; Feldman et al. 2002; Florence et al., 1998; Jenkins et al. 2011; Jones & Pons, 1998; Juliono et al. 1994; Ramachandran 1993; Strauss et al. 2002; Weiller et al. 1993). In fact, synaptic, neural pathway, and dendritic changes have been repeatedly reported in the adult brain during or following learning (Buchel et al., 1999; Davies, et al. 1989; Gustafsson & Wigstrom, 1988; Lynch, 2006; Lynch et al. 2011; Miltner et al., 1999; Paus et al., 1999; Quartz & Senjowski, 1997; Singer, 2011; Stevens, 1989).
EXPERIENCE-EXPECTANCIES FOR SOCIAL-EMOTIONAL STIMULATION
Initially synapses and interconnections may be formed randomly and in excess numbers, such that those which are not stimulated drop out. That is, synapses and neural interconnections are established at a certain time period in expectation that experience will activate those which are most appropriate. As summed up by Greenough et al. (2007, p. 540) "since the normal environment reliably provides all species members with certain experiences, such as seeing contrast borders, many mammalian species have evolved neural mechanism that take advantage of such experiences to shape developing sensory and motor systems. An important component...appears to be the intrinsically governed generation of an excess of synaptic connections among neurons, with experiential input subsequently determining which of them survive."
Because developing neurons are "experience-expectant" (Greenough & Black, 2002) when denied that experience, they may cease to function normally, or come to be inhibited by competing neural assemblies (Casagrande & Joseph, 1978, 1980; Freeman & Bradley, 1980; Joseph & Casagrande, 1980). Likewise, if the developing limbic system is denied sufficient emotional and social stimulation, or if the child is subject to abuse and emotional stress, it too may become "blind" and fail to respond or respond in an abnormal fashion to social-emotional nuances even when normal input is later provided. Indeed, children (Ainsworth, Blehar, Wates, & Walls, 1978; Bowlby, 2012; Cicchetti & Tucker, 1994; Koluchova, 1976; Spitz, 1945) and non-human primates and mammals (Geyer et al., 1993; Harlow & Harlow, 1965a,b; Joseph, 2009; Joseph & Casagrande, 1980; Joseph & Gallagher, 1980) reared under maternally deprived, abusive, or neglectful conditions, suffer severe social, emotional, cognitive, perceptual, and intellectual deficits. These disturbances include diminished curiosity and a lack of exploratory behavior, a reduced ability to anticipate consequences or to inhibit irrelevant or inappropriate behaviors which lead to punishment, and an impaired ability to form loving attachments. Even the ability to function in a sexually normal fashion is disrupted (Fritz & Fritz, 1985; King & Mellen, 1994; Langmeier & Matejcek, 2005; Moberg, 1985).
Some severely neglected and deprived children, including those who have been severely sexually abused, fail to shown any sexual drive, or they may repeatedly expose themselves and masturbate, or sexually attack other children (reviewed in Langmeier & Matejcek, 2005). Sexuality often becomes abnormal, because sexuality is associated with the functional integrity of the limbic system, the amygdala, septal nuclei, and hypothalamus in particular (see chapter 13). If sexually abused, these structures may become abnormally sexually differentiated and/or develop other neural abnormalities which result in abnormal behavior, including "traumatic sexualization" (see below).
LIMBIC SYSTEM STIMULUS HUNGER AND SOCIAL EMOTIONAL DEPRIVATION
When experiencing hunger or thirst, the organism is motivated to seek food or water. Likewise, the experience-expectant limbic system actively seeks social stimulation, and requires emotionally "sensitive" maternal contact in order to develop normally (Joseph, 2002, 2000a). Hence, for the first 8 months of postnatal development infants will indiscriminately seek contact and will smile at the approach of anyone, even complete strangers (Charlesworth & Kruetzer, 1973; Spitz & Wolf 1946; Sroufe, 1996). It appears that the immature "experience-expectant" limbic system in fact demands emotional and social contact, and will seek it out regardless of consequences.
Hence, young animals raised in social isolation will form attachments to bare wire frames, inanimate objects, and television sets, and will seek out animals that might maul them and creatures that might eat them (Cairn, 1966; Fisher, 1955; Harlow & Harlow 1965a,b; Hoffman & DePaulo, 1977). Infant humans, non-human primates, and a variety of young creatures will desperately seek contact with mothers and caretakers who reject, physically abuse, and who nearly kill them. For example, Melzack and Scott (1957) found that dogs who were denied social emotional stimulation for the first 7 to 10 months of life, desperately sought social contact and would hover excitedly next to the experimenter even when he repeatedly stuck pieces of glass or burning matches into their snouts. Similarly, Harlow and Harlow (1965a,b) report that young monkeys would persistently attempt to make maternal contact even when severely bitten and battered. Infant humans are no different.



This "paradoxical" abusive-contact seeking behavior is not entirely surprising as the limbic system appears to be genetically programmed to respond to aversive or threatening conditions by seeking safety and contact comfort from the mother or surrogate caretaker (Joseph, 2002, 1999b). Hence, the infant is unable to reconcile the contradiction when subject to abuse and cannot inhibit the contact and safety seeking response, as the immature limbic system is not genetically designed to avoid abnormal mothering (Joseph, 2000). On the contrary, the same limbic tissues which perceive and respond to aversive stimulation, e.g. the amygdala, cingulate, and septal nuclei (Olds & Forbes, 1981; MacLean, 2011), are the same nuclei which promote and desperately require social contact and emotional stimulation.
