Rhawn Gabriel Joseph, Ph.D.
Brain Research Laboratory
BrainMind.com
Blood Supply to the brain
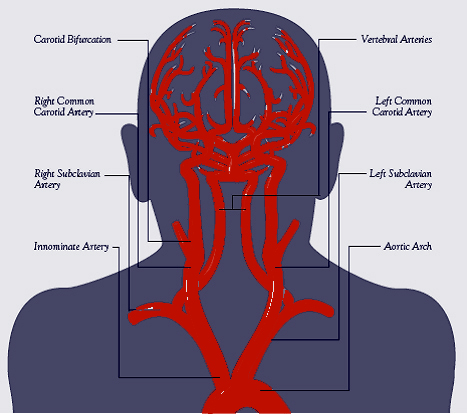
STROKE
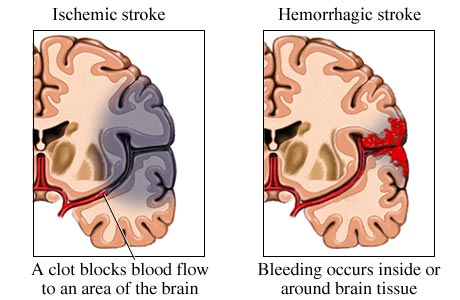
Stroke is the third most common cause of death (after heart disease and cancer) in the U.S. and Europe. Thrombosis and embolism (blood clots) account for approximately 75% of all strokes, whereas about 20% are due to hemorrhage. Up to 70% of all major stroke victims are usually permanently and significantly disabled. Of those who survive, the five year accumulative risk of repeated stroke is about 40% in men and 25% in women.
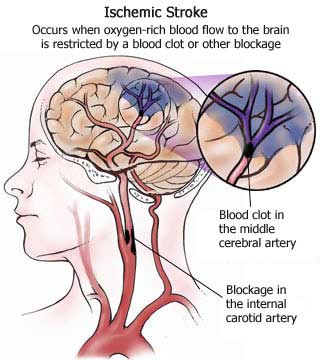
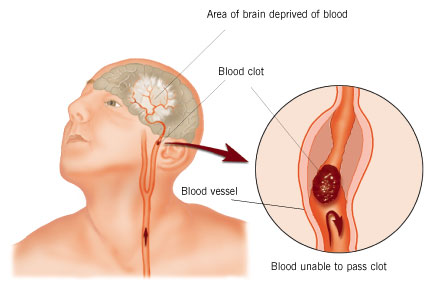
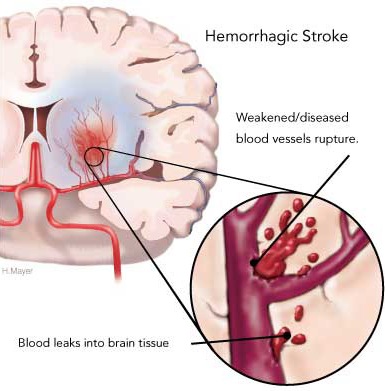
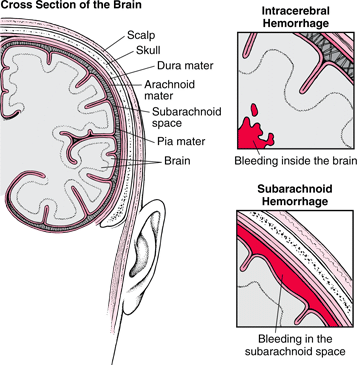
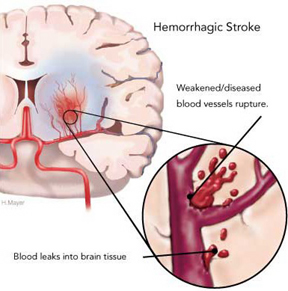
Hemorrhage can occur anywhere throughout the brain and may be due to a number of causes, e.g., head injury, hypertension, rupture of an aneurysm or arterial venous malformation (AVM), the weakening of a segment of the vasculature secondary to emboli or thrombus, or vessel wall necrosis due to occlusion and ischemia (Adams & Victor 2014; Roos et al. 2015).
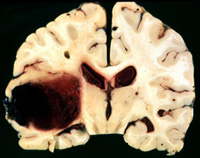
Hemorrhages are frequently classified in terms of gross anatomical location. These include, extradural, subdural, subarachnoid, intercerebral/cerebral, and cerebellar. Extradural and subdural hemorrhages are frequently secondary to head injury, whereas subarachnoid, cerebral and cerebellar hemorrhages are often related to arterial abnormalities. Bleeding from a hemorrhage may be minute and inconsequential, or profuse and extensive such that a large pool of blood rapidly develops. In some cases bleeding may occur at a very slow, albeit continuous pace such that the adverse effects are not detected for days.
SUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage results from any condition which causes blood to leak into the subarachnoid space. Massive subarachnoid hemorrhage is usually due to rupture of an intracranial aneurysm, or bleeding from a cerebral angioma--in either case there is no warning and the onset is quite sudden and abrupt (Adams & Victor, 2004; Roos et al. 2015; Schievink et al. 2015). If the hemorrhage is severe it may lead to immediate coma and death, particularly if there is a buildup of over 20 ml of intraventricular blood (Roos et al. 2015). If moderate, the patient may pass into a semi-stuporous state, and/or become confused and irritable. If minor patients may complain only of severe headache and possibly develop focal deficits after hours or over the course of the first few days or weeks following hemorrhage. Vasospams and rebleeding are very common during the first two weeks.
Subarachnoid hemorrhage has a mortality of over 50% , a third of whom will die immediately or within the first 24 hours (Schievink et al. 2015). Only approximately 25% who survive will make a good recovery (Hijdra et al. 2011).
Subarachnoid hemorrhage occurs most often among individuals above age 50. When it occurs among among younger individuals it is often secondary to congenital vascular abnormaltiies, including angioma, ruptured aneurysms or via the rupture of an AVM on the brain surface (Adams & Victor, 2014; Brown et al. 2014; Schievink et al. 2015; Toole, 1990). Rupture or seepage into the ventricular system is not uncommon (Roos et al. 2015), and CSF is bloody in 90% of cases. Anemic and hemorrhagic infarction may coexist in the same lesion.
Headache and vomiting are immediate common sequela of hemorrhage (as well as cerebrovascular disease) and most patients will complain of severe backache and neck stiffness in the absence of demonstrable focal signs which develop later (Gorelick et al. 2011; Portenoy et al. 1984). Onset is sudden and the intensity is described as severe or violent. These headaches are diffusely distributed over the cranium and/or are localized to the frontal-parietal region (Gorelick et al., 2011). Headaches are usually due the pressure effects of escaping blood which distend, distort, or stretch pain-sensitive intracranial structures. Pain in or behind the eye is often associated with hemorrhage of posterior communicating-carotid aneurysms (Gorelick et al., 2011).
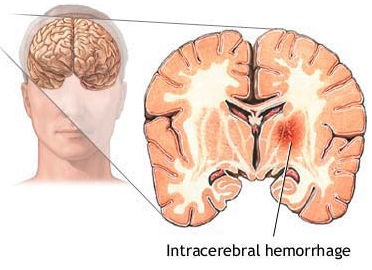
CEREBRAL/INTRACEREBRAL HEMORRHAGE
Cerebral hemorrhage is commonly caused by hypertension and associated rupture aneurysm and degenerative changes in the vessel wall of penetrating arteries which make them suceptible to rupture (Kase, 2011; Schievink et al. 2015). Onset is always sudden.
The rupture may be brought on by mental excitement of physical effort, or may ocur during rest or sleep. Usually the the patient complains of sudden headache and may vomit and become confused and dazed with progressive impairment of consciousness over several minutes or hours time (except in the most mildest of cases). However, it may evolve gradually taking hours or days to become fully developed, and there may be no warning signs (Mayer et al. 2004).
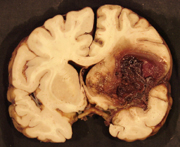
After a large hemorrhage the affected hemisphere becomes larger than the other due to swelling. As the pool of blood increases and begins to clot, surrounding tissues become compressed, the convolutions become flattened and pressure may be exerted against the opposite half of the brain causing damage in this region also. With large hemorrhages, coma and death may ensue due to compression of midline and vital brainstem nuclei (Adams & Victor, 2004; Mayer et al. 2004). If the patient survives and the clot is not surgically removed it is eventually absorbed and replaced by a glial scar. In these instances, however, patients are commonly incapacitated to varying degrees. Cerebral hemorrhages occur most often in the vicinity of the internal capsule, corona radiata, frontal lobe, pons, thalamus and putamen (Adams & Victor, 2004; Kase, 2011).
The neurological deficit is never transitory (good functional recovery being attained by less than 40% of the survivors) and 30 to 75% die within 30 days (Adams & Victor, 2004; Fieschi et al. 2012; Portenoy et al., 2011). Most patients suffer persistent, permanent, and severe neurological abnormalities. Good clinical outcome is related to lower age, the size of hemorrhage, the time period the patient was unconscious, high scores on the Gasgow Coma Scale, and post-operative neurological events (Meier 2014; Portenoy et al. 2011; Tidswell et al. 2015; Toole, 1990).
In over 60% of the cases, intracerebral hemorrhage is related to hypertensive cerebrovascular disease (Mohvr et al. 2008) which makes vessels susceptible to rupture. That is, hypertension can induce degenerative changes and may in fact induce the formation of microaneurysms particularly in the subcortical and perforating arteries (Kase, 2011). However, not all cerebral hemorrhages are due to hypertension.
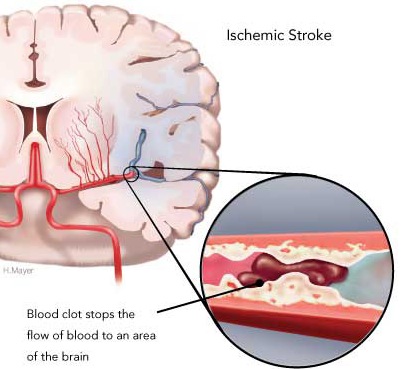
When emboli and other debris build up or occlude the artery, the arterial wall will begin to die and then rupture, and the vessel with hemorrhage out its contents. Approximately 65% of those with a cerebral embolic stroke develop hemorrhagic infarction. Conversely, less than 20% of those with thrombosis will develop hemorrhage (Ott et al. 2011). Similarly, approximately 50% of all hemorrhagic infarcts are associated with embolic strokes (Fisher & Adams, 1951; Hart & Easton, 2011). Hence embolic strokes have a special propensity for hemorrhagic transformation and hemorrhagic infarction nearly always indicates embolism.
