Rhawn Gabriel Joseph, Ph.D.
MOVEMENT AND THE MOTOR AREAS OF THE FRONTAL LOBES
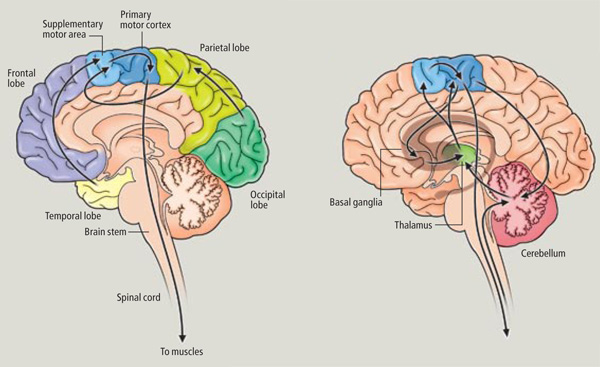
Movement and motor functioning are dependent on the functional integrity of the basal ganglia, brainstem, cerebellum, cranial nerve nuclei, the motor thalamus, spinal cord, as well as the primary, secondary and supplementary motor areas of the frontal lobes (Passingham, 2007; Schmahmann, 2007). Indeed, these areas are all interlinked and function as an integrated system in the production of movement (Mink, 2007; Mink & Thach, 1991; Parent & Hazrati 2013; Passingham, 2007).
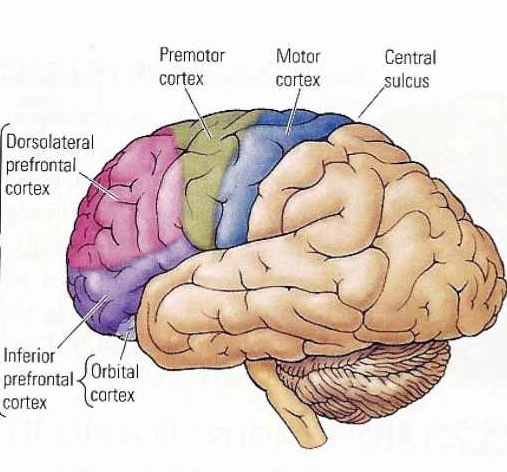
However, as to fine motor movements including those involved in the articulation of speech, these are almost completely dependent upon the functional integrity of the primary motor areas located along the percental gyrus (area 4), and within which are represented the muscles controlling the hands, fingers, and oral laryngeal musculature.
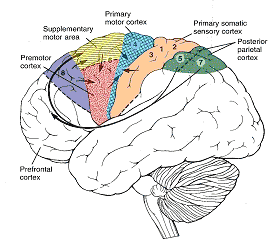
And yet, as noted above, the primary motor areas are in turn dependent upon motoric impulses which are organized in premotor and the supplementary motor cortex--the latter of which is located along the medial wall of the hemispheres. Considered from a very broad and simplistic perspective, it could be said that primary area is programmed and under the control of the secondary and supplementary motor areas as well as the "prefrontal" and other areas of the cerebrum, although neurons in the primary area also become active prior to and during movement (Passingham, 2007).
For example, Exner's writing area is in part, within areas 6 and becomes active prior to (as well as during) hand movements and appear to program hand movements, whereas the frontal eye fields (within areas 6,8,9) becomes active prior to (as well as during) eye movements and appears to program eye movements. As noted above, the primary area representing the oral-laryngeal musculature, is programmed by Broca's expressive speech area areas 45, 46 (Foerster 1936; Fox 2013; LeBlanc 1992; Petersen et al. 1988, 1989). Broca's area also becomes active prior to vocalization and during subvocalization as indicated by functional imaging.
Hence, like the sensory areas where information is generally received in the primary zones before transmission to the association areas as well as in the association areas independently of the primary areas (Zeki, 2007) motor impulses are processed and acted on in parallel (Passinghma, 2007), though in general, they generally begin their organizational journey in the supplementary motor areas (Alexander & Crutcher, 1990; Crutcher & Alexander, 1990) and/or the cingulate and other forebrain structures (Passingham, 2007; Stephan, et al., 2011), which are then transmitted to the primary regions where they are acted upon.
Again, however, there are areas within the primary region which also become active prior to movement, though these same areas become increasing active during movement (Passingham, 1993).
Because, in general, the impulse to move originates outside the primary motor cortex, direct electrical stimulation of this region does not give rise to complex, coordinated, or purposeful movements (Penfield & Boldrey, 1937; Penfield & Jasper, 1954; Penfield & Rasmussen, 1950; Rothwell et al. 1987). Rather stimulation will only induce, for example, twitching of the lips, flexion or extension of a single finger joint, protrusion of the tongue or elevation of the palate. It is noteworthy that patients never claim to have willed these movements, which again suggests the will-to-move is initiated elsewhere.
However, if electrical stimulation is applied when the patient is attempting to move, the result is paralysis (Penfield & Jasper, 1954; Penfield & Rasmussen, 1950). Presumably the reception of impulses-to-move (which are initiated and organized elsewhere) are blocked by primary motor electrical stimulation.