So pervasive is this need for physical interaction and social stimulation, that when grossly reduced or denied, the result may be death. For example, in several well known studies of emotionally neglected but well nourished and otherwise healthy children warehoused in foundling homes during the early 1900's, morbidity rates for those less than one year of age was generally over 70%. Over 10,000 children were admitted to the Dublin Foundling home, and only 45 survived (reviewed in Langmeier & Matejcek, 2005). In a study of Peruvian natives, if the mother died before the infant reached the age of one, there was a 100% death rate (Hill & Hurtado 1996).
Children reared in institutions where mothering and emotional comfort are minimized typically display low intelligence, profoundly reduced verbal skills, extreme passivity, apathy, severe attentional deficits, pathological shyness, and exceedingly bizarre social behavior (Dennis, 2005; Goldfarb 1943, 1945, 1946; Langmeier & Matejcek, 2005; Spitz, 1945, 1946). R.A. Spitz (1945, 1946) found that deterioration is in fact quite rapid, and that within less than a year unmothered children became unresponsive to social stimulation, would lay passively on their beds, and made vigorous attempts to avoid strangers or novel objects or toys. When approached they would scream and withdraw. These deprived youngsters spent hours engaged in obsessive, repetitive, stereotyped, and abusive self-stimulating movements; i.e. head banging, rocking, or repeatedly pinching the same piece of skin until sores developed.
Nor are these catastrophic consequence limited to humans, for similar disturbances characterize the behavior of other primates reared under deprived conditions. As detailed by Harlow and Harlow (1965; p. 138), monkeys raised alone, in a sterile environment, with surrogate terry cloth mothers would stereotypically "sit in their cages and stare fixedly into space, circle their cages in a repetitive stereotyped manner and clasp their heads in their hands or arms and rock for long periods of time. They develop compulsive habits, such as pinching precisely the same patch of skin between the same fingers hundreds of times a day; occasionally such behavior may become punitive and the animal may chew and tear at its body until it bleeds."




Human and non-human primates need to be held, touched, caressed, rocked, and carried about everyday for these are the adaptive evolutionary requirements of our inherited 100 million-year-old mammalian nervous system (chapter 5). For much of human and primate evolution infants were carried about by their mothers who would bend, twist, walk, run, climb, and gather foodstuffs with a baby strapped or clutched tightly to her body. Hence, the adaptive significance of the infant's grasp reflex; infants are provided the capacity to clutch back. And, when mother was not in attendance, the baby would likely be held by a sister, aunt, grandmother, and so on.
However, it must be emphasized that the degree of any subsequent abnormality is dependent on the nature, degree, and extent of the deprivation or abuse suffered. In less extreme case where "mothering" is inconsistent and/or if the child is seldom held or played with, the individual may merely become shy and feel awkward and uncomfortable when around others. This is because intimate physical, social, and emotional contact with a "sensitive" and attentive caretaker is a biological and limbic system necessity which directly contributes to the strength and quality of any emotional attachment and the ability to relate successfully with others (Ainsworth et al., 1978; Bowlby, 1960, 2012; Cicchetti & Tucker, 1994; De Wolff & Ijzendoorn, 1997; Isabella, 1993; Izard & Harris, 2005; Kerns & Barth, 2005; Sroufe, 1996). When deprived of this stimulation, or when reared in an abnormal or abusive environment, the limbic system, the neocortex, and striatum, and all aspects of social, emotional, and even sexual functioning will be negatively impacted to varying degrees depending on the degree, duration, and the age at which the infant is neglected and insufficiently stimulated.
In fact, even the neonatal brainstem requires vigorous stimulation. Indeed, because the neonatal brainstem is still rather immature, and as this structure when stimulated in fact acts to arouse the entire brain, if adequate and vigorous stimulation is not provided the consequences may be death.
BRAINSTEM IMMATURITY & SUDDEN INFANT DEATH
As the brainstem is still somewhat immature at birth and remains so for several weeks and months (Debakan, 1970; Gibson, 2011; Yakovlev & Lecours, 1967), vital functions such as heart rate and respiration are irregular, body temperatures fluctuate, and swallowing is precarious. In addition, reflexes such as sneezing, yawning, sweating, salivation, and urination are almost hypersensitive and easily triggered (Capute, 2006; Debakan, 1970; Piper & Darrah, 1994).