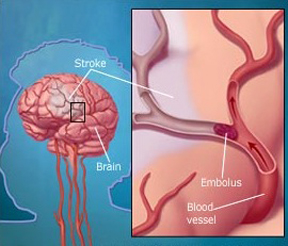
Blood vessels effected by embolism characteristically hemorrhage within 12-48 hours (Cerebral Embolism Study Group, 1984; Hart & Easton, 2011). However, the hemorrhage may take several days to fully develop (Laureno et al. 2011). Hemorrhagic transformation occurs when the emboli disintegrates and/or migrates distally thus allowing reperfusion of the damaged vessel (Fisher & Adams, 1951: Jorgensen & Torvic, 1969). That is, when a vesssel is occluded, it is damaged and weakened, and both the brain and the involved portion of the vessel may become necrotic. When the weakened portion of a previously occluded vessel is subsequently reexposed to the full force of arterial pressure it ruptures and hemorrhages. Fortunately, blood vessels have he capacity to regenerate.
Frequently embolic hemorrhages are asymptomatic (Hakim et al. 2009; Ott, et al., 2011) and supposedly benign since the tissue involved has already been damaged. However, if the hemmorhage is secondary to anticoagulant therapy the bleeding may be more profuse and cause significant neurological deterioration and even death.
Hemorrhage may occur secondary to drug use, anti-coagulant therapy, medication, AVMs, aneurysms, vessel wall necrosis, brain tumors, and various types of arterial pathology, including small vascular malformations, and cerebral amyloid angiopathy.
ISCHEMIA & HEMORRHAGIC INFARCTS
Ischemia not only results in the death of brain cells but necrosis of the local vasculature which is also deprived of metabolic support (Welch & Levine 2014). These vessel wall ischemic structural alterations make them very susceptible to rupture. Indeed, over 40% of those with cerebral ischemic infarction will become hemorrhagic within one to two weeks (Hornig et al. 2011).
These ischemic related hemorrhagic infarcts (HI) are not limited to a single vessel, however. Bleeding may be mutlifocal, particularly if the patient had suffered a large stroke.
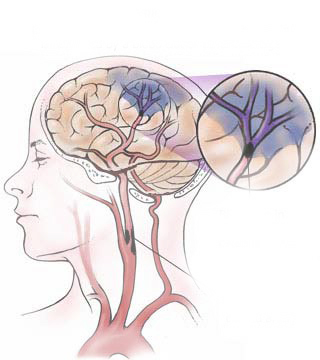
In part, this is also due to the more extensive edema associated with large strokes. When swelling occurs not only is brain tissue compressed but the endothelium of various small vessels is also crushed and damaged. When these vessels are compressed blood flow is prevented which in turn makes these same vessels and their distal extensions more susceptible to rupture when blood flow is reestablished (Garcia et al., 2009). That is, with blockage of a vessel, the distal part of the vessel may become necrotic. Even with mild degrees of edema there is compressions and subsequent damage to various small vessels surrounding the lesion (Welch & Levine 2014).
Hemorrhagic infarcts are not usually associated with chronic hypertension (Hart & Easton, 2011). Frequently, however, they are secondary to the reestablishment of blood flow following occlusion. Because of this when anticoagulants are employed so as to remove the clot, the necrotic vessels rupture and hemorrhage. Hence anticoagulants can increase the risk of secondary hemorrhagic infarction particularly when used following large strokes and/or those accompanied by gross neurological disturbances (Cerebral Embolism Study Group, 2009; Hornig et al., 2011).
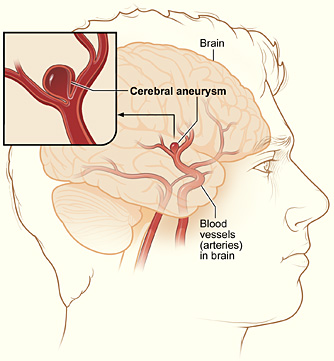
ANEURYSM
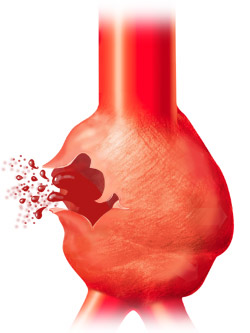
Aneurysm (also called saccular or berry aneurysms) take the form of small, thin walled blisters protruding from the various cerebral arteries (Brown et al. 2014). Aneurysms may be single or multiple, and are presumed to be due to developmental defects; e.g., a congenital weakness at the junction of two arteries. Often they are located at the bifurcations and branches of various arteries, particularly the internal carotid, the middle cerebral, or the junction of the anterior communicating and anterior cerebral arteries (Adams & Victor 2004; Brown et al. 2014).