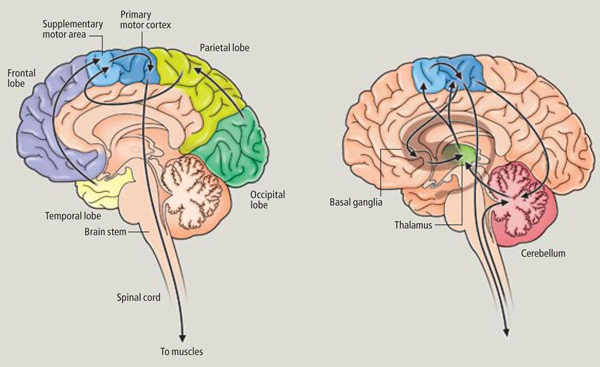
Motor functioning and movement, however, is also dependent on the parietal lobes, thalamus, basal ganglia, brainstem, cerebellum, and spinal cord, structures which are directly or indirectly interconnected (Kaas, 1993; Mink, 2007; Passingham, 2007; Schmahmann, 2007).
For example, smooth, purposeful, coordinated movement requires sensory feedback which is provided by the primary and association/secondary somatosensory areas located in the parietal lobe and which contain neurons which guide the hand and arm in visual space (see chapter 20). These cortices are intimately linked with the primary motor and the motor association/secondary and supplementary motor areas as well as the basal ganglia, brainstem, and cerebellum--regions which become highly active during and often prior to movement initiation (Passingham, 2007). For example, when human subjects learn a sequence of finger movements, functional imaging reveals increased activity in the primary and premotor cortex, and the primary and association somesthetic cortex, as well as in the cerebellum, striatum, ventral thalamus and cerebellum (Passingham, 2007). In fact, most of the "motor" area also contain independent motor maps of the body, which is why, if a subject moves an arm, leg, or shoulder, each of these areas becomes active almost simultaneously--at least as demonstrated with functional imaging.
On the other hand, as based on functional imaging, it has also been shown that there is increased activation of the primary motor area when subjects actually make a movement, as compared to merely preparing to make the movement (Passingham, 2007).
Hence, although some movements are programmed in parallel by a number of motor areas simultaneously, others are programmed in temporal sequences such that the SMA programs the premotor area which acts on the primary area which becomes maximally active when movements are actually performed. IN this regard it is noteworthy that whereas stimulation of the SMA can prevent movement, stimulation of the premotor area can give rise to the illusion that a movement is about to be made, that is, of an impending movement (Penfield, 1938), whereas activation of the primary area can trigger twitching, and minor motor movements--which again suggests a hierarchical organization.

According to Carpenter et al., (2011, p. 1755), although "individual cells prove largely independent information about the items in the sequence... during different epochs of presentation of the stimuli, different patterns of distributed activity in even small ensembles of motor cortical cells are sufficiently distinct and robust to provide a basis for encoding the sequence," which in turn allows for memory scanning and retrieval.
In addition, "motor" neurons in the inferior-ventral portion of the (monkey) premotor cortex can code for the spatial location of nearby sounds, particularly those originating near the head (Graziano et al., 2011). Indeed, these neurons appear to be "multi-modal" and can also respond to visual and tactile stimuli that impinge near or upon the face (Graziano et al., 2011).
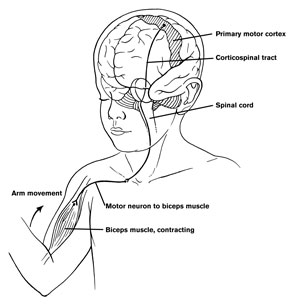
Only approximately 31% of the corticospinal tract arises from the pyramidal cells of agranular primary motor area 4, with another 30% arising from areas 6, 8, and the SMA, and another 30% from the primary and secondary/association somesthetic areas in the parietal lobe, and the remainder arising from the deep layers of the temporal, occipital, and orbital frontal lobes, as well as from pyramidal cells scattered throughout the amygdala, cingulate, and striatum. Hence, widespread areas of the neocortex control or influences movement by acting directly on the brainstem and spinal cord via the corticospinal tracts.
Presumably, those corticospinal axons arising from the parietal lobe project to the dorsal horns of the spinal cord so as to regulate sensory inflow rather than directly controlling movements. Hence, as two thirds of the corticospinal tract arise in the frontal motor area as these project to motor neurons, it is the frontal motor areas which dominate and exert controlling influences over most aspects of purposeful motor behavior. Because the motor cortex is specialized for controlling movement, the functional organization of the motor cortex differs from that of other brain areas.
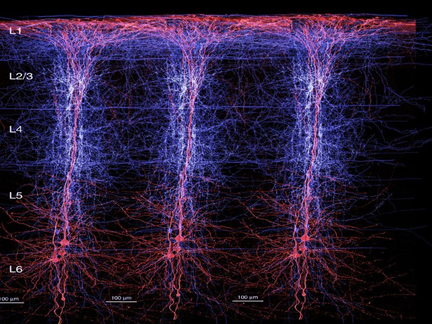
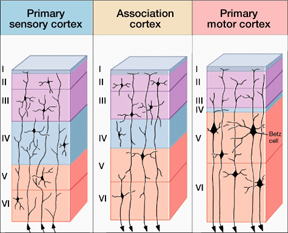
For example, the neocortex of the motor areas are relatively thick and "agranular" in structure. This is because internal "granular" layer IV is obscured by the increased size of layers III and V which are packed with large numbers of exceedingly large pyramidal cells including the giant cells of Betz. By contast, in somesthetic areas 3,1,2, layers III and V are much smaller, whereas layer IV is much thicker.