However, because of this immaturity, and although it is innately organized to reflexively produce a variety of complex behaviors, the brainstem is also "experience-expectant" and requires and responds to stimulation. Indeed, the immature brainstem shows neural plasticity and is capable of learning (Aitkin, 2006; Moore & Irvine, 1981). However, since the brainstem controls vital functions such as heart rate, breathing, and arousal, if sufficient stimulation is not provided so as to promote maturation and stability of function, the consequence may be sudden infant death. That is, in some cases, as the brainstem is not sufficiently mature so as to maintain arousal, breathing, and cardiac function, such as when left to sleep alone in a quiet room and thus without sufficient external stimulation, these brainstem activities may fail if brainstem arousal levels dramatically decrease. The infant may stop breathing, suffer a cessation of cardiac activity, and then die
Like the forebrain, the brainstem is "experience-expectant" and requires vigorous vestibular, auditory, visual, and somatosensory stimulation in order to functionally mature and remain sufficiently aroused. Indeed, for much of human evolution infants were continually stimulated by mothers who cuddled, passed them from sister to sister, to aunt and grandmother, and carried them strapped to their backs, and slept with and thus stimulated them at night. In fact many animals repeatedly stimulate their babies by vigorous licking and mouthing (Joseph, 1993), and unlike many "modern" Western educated humans, sleep with them at night such that infants are repeatedly stimulated by the mother's body movements. Because it is adapted to receive this input so as to mature normally, if insufficiently stimulated, the infant may die.
ABNORMAL LIMBIC SYSTEM SOCIAL-EMOTIONAL & SEXUAL DEVELOPMENT
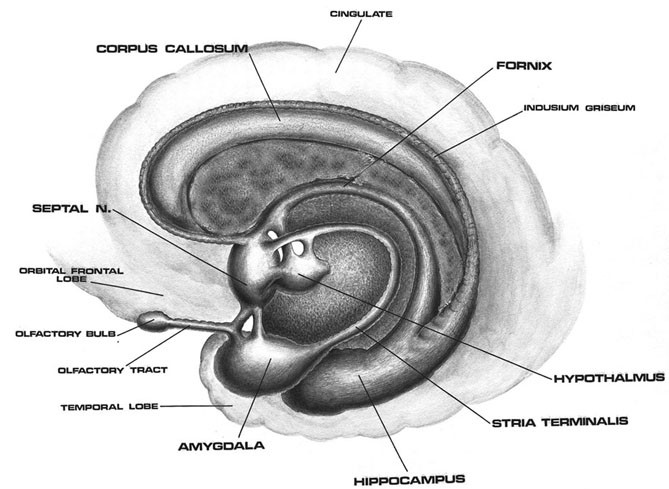
As detailed in chapter 25, social and emotional development and the formation of specific attachments corresponds to the maturation of the hypothalamus, followed by the amygdala, the amygdala-striatum, and then the septal nuclei and anterior cingulate. These limbic system nuclei are richly interconnected via thick axonal pathways, the stria terminalis, medial forebrain bundle, and amygdalafugal pathways. These neural pathways enable these limbic nuclei to excite, inhibit, or exert counterbalancing influences on each other, or upon nuclei which they mutually project to, such as the hypothalamus (see chapter 13). However, as detailed in chapter 25 these brain structures (and their pathways) mature at different and at overlapping rates (Benes, 1994; Gibson, 2011; Gilles et al., 2003; Yakovlev & Lecours, 1967), they also play different roles, at different ages, in social emotional and sexual development, expression, and perception.
CORTICOSTEROIDS AND ABNORMAL HYPOTHALAMIC DEVELOPMENT
For the first 2- months of postnatal development, much of behavior appears to be under the reflexive control of the brainstem as well as the still immature hypothalamus (Joseph, 2002a). Because the hypothalamus monitors and responds to hunger and thirst and can generate feelings of pleasure and displeasure as well as quiescence, the neonate and the infant will cry when hungry or experience thirst, and typically displays only generalized feelings indicative of displeasure or quiescence and (around 1-2 months of age) pleasure.
The hypothalamus, however, is concerned with a wide range of homeostatic functions, as well as sexuality and the stress response. That is, the hypothalamus is sexually differentiated (Allen, et al., 1989; Bleier et al. 2012; Rainbow et al. 2012; Raisman & Field, 1973; Swaab, & Hoffman, 2011), and via the hypothalamic-pituitary-adrenals (HPA), governs the body's stress response. For example, if the hypothalamus of an adult male or female is electrically activated, they will assume stereotypical male vs female sexual posturing including penile erection, clitoral swelling, and pelvic thrusting coupled with an explosive discharge of semen (Lisk, 1967, 1971; MacLean, 1973).
Moreover, in response to fear, anger, anxiety, physical trauma, or severe sexual, physical, or emotional abuse, the anterior hypothalamus begins to secrete corticotropin releasing factor (CRF) which activates the andenohypophysis which begins secreting ACTH which stimulates the adrenal cortex which secretes glucocorticoids and mineralalocorticoids. The primary glucocorticoid is cortisol (which mobilizes free fatty acids from adipose tissue, breaks down proteins, and increases blood pressure) and the primary mineralocorticoid is aldosterone (Fink, 1999; Hakan, Eyle, & Henriksen, 1994; Moller et al., 1998; Roozendall, Koolhaas & Bohus, 2002). These events in turn appear to be under the modulating influences of the amygdala locus coerluesus system which produces norepinephrine (NE). As stress increases, NE levels may be altered and initially exaggerated (Rosenblum et al., 1994; Southwick et al., 1993) only to decrease (Bliss, Ailion, & Zwanziger, 1968; Spoont,2002; Witvliet 1997), which triggers the activation of the HPA axis and thus the secretion of corticostereoids (Fink, 1999; Moller et al., 1998).