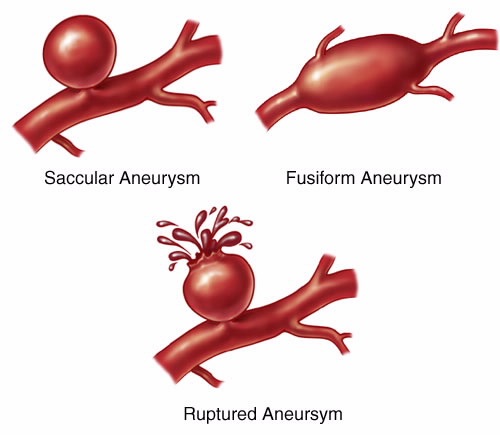
Symptoms secondary to aneurysm (due to rupture or compression) may occur at any age. Prior to rupture they are usually asymptomatic. However, as there is a tendency for them to enlarge over time, which in turn makes them more suceptible to rupture, with increasing age there is increasing risk. The peak incidence of rupture is between 40 and 55 (Adams & Victor, 2004).
Aneurysms may rupture due to sudden increases in blood pressure, while engaged in strenuous activity, during sexual intercourse, or while straining during a bowel movement (Adams & Victor, 2004). One patient I examined suffered a ruptured aneurysm when hyperventilating in his swimming pool so that he could remain submerged for a long time period.


Occasionally, if large and located near the base of the brain they may compress the optic nerves, hypothalamus or pituitary; and if within the cavernous sinus, compress the 3rd, 4th, 6th, or opthalamic division of the 5th nerve. Hence, a variety of visual, endocrine, and emotional alterations may hearld the presence of an aneurysm prior to rupture (Brown et al. 2014).
With large aneurysms, when rupture occurs, blood under high pressure may be forced into the subarachnoid space, and the patient may be striken with an excrutiating generalized headache and/or almost immediately fall unconscious to the ground, or they may suffer a severe headache but remain relatively lucid (Adams & Victor, 2004; Brown et al. 2014; Jorensen et al. 2004; Schievink et al. 2015). If the hemorrhage is confined to the subarachnoid space there are few or no lateralizing signs and no warning symptoms. In some cases, however, patient may complain of headache, transitory unilateral weakness, numbness or tingling, or speech disturbance in the days/weeks preceeding the rupture --due to minor leakage of the aneurysm.
Often those who become unconscious following rupture develop decerebrate rigidity. This is usually due to compression effects (such as herniation) on the brainstem. Persistent deep coma is accompanied by irregular respiration, attacks of extensor rigidity, and finally respiratory arrest and circulatory collapse. In mild cases, consciousness, if lost, may be regained within minutes or hours. However, patients remain drowsiness, confused, and complain of headache and neck stiffness for several days (Jorgenson et al. 2004). Unfortunately, in mild or severe cases there is a tendency for the hemorrhage to reccur.
Cerebral amyloid angiopathy
Amyloid angiopathies are associated with the development of microaneurysms and the occlusion of arteries in the superfical layers of the cerebral cortex (Kase, 2011). Following amyloid occlusion, the arteries are often weakened thus making them susceptible to rupture.
Amyloid angiopathies are often associated with recurrent hemorrhages over a period of months which in turn may lead to the develpment of intracerebral hematomas. Sometimes a head trauma can trigger these later form of hemmorhages.
ARTERIOVENOUS MALFORMATIONS (AVM)
Arteriovenous malformations consist of a tangle of dilated blood vessels and are sometimes referred to as angiomas. It is a developmental abnormality, and may become symptomatic at any age, but most commonly between the ages of 20-30.
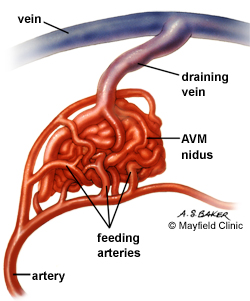
Frequently AVMs form abnormal collateral channels between arteries and veins thus bypassing the capillary system (Brown et al. 2014). When this occurs there may be an abnormal shunting of blood from the arteries to the veins. In consequence underlying brain tissue is not adequately irrigated and may become ischemic depending on the size of the AVM.
AVMs vary in size and tend to be located in the posterior portion of the cerebral hemispheres, near the surface, as well as deep within the brainstem, thalamus, and basal gnaglia. Frequently they are multiple and may be found in a variety of separate locations (Brown et al. 2014; Toole, 1990). They tend to be more common among males.