Within the frontal motor areas, it is layers III and V which gives rise to the corticospinal tract, and which consists of large and medium size pyramidal cells. Layer V also gives rise to the corticbulbar, corticopontine, and corticorubral fibers.
Approximately 90% of corticospinal axons range from 1 to 4 um in diameter. The largest fibers are axons from the giant Betz cells which are located in layer V of the primary motor area. About 60% of the axons of the corticospinal tract are myelinated.
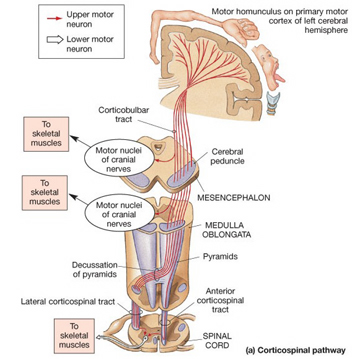
Specifically, the axons of the corticospinal (and related) tracts arise from large pyramidal neurons located in layers III and V of the neocortex and descend via the internal capsule to the midbrain, some of which make contact and terminate in the red nucleus, the periaqueductal gray, and a variety of other brainstem motor nuclei. Collectively these latter fibers are actually referred to as the corticobulbar tract and many of its axons arise in the orbital and medial frontal lobe as well as the inferior lateral convexity.
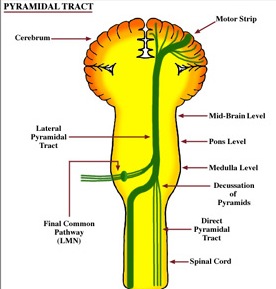

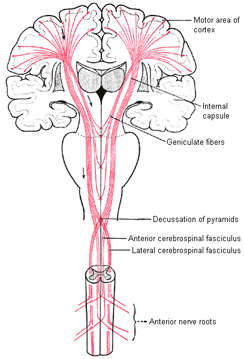
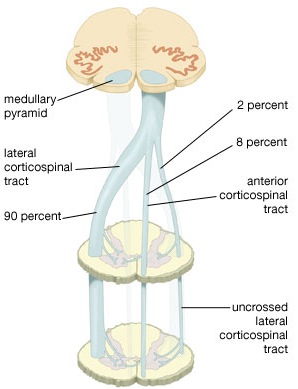
By contrast, corticospinal axons descend through the pons where they separate into tiny nerve bundles before regrouping within the medulla to form the medullary pyramid. However, at the spinal-medulla border, about three fourths of these axons cross over at the midline of the medulla to form the pyramidal decussation, with the crossed fibers forming the lateral corticospinal tracts and the remaining uncrossed axons forming the ventral corticospinal tract.
Hence, the corticospinal tract aconsists of two divisions, the lateral and ventral.The lateral tract projects to the lateral motor nuclei of the ventral horn and to intermediate zone interneurons, whereas the ventral tract projects bilaterally to the medial cell column which is concerned with the axial muscles. Most of the ventral (uncrossed) axons originate in Broadmans areas 4, 6 and 8, whereas the lateral tract also originates in frontal motor areas 4 and 6, and parietal somesthetic areas 3,1,2,5,7, though yet others have their source throughout the neocortex as well as in the limbic system.
Thus, these two divisions consist of a number of separate fiber bundles which originate not just within the pyramidal cells of the somatomotor areas, but within the striatum, cingulate and amygdala. These fiber bundles, therefore consist of a commingling of axons which originate throughout the forebrain, and which arise in discrete neocortical locations. Again, widspread areas of the brain contribute to purposeful movement. Moreover, although these fiber bundles descend together through the internal capsule, they innervate different brainstem targets, and are thus referred to as the corticobulbar, corticopontine, corticorubral and corticospinal tracts.
The vast majority of descending motor fibers, however, penetrate the brainstem and establish direct synaptic interconnections with spinal motor nuclei. These latter fibers are referred to as the corticospinal tracts.
Specifically, corticospinal axons descend through the pons where they separate into tiny nerve bundles before regrouping within the medulla to form the medullary pyramid. At the spinal-medulla border, about three fourths of these axons cross over at the midline to form the pyramidal decussation, with the crossed fibers forming the lateral corticospinal tracts, and the remaining uncrossed axons forming the ventral corticospinal tract. That is, about 90% cross over, such that the left half of the brain controls the right half of the body (and vice versa).