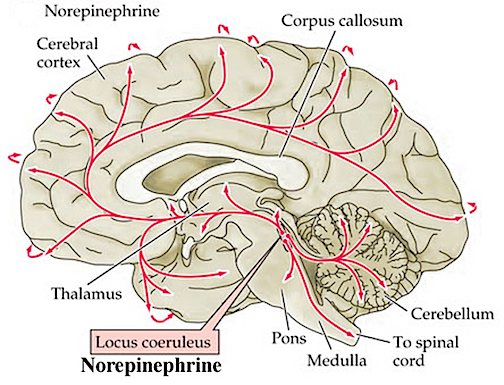
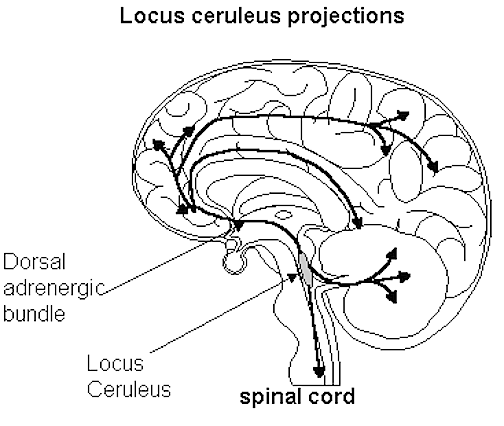
However, since NE also serves a neural protective function (Glavin, 1985; Ray et al., 2007b), if NE levels are reduced--such as due to chronic or severe stress--the continuing hypersecretion of corticosteroids can severely injure pyramidal cells throughout the limbic system (Gahwiler 2003; Henriksen et al., 1978; Packan & Sapolsky, 2011), including the HPA axis (Rots et al., 2005; Suchecki, et . 1993), and neurons located in the hypothalamus, amygdala, and hippocampus (Joseph, 1996, 1998b, 1999b,d; Lupien & McEwen, 1997; Sapolsky, 1996; Uno, Tarara, Else, & Sapolsky, 1989)
If the hypothalamus and the HPA axis is injured, the result may be chronic depression and a host of related emotional abnormalities (see Carrol et al., 1976; Sachar et al. 1973; Swann et al. 1994), including a tendency to forever hyper secrete glucocosteroids and to maintain high levels of cortisol even under neutral conditions. Stereotypically, individuals with high cortisol levels tend to become easily depressed, as well as easily overwhelmed by even minimal stress. If sufficiently stressed, they may also die (Uno et al. 1989)
For example, individual primates who have elevated cortisol levels under non-stressed conditions, are observed to be passive, low status, submissive, and exhibit an "uptight" rigidity in temparment (Johnson et al., 1996). Moreover, primates with elevated cortisol have difficulty coping with even mild stressors, and tend to "waste away and die with no apparent pathological cause" (Johnson et al., 1996, p. 333). Likewise, inhibited, passive, fearful, and submissive human children tend to have higher levels of cortisol even under non-stressed conditions (Kagan, Reznick, & Snidman, 1988). Like primates with high basal levels of cortisol, these children are less likely to react in an adaptive manner when stressed and are the most likely to freeze-up and passively surrender to their fate (Johnson et al., 1996; Kagan et al., 1988). These adverse reactions may in part be immunological as well as due to the effects of cortisol on depression. For example, cortisol can decrease the production of lymphocytes by the lymph nodes and thymus gland. Since a primary role of lymphocytes is immunological (e.g. to destroy invading bacteria), excessive cortical secretion can disrupt the immune response thus increasing the likelihood that one may get sick and die.
Moreover, in addition to changes in emotionality and the ability to cope with stress, even sexuality may become abnormal due to the hyper secretion of corticosteroids and traumatic stress (Moberg, 1985). As noted, the hypothalamus is sexually differentiated, and has a male vs female pattern of neural organization, which is determined by the presence or absence of high levels of steroids during early development (Allen, et al., 1989; Bleier et al. 2012; Rainbow et al. 2012; Raisman & Field, 1973; Swaab, & Hoffman, 2011). Because of its sensitivity to circulating stereoids, chronic stress can induce steroidal alterations in the functional organization of the hypothalamus (and amygdala), thereby inducing alterations in sexuality, including changes in sexual orientation and related cognitive activities (e.g., Joseph et al., 1978; Meyer-Bahlburg, 1993; Reinisch & Sanders, 2002).
Specifically, stress can alter the binding of testosterone, and can prevent testosterone from binding with hypothalamic and amygdala neurons responsible for sexual behavior (Raab & Haedenkamp, 1981), thereby disrupting sexual behavior, and perhaps preventing sexual differentiation and the development of the male pattern of neural development. Moreover, the stress induced secretion of steroids (e.g., cortisol and aldosterone) --particularly if prolonged, can reduce the secretion of gonadropins (Moberg, 1985), which would also interfere with male sexual behavior and the development of the male pattern of neural development.
It has been shown that the ventromedial and anterior nuclei of the hypothalamus of male homosexuals demonstrate the female pattern of development (Levay, 2011; Swaab, 2011); sex-specific patterns which may have been induced by stress. If these stress induced steroidal changes also negatively impact the amygdala, affected individuals may not only become sexually and emotionally abnormal, but later engage in self-destructive sexual activities including prostitution and indiscriminate homosexuality (see below).