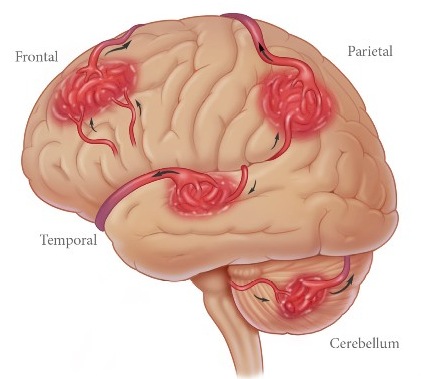
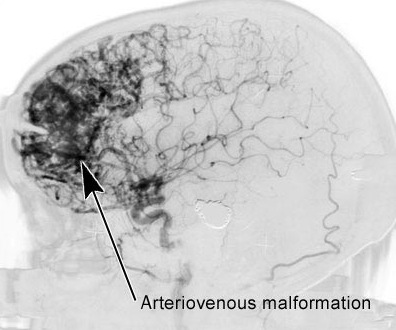
Like aneurysms, AVMs are present from birth and can grow larger and more complicated over time. It has been estimated that AVMs can increase in size by 2.8% per year and can become 56% larger over the span of a 20 year time period (Mendelow et al. 2011). As they increase in size the risk of them becoming symptomatic increases as there is a greater likelihood of collateral shunting.
AVMs are often a cause of intracerebal and subarannoid hemmorhage (Brown et al. 2014; Drake, 2008). When hemorrhage occurs blood may enter the subarachnoid space thus mimicking an aneurysm. However, although the first symptom is usually a hemorrhage, 30% of patients with this disorder may suffer a seizure and 20% headaches or focal neurological symptoms.
Small vascular malformations often become symptomatic during the 30s and 40s and occurs more often among females (Kase, 2011). These often involve the subcortical white matter of the convexity.

Brain tumors can give rise to hemorrhage in a variety of ways, particularly if the tumor is malignant and is richly vascularized. That is, certain tumors have a tendency to become spontaneously necrotic. When this occurs, there supporting vasculature ruptures. However, frequently tumors are transmitted to the brain via the arterial system, whereas others, such as carcinomas, tend to invade the walls of blood vessels. In either instance, by adhering to or penetrating the walls of the blood vessels, tumors can make them more suceptible to rupture (see chapter 34).
DRUG INDUCED HEMORRHAGES
Individuals with possible cerebrovascular abnormalities (such as aneurysm, AVM, or even tumor), and who abuse cocaine or amphetamines are at risk for suffering an intracranial hemorrhagic infarct (Golbe & Merkin, 2011; Lichtenfeld et al. 1984; Schwartz, & Cohen, 1984; Wojak & FLamm, 2011). Presumably these drug induced hemorrhages are due to transient increases in blood pressure and/or vasospasm which in turn act to rupture abnormal vessels.
Internal Capsule Hemorrhage
A patient who suffers an internal capsular hemorrhage is usually unconscious, pulse rate is slow, and there may be Cheyne-Stokes respiration. The head and eyes deviate to the side of the lesion (due to paralysis), and a divergent squint is common (Adams & Victor 2004). The corneal reflex is often lost opposite to the lesion, and may be lost on both sides if coma is profound. Also there is paralysis of the contralateral side of the body, and no response to pin prick on paralyzed side. The limbs are extremely hypotonic such that when lifted by the physician they will fall inertly.
Thalamic Hemorrhage
Thalamic hemorrhages also produce a hemiplegia or paresis due to compression of the adjacent internal capsule. Thalamic syndromes includes severe sensory loss from both deep and cutaneous (contralateral) receptors, and transitory hemiparesis (Adams & Victor 2004). Sensation may return to be replaced by pain and hyperatheia. Sensory deficits usually equal or outstrips the motor weakness, and an expressive aphasia may be present if the hemmorhage involves the left thalamus. The eyes may deviate downwards, with palsies of vertical and lateral gaze, and inequality of pupils with absence of light reaction.
Pontine Hemorrhage.
Hemorrhage of the pontine brainstem is usually fatal. Deep coma ensues in a few minutes and there may be total paralysis, decerebrate rigidity, and pinpoint pupils which do not react to light. The head and eyes are turned toward the side of the hemorrhage if unilateral. Usually, however, even unilateral brainstem hemorrhages exert bilateral brainstem compression (Adams & Victor 2004). Eyeballs are usually fixed.
Cerebellar Hemorrhage.
Hemorrhage involving the cerebellum usually develops over a period of hours (Adams & Victor, 2004). However, there may be sudden onset with occipital headache, vomiting, inability to stand or walk, and loss of consciousness. There is a paresis of conjugate lateral gaze to the side of the hemorrhage, and forced deviation contralaterally. Pupils are small and unequal but react to light. Also, there may be involuntary closure of one eye, as well as ocular bobbing.
Frontal, Parietal, Temporal, & Occipital Hemorrhage.
These hemorrhagic conditions initially give rise to slow or rapidly developing focal syndromes including headache (Jorgensen, et al. 2004). However, as the hemorrhage increases in size, syndromes becomes more global with impaired consciousness.
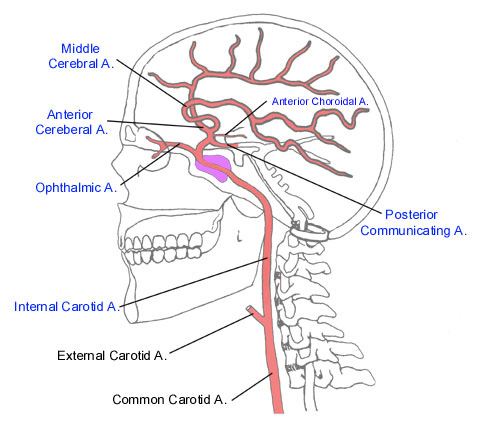
Internal Carotid Artery Syndromes.