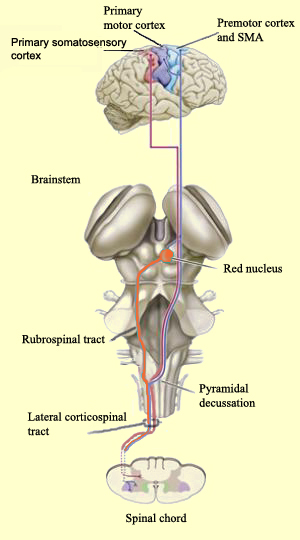
By these long descending pyramidal axons, neocortical motor control is gained via neocortical pyramidal axons which innervate the motor nuclei of the brainstem and spinal cord at all levels.
Hence, due to early left hemispheric motor development, the left corticospinal tract grows more quickly and descends into the brainstem, and then crosses at the pyramidal decussation, and then descends into the spinal cord in advance of those fibers from the right (Kertesz & Geschwind 1971; Yakovlev & Rakic 1966). This provides the left hemisphere and the right hand with a competitive advantage in establishing both motor control and thus right hand dominance.
Because the corticospinal tract does not reach advanced levels of myelination and does not become functionally mature until the 8th-12th postnatal month (Yakovlev & Lecours, 1968) it is only as the infant approaches their first birthday that handedness become obvious or apparent.
It is also around the 8th to 12th month that the motor and somatosensory areas become increasingly mature (Bell & Fox, 2014; Brody et al., 1987; Chugani, 2014; Hudspeth & Pribram, 1992; Huttenlocher, 1990; Schore, 2014) and the secondary motor and sensory receiving areas display increased myelination (Flechsig, 1901).
As based on PET scans, the frontal lobes also display increased metabolic activity from 8 to 12 months of age (Chugani, 2014), and around the end of the first year the neocortical EEG begins to increasingly resemble a more adult pattern (Kellaway, 1979; Ohtahara, 1981). In addition, around the 8th to 12th month, the corticospinal and pyramidal axons begin to approximate adult level of cellular development, myelination, and organization, and then become increasingly well myelinated over the course of the second year (Debakan, 1970; Langworthy, 1937; Yakovlev & Lecours, 1967)--a process which continues into late childhood (Paus et al., 2011).
Hence, around the 8-12th month the neocortex and frontal motor areas begin to increasingly exert hierarchical control over the limbic forebrain, brainstem, and musculature, and this process continues to develop over the course of the ensuing decade and requires considerable coordinated interaction between the parietal and motor areas of the frontal lobe as well as the striatum, limbic system, and cerebellum.
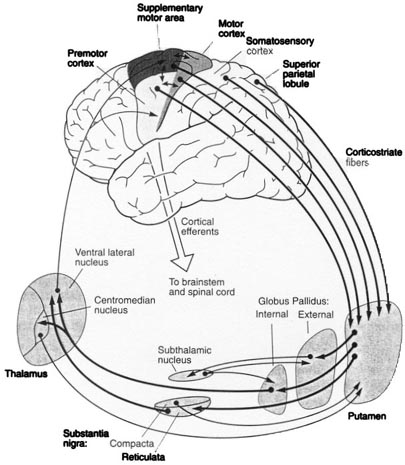
The SMA is tightly interlinked with the striatum, anterior cingulate, amygdala, as well as the motor thalamus, and contributes axons to the corticospinal tract and makes synaptic contact with the brainstem, cerebellum, and spinal cord (Brodal, 1981; Carpenter, 1991; Schmahmann, 2007). Moreover, the SMA receives input from and transmits to the premotor and primary frontal motor areas as well as from and to the parietal lobe. Hence, this structure is situated at the cross-roads where divergent impulses and sensory impression are received and apparently acts to integrate these signals. Presumably because of its integrating role, the SMA also becomes highly active during the modification, learning and establishment of new movement programs (Brinkman & Porter, 1979; Roland et al.1980; Tanji et al. 1980), and may well act to make decisions regarding possible movements based on these signals well before a movement is initiated.
In fact, single cell recordings (Brinkman & Porter, 1979; Tanji & Kurata, 1982) and studies of blood flow studies (Orgogozo & Larsen, 1979; Shibasaki et al. 1993) and movement related evoked potentials (Ikeda et al. 1992) indicate increased activity in this area when simply imagining as well as when performing complex movements of the fingers and hands.
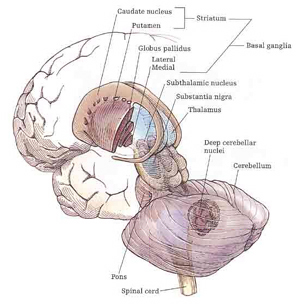
In general, however, and as based on single cell recording (vs functional imaging) activity begins in the SMA well before movements are initiated and prior to activation within the premotor and primary motor areas (Alexander & Crutcher, 1990; Crutcher & Alexander, 1990; Mink, 2007; Mink & Thach, 1991). For example, when anticipating or preparing to make a movement, but prior to the actual movement, neuronal activity will first begin and then dramatically increase in the SMA, followed by activity in the premotor and then the primary motor area, and then the caudate and last of all the putamen and globus pallidus (Alexander & Crutcher, 1990; Mink, 2007; Mink & Thach, 1991).
Presumably the putamen, in conjunction with the caudate, transmits this information to the globus pallidus which in turn projects to the motor thalamus, brainstem reticular formation, as well as to the motor neocortex, thus creating a very elaborate feedback loop (Mink, 2007; Mink & Thach, 1991; Parent & Hazrati 2013) which is also influenced by the anterior cingulate and amygdala.