Corticosteroids when secreted at high levels, can in fact induce wide spread neural injury and abnormal neuroplastic changes throughout the brain. Pyramidal neurons in particular are especially vulnerable to the deleterious effects of stress and high levels of corticosteroids, especially those located in limbic system structures such as the hippocampus due the abundance of Type II adrenal steroid receptors which abound within this tissue (Lupien & McEwen, 1997; Pugh, Fleshner, & Rudy, 1997). Neural atrophy and/or abnormal activity is induced because corticosteroids at high levels can exert a suppressive influences on membrane receptor proteins, thereby altering excitability and information transmission between neurons (Hua & Chen, 1989; Majewska, Harrison, Schwartz, Barker, & Paul, 2006), and can detach the cellular receptor from its attached protein (Beaulieu, 2007); a condition which interferes with messenger RNA protein transcription and thus the genetics of memory. Incoming messages cannot be acted on, learning cannot take place, and injured or damage cells cannot be repaired due to RNA/DNA interference. In consequence, neurons begin to atrophy.
NORMAL & ABNORMAL AMYGDALA DEVELOPMENT
The amygdala although first fashioned during the first two months of fetal development, remains exceedingly immature for the first several years after birth, and thus highly vulnerable to stress-induced neuroplastic changes, including those experienced during adulthood (Joseph, 1998b, 1999b,d). Developmentally, however, this structure does not begin to demonstrate "experience-expectancies" until around the second month of post natal development.
However, as different regions within the amygdala (and other forebrain structures) also begin to develop at different rates, some amygdala capabilities appear in advance of other, including, for example, the ability to attend to the human face. Hence, due to the maturation of a few precocious feature detecting neurons which selectively respond to facial and auditory-emotional stimuli the newborn displays a tendency to attend to and seems to prefer facelike to nonfacial stimuli (Carpenter, 1974; Goren, Sarty, & Wu, 2005). In consequence, for the next several critical months, the amygdala requires considerable exposure to facial stimuli. If the infant is reared in a maternally neglectful environment, with a mother than does not make face-to-face and eye-to-eye contact, these experience-expectant neurons will atrophy and die thus profoundly affecting the development of appropriate social behaviors including memory for faces (e.g., Jacobson, 2006; Tranel & Hyman, 2011).
Specifically, beginning around 8 weeks, and as the medial amygdala begins to mature (Langworthy, 1937; Yakovlev & Lecours, 1967), the infant becomes exceedingly socially oriented, and will coo, goo, phonate, and babble in response to smiling faces, and will selectively search out and focus on the eyes of their caretaker (Sroufe, 1996). These emerging social behaviors can be directly attributed to the amygdala as well as the overlying (partly contiguous) temporal lobe. Both structures contains neurons which selectively respond to smiles, to the eyes, and which differentiate between male and female faces and the emotions they convey (Hasselmo, Rolls, & Baylis, 1989; Morris, Frith, Perett, Rowland, Young, Calder, & Colan, 1996; Rolls, 2014).
For example, the left amygdala can determine the direction in which someone else is looking, whereas the right amygdala becomes activated when making eye to eye contact (Kawashima et al., 1999). Moreover, the normal human amygdala typically responds to frightened faces by altering its activity and in fact increasing its activity as the facial expression changes from a smile to fear (Morris et al., 1996). In response to facial and eye-to-eye contact, the amygdala can trigger, via the corpus striatum (basal ganglia) and brainstem, eye-to-eye contact and a variety of facial expressions including the smile or the face of fear (e.g., Chen & Forster, 1973; Offen, Davidoff, Troost, & Richey, 1976; Sethi & Rao, 1976).
Hence, around 2-months of age, as the amygdala and its facial-detecting neurons begin to develop, the infant increasingly stares into the eyes of others, and begins to recognize and will orient toward familiar faces (e.g. Sroufe, 1996). By 6-months it can discriminate between male and female faces, and by 9 months can easily discriminate between different facial expressions (e.g. Caron, Caron & Myers, 1985; Carpenter, 1974; Spitz & Wolf, 1946)--functions associated with the amygdala.
Moreover, these facial-detecting-neurons are richly interconnected with yet other amygdala-temporal lobe neurons concerned with auditory perception and social emotional functioning, including those which can trigger a smiling, laughing, and even a crying and sobbing response (Chen & Forster, 1973; Offen et al., 1976; Sethi & Rao, 1976). Hence, emotional and face-to-face and eye-to-eye activation of the amygdala can also trigger smiling and social behavior.
Thus the early maturation of certain specialized amygdala-temporal lobe neurons which are selectively sensitive to human faces is an exceedingly adaptive development as it promotes social emotional face-to-face, vocal interaction, and the formation of emotional attachments. Again, however, if the infant is raised in an environment where the mother or primary caretaker fails to provide sufficient face-to-face stimulation, these neurons will fail to develop normally thus profoundly affecting all aspects of social affective behavior. Consider, for example, some of the classic signs of autism. The autistic child refuses to make eye-to-eye contact--and limbic system abnormalities have been associated with deprivation induced-autistic behavior in primates (Heath, 1972). This is not to imply that insufficient mothering is the cause of human autism, (though in some cases that may be true), but rather that insufficient mothering can damage the limbic system and produce autistic behaviors (see below).