Because the cerebral arteries arise from the internal carotid, hemorrhage or occlusion of this artery may be associated with extremely variable as well as widespread symptoms. As the artery becomes increasingly occluded an occasional patient may complain of hearing a disturbing noise (bruits)--a result of turbulence from stenosis of the carotid artery being relayed through the blood supply of the ear.
Stenosis of the internal caroid artery may cause massive infarction involving the anterior 2/3 of all of the cerebral hemisphere, including the basal ganglia, and can lead to death in a few days. Usually it produces a picture resembling middle cerebral artery occlusion. For example, if the left internal carotid is occluded the patient may become hemiplegic and globally aphasic. Because occlusion is accompanied by ischemia, the swelling of cerebral tissue may simulate an intracranial neoplasm. If there is massive edema, tentorial herniation and death may ensue.
Since this artery gives rise to the opthalamic which nourishes the optic nerve and retina, carotid artery insufficiency may produce transient monocular blindness just prior to stroke onset. Unilateral blindness is the only feature distinguishing the carotid syndrome from that produced by obstruction of the middle cerebral artery.
Nevertheless, since the carotid also gives rise to the middle and anterior cerebral arteries the most distal parts of the vascular territories of these vessels will suffer as they are maximally subject to the influences of ischemia. That is, the most distal portion of any occluded artery is the most severely affected. These zones are also the most vulnerable to TIAs--giving rise to weakness or paresthesias of the arm, and if extensive, the face and tongue.
In some cases of internal carotid obstruction, the numerous branches of the external carotid (occipital, superficial temporal and maxillary arteries) can serve as collateral blood supply channels. In these instances, although occluded, symptoms are mild or nonexistant.

Middle Cerebral Artery Syndromes.
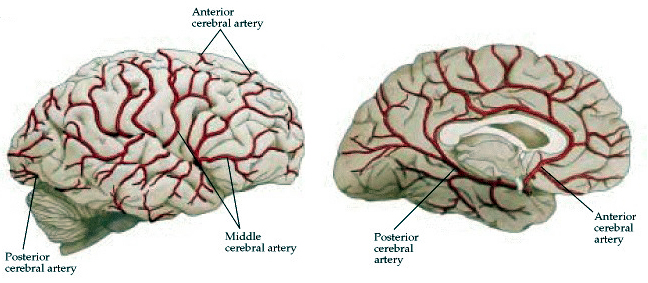
The middle cerebral artery through its branches supplies the lateral part of the cerebral hemispheres, including the neocortex and white matter of the lateral/inferior frontal lobes (and motor areas), the superior and inferior parietal regions, and the superior portion of the temporal lobe (Carpenter 2014; Parent 2015). Its penetrating branches irrigate the putamen, caudate, globus pallidus, posterior limb of internal capsule and the corona radiata. Whether caused by trauma, embolus, or atherosclerosis, contralateral hemiplegia is the hallmark of infarction in the territory supplied by this artery (Adams & Victor, 2014; Absher & Toole 1996). The classic picture of total occlusion is contralateral hemiplegia, with hemiparesis involving the face and arm more than the leg. Sensory deficits may be severe or mild, with disorders of pain perception, touch, vibration, and position (Adams & Victor, 2014; Absher & Toole 1996). This includes extinction of pin-prick or touch, deficits in two-point discrimination, astereognosis, and perhaps dense sensory loss if the parietal lobe is involved. Visual field defects consists of a homonymous hemianopsia or inferior quadrantanopia.
If the left hemisphere is involved global aphasia is present. However, if the right hemisphere is effected, there may occur neglect, denial, and indifference, confabulation and manic-like states, as well as disturbances involving visual-perceptual functioning and the ability to perceive and/or express musical and emotional non-verbal nuances.
It is important to note that only a branch of this artery may be obstructed. If the posterior branch of the middle cerebral artery is occluded, the parietal and inferior parietal lobe may be affected. If the left anterior branch is occluded, the patient may develop expressive aphasia.
ANTERIOR CEREBRAL ARTERY SYNDROMES.
Through its branches the anterior cerebral artery supplies the anterior 3/4 of the medial surface of the hemispheres, including the medial-orbital frontal lobe, the anterior 4/5 of corpus collosum and the head of caudate nucleus and putamen (Carpenter 2014; Parent 2015). Hemorrhage is most often due to rupture of an aneurysm. With total occlusion or hemorrhage patients become obtunded, mute, develop grasp, sucking and snout reflexes, gait apraxia, gegenhalten, waxy flexibility, catatonic-like postures, and are incontinent (Bekson & Cummings 2014; Joseph, 2013a; Starkstein & Robinson 1992). For a further discussion of orbital and medial symptoms see Chapter 19.