The SMA, however, is not concerned with fine motor functioning, as this is the province of the primary motor areas. Rather, it is concerned with coordinating gross and bilateral movements of the hands and extremities, including grasping functions (Andres et al., 2011; Passingham, 2007; Stephan et al., 2011). Electrical stimulation of the SMA has produced complex semipurposeful movements, vocalization (Penfield & Jasper, 1954), and postural synergies involving the trunk and extremities bilaterally (Van Buren & Fedio, 1976), but not fine motor movements.
Because of its role in guiding and programming gross body movements, if this structure is damaged, the body may become stiff and movements tend to be slow and incoordinated--a condition also seen with mild lesions (Penfield & Jasper, 1954). Patients demonstrate clumsiness, severe agraphia, impairments of bimanual coordination and difficulty performing rapid or alternating movements (Brinkman, 1981; Gasquione 1993; Goldberg & Bloom 1990; Goldberg et al. 1981; McNabb et al. 1988; Penfield & Jasper, 1954; Stephan et al., 2011; Travis, 1954; Truell et al. 2013; Watson et al. 2007). Patients may walk with short steps and suffer disturbances involving posture, balance, and gait. As detailed above, with extensive and massive injuries patients may become mute (McNabb et al., 1988; Watson et al., 2007) and so stiff and unmoving that they appear to demonstrate all the classic signs of catatonia including gegenhalten and waxy flexibility.
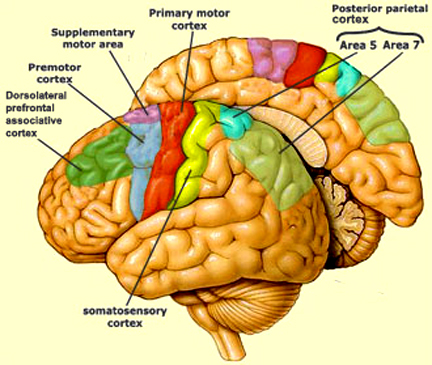
In addition to its interconnections with the SMA and primary motor areas (Jones et. al., 1978; Jones & Powell, 1970), the premotor area receives information directly from the primary and secondary somesthetic and visual (area 17, 18, 19) cortices (Jones & Powell, 1970; Pandya & Kuypers, 1969). It is precipally concerned with the guidance and refinement of movement via the assimilation of sensory information provided by the sensory areas (Godschalk et al. 1981; Porter 1990) and interacts with the basal ganglia, motor thalamus, and SMA so as to achieve these goals (Alexander & Crutcher, 1990; Crutcher & Alexander, 1990; Mink & Thach, 1991; Parent & Hazrati 2013).
As noted, the SMA becomes activated prior to the premotor area, which becomes activate prior to the primary motor area. Hence, whereas neurons in the primary motor region become active during movement, excitation in the premotor cortex precedes cellular activation of the primary region (Weinrich et al. 1984). Moreover, cells in the premotor cortex become activated before movements are even initiated, whereas electrical stimulation of this area induces the illusion of a impending movement (Penfield, 1938). These and other findings suggest that the premotor area may be modulating and exerting controlling and integrative influences on impulses which are to be transmitted to the primary region for expression.
Indeed, the premotor area appears to be highly involved in the programming of various gross and fine motor activities, and becomes highly active during the learning of new motor programs (Passingham, 2007; Porter 1990; Roland et al. 1981). Moreover, electrical stimulation elicits complex patterned movement sequences as well as stereotyped and gross motor responses such as head turning or torsion of the body (Fulton, 1934; Passingham, 1981, 1993).
Unlike the primary area, damage limited to the pre-motor cortex does not result in paralysis but disrupts fine motor functioning and dexterity, including simple activities such as finger tapping (Luria, 1980). With extensive damage fine motor skills are completely lost and phenomena such as the grasp reflex are elicited (Brodal, 1981); i.e. if the patient's hand is stimulated it will invulantarily clasp shut.
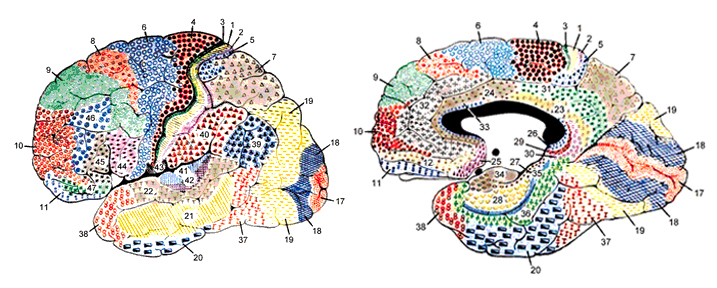
Functionally and structurally the primary motor cortex appears to extend well beyond the confines of the precentral gyrus (area 4) and includes portions of area 6 and the somatosensory (1, 2, 3, 5) regions (Brodal, 1981; Carpenter, 1991). These areas are richly interconnected (Jones et al. 1978; Jones & Powell, 1970; Kuypers & Catsamn-Berrevoets, 1984), the somatosensory projections providing information important in the sensory guidance of movement (Godschalk et al. 1981; Kaas, 1993; Lebedev et al. 2014).