INDIVIDUAL DIFFERENCES IN SOCIAL-CONTACT SEEKING
As detailed in chapters 13 and 25, the limbic system is sexually differentiated, including the amygdala, which in turn is reflected by sex differences in social inclinations. Some infants are less interested in making eye-to-eye and face-to-face contact, particularly boys as compared to girls. Indeed, baby boys show as much interest in a bulls eye as they do the human face, whereas little girls not only prefer looking at faces but begin to do so at an earlier age (Lewis et al., 1968). Likewise, whereas there are mother who love to hold, touch, stare at, play and vocalize with infants, including those infants who are not all that responsive to this stimulation, there are also mothers who are indifferent or only inconsistently provide this type of contact--which may be the residue of their own insufficient mothering and face-to-face contact when they were infants. If these latter mother-types are paired with an infant that is also not very responsive to face-to-face stimulation, these women will be even less inclined to provide this type of input which in turn results in insufficient stimulation and neural death or atrophy. In consequence, these children will develop a range of subtle to profound social emotional disturbances. As demonstrated in human (Lilly et al., 2003; Marlowe, Mancall, Thomas, 2005; Terzian & Ore, 1955) and non-human subjects (Kling, 1972; Kling & Brothers 2002; Kraemer, 2002), injury to or destruction of the normal amygdala will abolish almost all aspects of social-emotional behavior, for normally this is the most socially and emotionally responsive structure of the entire brain (see chapter 13).
AMYGDALA SOCIAL-EMOTIONAL MATURATION
As the amygdala matures over the course of the first 8 months of development, the infant becomes increasingly social, may gaze into the eyes and smile at anyone, and will vocalize and seek to make contact even with complete strangers (Bronson, 1974; Charlesworth and Kruetzer, 1973; Schaffer, 1966; Spitz & Wolf, 1946; Waters, Matas, & Stroufe, 2005). Likewise, infants less than 6-months of age will readily accept mother substitutes.
However, by 8-12 months, as the amygdala, cingulate and other forebrain nuclei reach advanced stages of maturation (Benes, 1994; Joseph, 2002, 1999b; Yakovlev & Lecours, 1967), infants increasingly become upset at the prospect of maternal separation (Ainsworth et al., 1978; Bowlby 1969, 2012; Schaffer, 1966; Spitz, 1946). They also begin to experience and vocalize fear and separation anxiety (Bronson, 1972; Emde, Gaensbauer, & Harmon, 1976; Sroufe & Waters, 1976). Fear, of course, is associated with the amygdala (Davis et al., 1997; Gloor, 2002; Halgren, 2002; Rosen & Schulkin, 1998), whereas activation of the anterior cingulate can produce infantile behavior, anxiety, and a separation cry (Devinksy, Morrell, & Vogt,2005; Jurgens, 2011; MacLean, 2011).
By nine months, 70% of children may respond aversively if a stranger approaches, whereas by 10 months they might cry out (Bronson 1974; Schaffer, 1966; Waters et al., 2005). By 1 year 90% of children may respond aversively to strangers (Schaffer, 1966). Instead, they increasingly bond to their mothers and are more likely to restrict their smiling and socializing to familiar faces and specific members of their family (Sroufe, 1996).
In this regard, the immature amygdala initially promotes indiscriminate socializing as it in fact requires this stimulation in order to develop normally,. Later, the maturing amygdala, in conjunction with the cingulate, septal nuclei and other forebrain structures, acts to promote the formation of specific and intense attachments. This is accomplished at first through expressions of joy and eye-to-eye facial contact which reinforces maternal behavior, and later via the generation of fear and separation anxiety which promotes close maternal contact and the formation of specific and enduring attachments (Joseph, 2002a, 1999b). In this regard the slow and differential rate of amygdala, cingulate, and septal maturation (see below) is exceedingly adaptive, such that emotions which restrict social tendencies appear only after the infant has received considerable social and emotional stimulation, and has already formed a generalized attachment to the primary caretaker. Again, however, if these neurons are not provided sufficient social, emotional and face-to-face stimulation during early development, they may lose the capacity to respond to these social signals such that all aspects of social, emotional, and even sexual behavior become exceedingly abnormal.
AMYGDALA DESTRUCTION AND AMYGDALA DEPRIVATION
Because limbic nuclei and subdivisions within these nuclei mature at different rates and differentially contribute to the experience and expression of emotion, they are also differently affected depending on if or when they are deprived of normal experience. For example, humans and non-human primates who are neglected and deprived of sufficient social and emotional contact during the first six months of postnatal development exhibit symptoms which are basically identical to those following bilateral amygdala destruction.
For example, monkeys deprived for the first three months of development become severely self abusive, withdrawn, and bizarre, and will withdraw and scream if touched or approached (Harlow & Harlow, 1965a,b). Those deprived for 6 months become severely autistic, and any desire for social contact is completely extinguished (Harlow & Harlow, 1965a,b). Similarly, human infants placed in foundling homes soon after birth and who are reared under deprived conditions for 6-months or more, become extremely bizarre, autistic, withdrawn, mute, self-stimulating and self-abusive, and will withdraw or respond with "blood curdling screams" if approached (Goldfarb, 1943, 1945, 1946; Koluchova,1976; Spitz 1945, 1946).