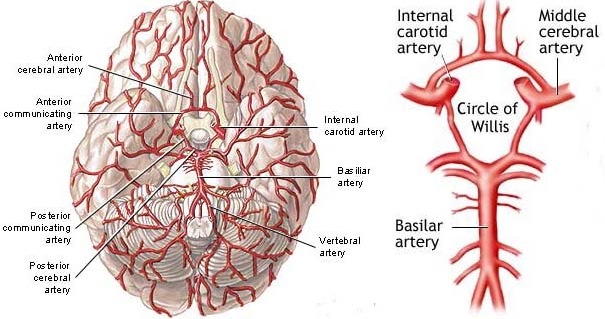
VERTEBRAL-BASILAR ARTERY SYNDROMES
Because there may be collateral circulation from the vertebral-basilar system, obstruction of this artery on one side may not result in symptoms. However, if later there occurs obstruction of the opposite vertebral artery there will result disasterous consequences. Heachade occurs in over 50% of those with ischemia, beginning as a pounding or throbbing behind the orbit or in the temporal region.
A wide variety of visual and vestibular symptoms also occurs when the vertebral-basilar system is compromised including diplopia, transient blindness or blurring, and visual halucinations and illusions. Patients will complain of severe pounding or throbbing headahces (usually localized behind the orbit of the ye or in the temporal region) and will experience episodes of dizziness and vertigo, with or without hearing loss. This is because the auditory and vestibular portions of the ear as well as the brainstem vestibular nuclei are supplied by the verterbral-basilar arterial system. In addition, other brainstem signs include numbness, diplopia, impaired vision in one or both fields, dysarthria, hiccups, and difficulty swallowing (Adams & Victor 2004).
LATERAL MEDULLARY SYNDROME.
The vertebral arteries are the chief arteries of the Medulla, and supply the lower 3/4 of the pyramids, the medial lemniscus, restiform body and posterior-inferior cerebellum (Carpenter 2014; Parent 2015). However, rarely does an infarct involve the pyramids or medial lemniscus. Rather, two prominent sites of lesion are the medial and in particular the lateral medulla.
The classic lateral medullary syndrome is due to an infarction of a wedge shaped area of the lateral medulla and inferior surface of the cerebellum. Onset is associated with severe vertigo, and vomiting may occur (Adams & Victor 2014). Symptoms typically include contralateral impairment of pain and thermal sense; ipsilateral Horners syndrome (miosis, ptosis, decreased sweating); ipsilaterial paralysis of the soft palate, pharnyx and vocal cord (due to involvement of nucleus ambiguous); 9th & 10th nerve dysfunction (hoarseness, dysphagia, ipsilateral paralysis of the palate and vocal cords); loss of balance such that the patient falls to the side ipsilateral to the lesion; loss of taste sensation; hiccup, nystagmus, and nausea. There may also be dysphagia and pain or paresthesia--a sensation of hot water running over the face. There is some degree of cerebellar deficiency, with nystagmus, hypotonia, and incoordination on the side of the lesion.
MEDIAL MEDULLARY SYNDROME.
This condition is a less common consequence of vertebral artery occulsion. Nevertheless, this causes contralateral hemiparesis, ipsilateral paralysis of the tongue due to 12th nerve involvement near the zone of infarction, and loss of position and vibratory perception with sparing of pain and temperature sensation. Vertical nystagmus implies a lesion at the pontomedullary junction, and a paralysis of gaze suggests a lesion above the medulla (in pons or midbrain).
Vertebral Artery Trauma.
The vertebral arteries are vulnerable to compressions or extremes in extension such as can occur during whiplash (Toole, 1990). Moreover, if the patient suffers from osteoarthritis of the cervical spine the space through which the vertebrals pass may become narrowed thus subjecting these arteries to pinching or other stresses with movement of the head. Hence, these patients may be subject to repeated instances of vertebral insufficiency.
BASILAR ARTERY SYNDROMES.
The basilar artery supplies the pons (Carpenter 2014; Parent 2015). Infarcts characteristically involve the corticospinal and corticobullar tracts, cerebellum, cerebellar peduncles, medial/lateral lemniscus, spinothalamic tracts, and 3-8th nerves (Adams & Victor 2004). Hence, the outstanding features of basilar artery infarct are a constellation of cranial nerve signs of both the sensory and motor varieties, cerebellar symptoms, bilaterial pyramidal tract signs, including ataxia, dysarthria, hemiplegia, and disturbances involving occular movement and paralysis of gaze. If convergence is preserved the lesion is in the middle of the inferior pons. If there is no convergence the lesion is in the superior pons (medial longitudinal fasciculus).
Often patients become comotose because of ischemic compression effects on the reticular formation. Early signs of increasing occlusion or hemorrhage may occur in different combinations: somnolence, visual hallucinations, disorders of ocular movement, delerium, and korsakoffs amnesic defects.
Medial pontine lesions result in ataxia of the ipsilaterial limbs, a contralaterial paresis of the face, arm, leg, and variable sensory loss. If Horners syndrome is present or if cranial nerve 5, 7, or 8 are effected, the lesion is laterally placed. If paralysis of cranial nerve 3, 4, 6, or 7, or involvement of the pyramidal tract occurs, the lesion is probably medially placed.
In general, infarct of the medial pons results in (variably) vertigo, nausea, vomiting, nystagmus, ipsilateral ataxia, ipsilateral Horners syndrome, paresis of conjugate gaze, contralateral loss of pain and temperture sensation over the face, arm, trunk and leg, and slurred speech.