As noted, axonal projections from the motor and somesthetic regions all give rise to the massive cortico-spinal (pyramidal) tract which innervates cranial nerve and sensory and spinal motor neurons (Brodal, 1981; Kuypers & Catsman-Berrevoets, 1984). For these reasons some authors have referred to the somesthetic and motor regions as the sensorimotor cortex.
The primary motor area is concerned with the coordination and expression of gross and fine motor functioning including finger movements (Colebatch et al., 1991; Luria 1980; Rao et al. 2013; Sanes et al., 2013; Shibasaki et al. 1993; Woolsey, 1958), and serves as the final common pathway where impulses organized in other brain areas are transferred for expression, particularly those involving the fingers, thumb, lips, tongue, and hand. Indeed, the region of area 4 that represents the hand is especially prominent and in fact forms a bulge upon the surface (Yousry et al., 2013).
Electrical stimulation of discrete points within the motor cortex while at rest can trigger contractions and movements of tiny muscle groups on the opposite side of the body (Penfield & Jasper, 1954; Penfield & Rasmussen, 1950; Rothwell et al. 1987). Conversely, however, activation of one half of the body can induce bilateral activation of the motor area.

The primary motor area, however, is organized in a dual fashion in regard to the control over the body musculature. There is a one to one correspondence between neurons located in the primary motor area and specific muscles, which in turn makes fine motor functioning possible (Penfield & Jasper, 1954; Penfield & Rasmussen, 1950). However, there are also convergent connections as well, such that a single neuron innervates numerous muscle groups which in turn are innervated by yet other motor neurons (Humphrey, 2007; Shinoda, et al., 1981). In this manner, a neuron innervating a specific group of arm muscles, can also act on other muscles so as to coordinate movement.
Hence, a given muscle is also influenced by a number of motor neurons, such that a single muscle is represented by multiple neurons (Humphrey, 2007; Shinoda, et al., 1981). Multiple representation enables the motor cortex to coordinate a variety of movements, such that wide areas of the motor area become activated during even discrete movements of, for example a single finger (Colebatch et al., 1991; Sanes et al., 2013). Thus, activity is distributed over the motor area even when performing discrete movements.
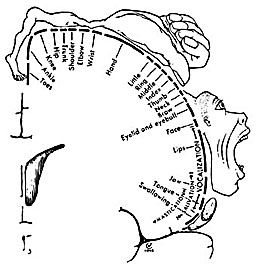
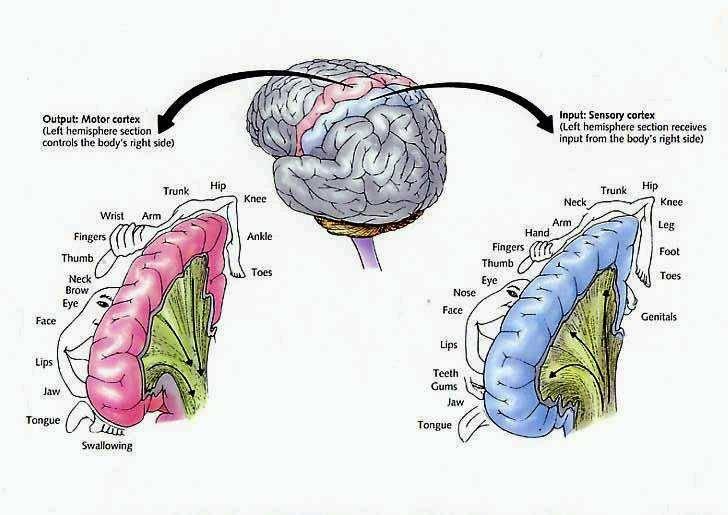
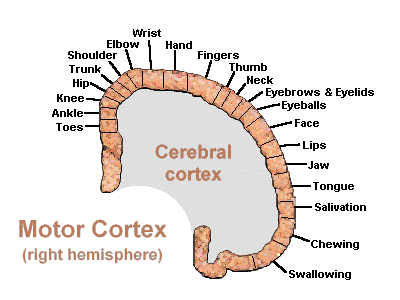
Hence, the entire musculature of the body is neuronally represented in the motor cortex. Nevertheless, since certain muscle groups play a proportionately greater role in the performance of complex (vs simple) movements, a relatively greater number of neurons are involved in their representation. Thus, the hand and fingers have extensive cortical representation (Boroojerdik et al., 2011; Penfield & Jasper, 1954; Penfield & Rasmussen, 1950; Rothwell et al. 1987) whereas a smaller neuronal field is concerned with the elbow. The motor homunculus, therefore, is quite distorted.