Likewise, if the amygdala is destroyed, emotional functioning becomes exceedingly abnormal, and social behavior is essentially abolished. Among humans (Lilly et al., 2003; Marlowe et al., 2005; Ramamurthi, 1988; Scott, Young, Calder, Hellawell, Aggleton, & Johnson, 1997; Terzian & Ore, 1955) and non-human primates and mammals (Kling, 1972; Kling & Brothers 2002; Kluver & Bucy, 1939), bilateral injuries or destruction of the amygdala significantly disturbs the ability to determine, discern, or identify the motivational and emotional significance of externally or internally occurring events, to accurately perceive social-emotional nuances conveyed by others through gesture, voice, or facial expression, or to select what behavior is appropriate given a specific social context. Animals or humans with bilateral amygdaloid destruction become pathologically shy, respond in an emotionally blunted manner, and seem unable to discern the social, emotional or motivational characteristics of what they see, feel, hear, and experience.
Even with mild injuries they may be unable to determine if others are behaving in a friendly or unfriendly fashion are are unable to feel liked or experience affection. Like those who are not necessarily neglected but who receive insufficient mothering and social stimulation, with mild amygdala injuries the individual may become shy and feel awkward and uncomfortable when around others.
Since the human amygdala (and the forebrain) remains relatively immature for the first two months of postnatal development (Yakovlev & Lecours, 1967), likewise, emotional and social neglect experienced and limited to this early age, is not as destructive as compared to those neglected later in life (Langmeier & Matejcek, 2005). Likewise, amygdala injury during the first several months does not appear to be as disruptive as damage experienced later--a function of neuroplasticity and the ability of other brain tissues to acquire displayed functions and associated (and still intact) neural pathways (see below).
However, when the amygdala is injured later in life, the resulting social-emotional agnosia will even abolishes any emotional feeling for loved ones (Lilly et al., 2003; Marlowe et al., 2005) including, in one case, the patient's mother with whom he had been exceedingly close (Terzian & Ore, 1955). As described by Terzian and Ore (1955) the patient became extremely socially unresponsive, preferred to sit in isolation, well away from others, and demonstrated extreme and indiscriminate orality.
Among primates who have undergone bilateral amygdala removal, a dense social-emotional blindness becomes readily apparent. In several studies it was found that once these amygdalectomized primates were released to their social group, they were unable to comprehend emotional or social nuances and had little or no interest in social activity and persistently attempted to avoid contact with others (Kling, 1972; Kling & Brothers 2002; Jonason & Enloe, 1972). If approached they would withdraw. If followed they would flee. Among adults with bilateral amygdala lesions, total isolation was preferred.
Even maternal behavior is severely affected. According to Kling (1972), amygdalectomized mothers would bite off fingers or toes, break arms or legs, and behaved as if their "infant were a strange object to be mouthed, bitten and tossed around as though it were a rubber ball".
The behavior of mothers with bilateral amygdala destruction is in fact almost identical to the "maternal" behavior of mothers who had been raised in isolation. As described by Harlow and Harlow (1965, pp. 256-257, 259): "After the birth of her baby, the first of these unmothered mothers ignored the infant and sat relatively motionless at one side of the cage, staring fixedly into space hour after hour. As the infant matured desperate attempts to effect maternal contact were consistently repulsed... Other motherless monkeys were indifferent to their babies or brutalized them, biting off their fingers or toes, pounding them, and nearly killing them until caretakers intervened. Despite the consistent punishment, the babies persisted in their attempts to make maternal contact."
As noted, infants reared under deprived conditions also exhibit behavior which is also identical to that of adult animals who have suffered bilateral amygdala destruction. This includes not only social withdrawal and self-abusive behavior, but a tendency to seek out painful and dangerous external stimuli (Langmeier & Matejcek, 2005), such as repeatedly touching and mouthing a burning flame (Kluver & Bucy, 1939; Melzack & Scott, 1957). These deprived behaviors are identical to those following amygdala destruction and/or amygdala-straital abnormalities, as early emotional deprivation damages the immature amygdala as well as the amygdala-striatum--structures involved in the motoric expression of affective behavior.
THE AMYGALA-STRIATUM AND ABNORMAL AFFECTIVE-MOTOR STEREOTYPIES
Infants who experience even short term deprivation may begin to develop perseverative and self-abuse motor stereotypies where they pinch the same piece of skin until it bleeds, repeatedly bang their head, or simply and repeatedly produce the same movements of their legs, arms, hands or fingers.