Specifically, represented deep in the inferior medial portion of area 4 and moving anterior toward the superior medial frontal lobe are the toes, ankle, knee, hip, anal sphincter and genitals respectively. Circling up and over the superior surface to the lateral convexity are the shoulder, elbow, wrist, and hand, with extensive areas of neocortex devoted to the fingers and thumb, followed by the brow, eyelids, larynx, lips, jaw, and tongue which is located at the most inferior opercular portion of the precentral gyrus. Like the somesthetic neocortex which maintains a double representation of the body surface (e.g. a double body image) the primary motor area contains multiple representations of the body's musculature (Colebatch et al., 1991; Humphrey, 2007; Sanes et al., 2013; Shinoda, et al., 1981).
As noted, based on single cell recording, activity begins in the primary motor area following activation of the premotor and SMA and becomes maximally active at the time of movement. However, as based on single cell recording and functional imaging, neurons in the primary motor area also become active just prior to movement, increase their activity during movement, and will become increasingly active in response to direction, velocity, and the amount of force required to perform the movement (Ashe & Gerogopoulos, 2014; Crutcher & Alexander, 1990; Passingham, 2007). Indeed, this has also been demonstrated with evoked potentials. Activity will begin in the primary motor area prior to movement, and will increase as the time of movement onset approaches, and increases in amplitude during the course of movement (Ikeda & Shibasaki, 1992). In addition, these neurons will increase their activity depending on the amount of force involved in the movement, and in reaction to the direction and velocity of movement (Ashe & Georgopoulus, 2014).
Moreover, the motor area is exceedingly plastic and as indicated above, is capable of learning and memorizing and remembering (e.g. Carpenter et al., 2011). If a single muscle is repeatedly activated and/or as an individual becomes increasingly skilled at performing a certain motor tasks, the cortical territory representing those muscles will significantly increase (Pascual-Leone et al., 2014).
Paralysis.
Damage to the motor areas, or to the descending cortico-spinal tract initially results in a flaccid hemiplegia such that the muscles are completely without tone contralateral to the lesion (Adams & Victor, 2014; Brodal, 1981). If the examiner were to raise and release an effected arm, it would drop in a limp rag doll fashion.
Over the course of the next several days the muscles develop increased tone and there is resistance to passive movements. Reflexes become very brisk and spasticity and hyperreflexia are manifest. With massive lesions extending into the medial regions (where the leg is represented), the leg will become permanently extended and the arm will assume a flexed position (Adams & Victor, 2014; Brodal, 1981). After several weeks or months a very limited capacity to perform gross movements reappears. Fine movements are usually permanently lost (Brodal, 1981).
Exner's area is dependent on Broca's expressive speech area with which it maintains extensive interconnections. In fact, Exner's writing center extends to and appears to become coextensive with Broca's area (Lesser et al. 1984)--which in turn was originally a hand area--at least in primates. Broca's area possibly acts to organize and relay impulses received from the posterior language zones to Exner's area in instances where written expression is desired. Exner's area, in turn, transfers this information to the secondary and primary motor areas for final expression.
Electrical stimulation of this vicinity in awake moving patients has resulted in the arrest of ongoing motor acts, including the capacity to write or perfrom rapid alternating movements of the fingers (Lesser et al., 1984). In some instances, writing and speech arrest were noted.

Agraphia.
Lesions or seizure activity localized to this vicinity lead to deficiencies involving the elementary motoric aspects of writing; i.e. agraphia (Penfield & Roberts, 1959; Ritaccio et al. 1992; Tohgi et al. 2013). Grapheme formation becomes labored, incoordinated, and takes on a very sloppy appearance. Cursive handwriting is usually more disturbed than printing. In cases of well circumscribed lesions, usually there are no gross deficiencies of motor functioning or speech, although mild articulatory disturbances may be observed (e.g. lisping) as well as abnormalties involving fine motor control (Cf Lesser et al., 1984; Goodglass & Kaplan, 2000; Levine & Sweet, 1982.)
In cases of pure (frontal) agraphia, spelling may or may not be affected, whereas with parietal lesions spelling as well as writing is often abnormal. Rather, with left frontal lesions more frequently there are disturbances of grapheme selection such that the patient may seem to have "forgotten" how to form certain letters and/or may misplace or even add unnecessary letters when writing (Hecaen & Albert, 1978; Tohgi et al. 2013). When spelling orally or typing the ability to spell is often better preserved.
Damage localized to this vicinity can be secondary to perinatal trauma, tumors, or vascular abnormalities. Disturbances involving constructional or manipulospatial functioning are not apparent. In fact, one such patient whose damage was secondary to birth injury, although able to write or print only with great difficulty, was able to draw and paint with some professional acumen. However, his ability to copy letters was severely effected. Hence, disturbances secondary to lesions localized to Exner's area limited to abnormalities involving linguistic-symbolic grapheme motor control.

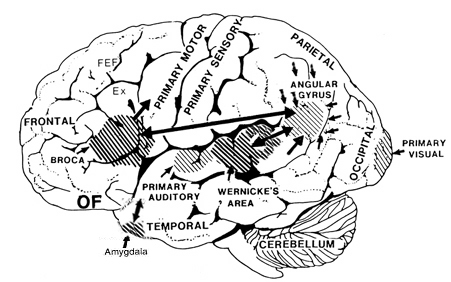
Ex=Exner's Writing Area; FEF=Frontal Eye Fields
The FEF receives projections from the primary and association visual cortices in the occipital lobe (17, 18, 19), the auditory association (22) and multimodal visual association areas (20) in the temporal lobe (Barbas & Mesulam, 1981; Jones et al., 1978; Jones & Powell, 1970), and the somatosensory association area (Crowne, 2003). It also shares interconnections with the caudate, superior colliculus, and oculomotor nucleus (Astruc, 1971; Knuzle & Akert, 1977; Segraves & Goldberg, 1987). Hence, the FEF receives information concerning the auditory, tactual, and visual environment and is multimodally responsive.