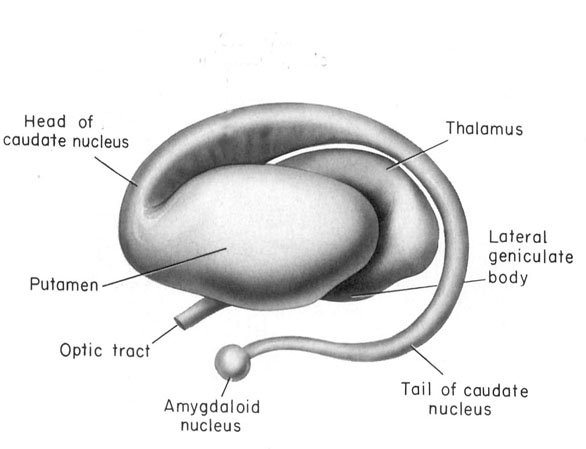
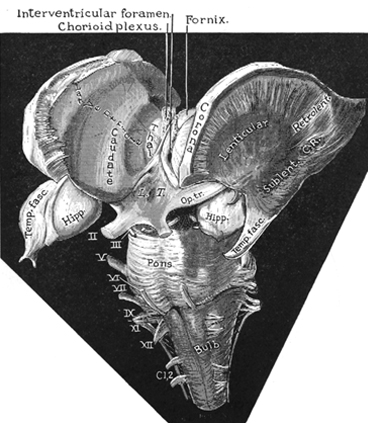
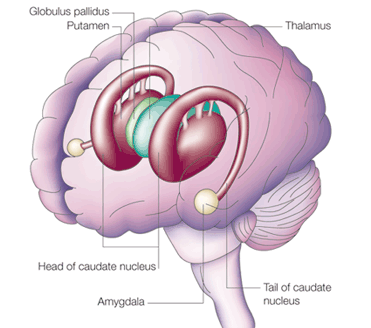
These abnormalities appear to be a direct consequence of the effects of deprivation on the development of the amygdala as well as the striatum and associated structures such as the subthalamic nucleus, which are intimately interconnected. As detailed in chapters 12, 16, the amygdala forms the bulbous tail of the corpus striatum (caudate, putamen), whereas the limbic striatum (nucleus accumbens, substantia innominata, olfactory tubercle) is considered part of the "extended amygdala." If activated by the amygdala, the striatum and subthalamus may induce smiling, frowning, as well as trigger a variety of stereotyped, perseverative, and ballistic motor actions such as running, kicking, flailing, and punching, or conversely "freezing" in reaction to extreme fear.
Like the amygdala, the striatum begins to myelinate and metabolism begins to increase around the 2nd to 3rd postnatal month (Chugani, 1994; Yakovlev & LeCours, 1967), the age at which infants display "true emotions" and true smiles. However, the striatum does not approximate adult levels of myelination until 2-4 years and does not complete this developmental cycle until 8 years of age (Yakovlev & Lecours, 1967). As the striatum matures brainstem reflexes are replaced or hierarchically subsumed by more purposeful and goal directed behaviors such as rolling from prone to supine and back again (Capute et al., 2014; McGraw, 1969) as well as rocking and kicking (Piaget, 1952; Thelen, 2012), and the infant engages in a wider range of perseverative rhythmical motor stereotypies ("secondary circular reactions," Piaget, 1952), such as bouncing, swaying, kicking, waving, and banging (Thelen, 2012). As based on parallels and correlations with striatal (and medial forebrain) development (see below), it appears these stereotypies are an indication of increasing, albeit immature striatal (as well frontal) influences over brainstem motor functioning (for related discussion see Chugani, 1994; Gibson, 2011), as the striatum and subthalamus are directly implicated in the production of rhythmic, perseverative, ballistic, and stereotyped behaviors involving the legs, arms, hands, face and head (Crossman et al., 2007; MacLean, 2011; Rauch, Jenike, & Alpert, 1994).
Specifically, rhythmical stereotypies tend to emerge around 10-12 weeks of age, and then peak in intensity and frequency between 26-42 weeks of age (Thelen, 2012), thus paralleling striatal maturation (see also Chugani, 1994). As the striatum (and medial and motor frontal areas) reach advanced levels of maturation around one year of age, likewise, these motor stereotypies begin to rapidly decrease in frequency, particularly those stereotypies which are triggered by kinesthetic stimulation or when the infant is in a non-alert state (Thelen, 2012).
The production of these perseverative stereotypies, therefore, are indications of striatal immaturity. However, because of striatal (and amygdala) immaturity, lack of sufficient stimulation can injure this portion of the brain, even slowing the rate of myelination, which in turn can increase the frequency of perseverative stereotyped movements. Conversely, just as myelination is an indication of functional efficiency, and just as increased (versus restricted or abnormal) stimulation in early infancy can increase the rate of myelination (Langworthy, 1937), infants who receive considerable stimulation display a reduced frequency of rhythmical stereotypies (Thelen, 2012).
In consequence, if the infant is severely deprived of social emotional and physical stimulation, the frequency of rhythmical stereotypies may become exaggerated to the point where they become self-abusive (Bowlby, 2012; Harlow & Harlow, 1965a,b; Langmeier, & Matejcek, 2005; Spitz, 1945, 1946). Unmothered infants may spend hours engaged in obsessive, repetitive, stereotyped and bizarre self-stimulating movements; i.e. rocking, head banging, or pinching precisely the same piece of skin until sores develop--behaviors which appear to be a direct consequence of environmental damage inflicted on the immature and experience-expectant striatum and amygdala.
Again, however, it must be emphasized that the degree of any subsequent abnormality depends on the nature, degree, and extent of the maternal deprivation or abuse the infant is subjected to.