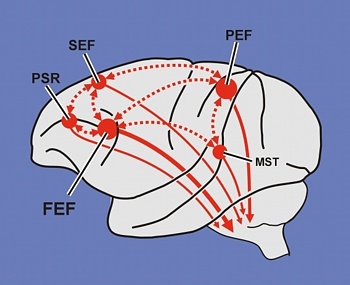
The FEF coordinates and maintaines eye and head movements, gaze shifts, and thus orienting and attentional reactions in response to predominantly visual, but also tactile and auditory stimuli (Barbas & Dubrovsky, 1981; Braun et al., 2006; Denny-Brown, 1966; Gottlieb et al. 2014; Latto & Cowey, 1971ab; MacAvoy, et al., 1991; Pragay et al. 1987; Segraves & Goldberg, 1987; Wagman et al. 1961). It is also involved in focusing attention on certain regions within the visual field, particularly the fovea (Wurtz et al. 1980; Segraves & Goldberg, 1987) as well as in making smooth pursuit movements (Gottlieb et al. 2014) and perhaps guiding eye movements while reading and when writing (e.g. Ritaccio et al. 1992).
In addition to visual tracking and supporting focused visual attention, neurons in the FEF demonstrate anticipatory activity (Gottlieb et al. 2014; Pragay et al. 1987); that is, firing before a response is made. In fact, these neurons will continue to fire at a high rate until the behavior is initiated. Yet other cells begin to fire only when the waiting period becomes prolonged (Pragay et al., 1987). In this regard, neurons in the FEF probably exert a countering influence so that attention does not drift and attention and eye position remains stabilized and focused on the primary retinal target.
Electrical stimulation of the FEF results in complete saccades of the eyes (Barbas & Dubrovsky, 1981; Wagman et al., 1981), as well as pupillary dilation. Moreover, cells in the FEF will fire slectively in response to stationary and moving stimuli, to objects which are within arms reach, as well as to tactual stimuli applied to the hands and/or mouth (Rizzolatti, Scandolara, Matelli, & Gentilucci, 1981ab). In fact, as an object approaches the face and mouth, some of these cells correspondingly increase their rate of activitity (Rizzolatti et al., 1981ab). Hence, cells within the FEF are highly involved in mediating sustained attention and orienting reactions of the head and eyes, maintaining visual fixation and modulating visual scanning, as well as coordinating eye-hand and hand to mouth as well as smooth pursuit eye movements.
Visual Scanning Deficits and Neglect.
Damage to the FEF can cause abnormalities in fixation, decreased sensitivity to stimuli throughout the visual field, slowed visual scanning and searching (Latto & Cowey, 1971ab; Teuber, 1964), inattention and neglect, as well as mislocation of sounds (Denny-Brown, 1966; Welch & Stuteville, 1958). Moreover, some subgroups of "schizophrenics" have been shown to suffer from smooth pursuit and sacadic abnormalities, which, based on neuropsychological tests, have been interpreted as indicative of FEF disturbances (O'Discoll, et al. 2013; Rosse et al. 1993).
With massive lessions, searching and responsiveness become so profoundly reduced that a complete unilateral neglect and failure to attend to any and all stimuli falling to one side of the body results (Heilman & Valenstein, 1972). Like confabulation, neglect is more frequently seen after right cerebral damage (chapter 10).
Even with less severe destruction performance on tasks involving Visual Search is disruped (Teuber, 1964) as is attention to visual detail (Luria, 1980). If shown and asked to describe a complex picture, patients may focus on only one detail, neglecting the remainder of the array, or look about haphazardly (Luria, 1980). It is probable that individuals with certain types of dyslexia suffer from similar abnormalities involving visual search and synthesis.
With large lesions (particularly when involving the right frontal area), they may tend to make leaps of judgment and impulsively guess and describe the meaning of the whole based on the perception of a fragment. For example, focusing only on a "drummer boy" in a battle scene they may describe the picture as being about "musicians" or "a rock band". Hence, such patients may erroneously extrapolate from an isolated detail around which they construct and confabulate a conclusion (Joseph, 2007a).
In the extreme, rather than a true analysis they may produce irrelevant associations as they not only have difficulty analyzing and synthesizing the different components of a visual array (Luria, 1980) but in correcting their impressions via seach and feedback. In these instances the lesion typically extends well beyond the confineds of the FEF.
With extensive damage involving the FEF and convexity, not only confabulation but Capgras syndrome (false identification) and reduplicative paramnesia have been reported--particularly if the damage is bilateral (Alexander, Stuss, & Benson, 1979; Benson, Gardner, & Meadows, 1979; Hecaen, 1964).