LOCKED-IN SYNDROME.
If occulsion spares the upper brainstem but involves the midpontine level, the "locked-in" syndrome may occur: the patient is alert, conscious of his surroundings, able to see and hear, but completely paralyzed and unable to communicate except through eye blinks. However, because the respiratory, vasomotor and thermoregulatory centers and pathways are affected, there might be associated abnormalities. Unfortunately, because patients can only interact via eye blinks it is extremely difficulty to ascertain how impaired the patient is in actuality.
POSTERIOR CEREBRAL ARTERY SYNDROMES.
The posterior cerebral arteries are a branch of the vertebral-basilar system and supplies via its own branches the inferomedial temporal, and medial occipital regions including areas 17, 18, 19, and the posterior hippocampus (Carpenter 2014; Parent 2015). Via deep penetrating branches it also supplies the thalamus, subthalamic nuclei, substantia nigra, midbrain, and pineal body.
Thalamic infarct or hemorrhage includes severe sensory loss and possible receptive aphasia. However, sensation may return to be replaced by pain and hyperathesia. Midbrain infarcts includes Weber syndrome (oculomotor palsy with contralateral hemiplegia) paralysis of vertical gaze, and stupor or coma (Adams & Victor 2004).
Cortical syndromes include anomia, alexia apraxia, prosopagnosia (depending on which hemisphere is involved) and related temporal-occipital, parietal-occipital disturbances. Involvement of the optic radiations or infarction of the calcarine cortex causes visual field impairments, such as scotoma and homonymous hemianopias, particularly of the upper quadrants. With bilateral occlusion the patient will become cortically blind.
Ischemic lesions in the occipital area may cause variations in the nature and form of visual field defects experienced by the patient from day to day--occasionally leading to an erroneous diagnosis of hysteria. TIAs in this vicinity are also reflected by fleeting visual field defects of a hemianopic distribution.
Distal occlusion also causes medial temporal infarction, with hippocampal involvement and memory loss. Transient global amnensia is also sometimes a consequence of transient occlusion of the posterior cerebral arteries.
CEREBELLAR ARTERY SYNDROME.
The cerebellar artery is also a branch of the vertebral-basilar system and it supplies portions of the pons as well as the cerebellum (Carpenter 2014; Parent 2015). Deafness and tinnitus may occur with lateral inferior pontine lesions due to occlusion of the anterior-inferior cerebellar artery. Infraction in this area may also cause vertigo, nystagmus, ipsilateral ataxia of limbs, and a contralateral hemiparesis with no sensory defect. With mild lesions the patient may walk with an unsteady wide based gait. With more severe lesions patients may have extreme difficulty walking or even looking to the side of the lesion, although the pupils are normal and reactive to light.
DIFFERENTIAL DIAGNOSIS: CAROTID VS. VERTEBRAL SYSTEM.
The development of deficits such as aphasia, agnosia, apraxia, constructional and manipulo-spatial deficits, emotional abnormalities and/or delusions indicate a carotid circulatory disturbance. Dizziness, diplopia, ataxia, nystagmus, cranial nerve signs, internuclear opthalmoplegia, dissociated sensory loss, and/or bilateral abnormalities are hallmarks of a brainstem lesion within the vertebral-basilar territory.
The major determinants for short-term mortality are intraventicular hemorrhage, pulmonary edeman, impaired consciousness, leg weakneness, respiratory diease and increasing age (Chambers et al. 2011; Lanska & Hoffman, 2013; Roos et al. 2015; Schievink et al. 2015)-with level of consciousnes following stroke being the single most important predictor of short-term survival (Chambers et al. 2011). The major determinants for long term mortality are low activity level, advanced age, male sex, heart disease and hypertension. However, those who suffer intraventricular hemorrhagic infarcts have a higher mortality rate than those with infarcts due to other causes (Chambers, et al. 2011; Roos et al. 2015; Schievink et al. 2015). As noted in chapter 10, those with right hemisphere damage tend to have poorer outcomes as well as higher mortality rates. In particular right parietal lobule infarcts are associated with very poor outcomes (Valdimarsson et al. 1982).
Hyperglycemia and diabetes are also associated with poor neurological recovery, and higher short-term mortality as well as increasing the risk for stroke in general. This is because diabetes and hyperglycemia both accentuate ischemic damage (Bruno et al., 2013; Pulsinelli et al. 2009; Woo et al. 2012). Hyperglycemia also appears to have a negative effect on energy metabolism due to the generation of severe lactic acidosis (Rehncrona et al. 1980) --factors which act to retard neuronal recovery.
Some authors have argued that luxury perfusion and increased cerebral blood flow (CBF) within an infarcted cite is often indicative of a good prognosis, whereas low CBF is a bad prognosis (Olsen et al. 1981). Presumably increased flow acts to nourish damaged tissue. Other studies however, indiate that initial CBF levels are not predictive of clinical outcome (Burke et al. 2011). Apparently this is because once damage occurs during the initial period of ischemia, these cells cannot be salvaged (Heiss & Rosner, 2009).
Hence, blood flow increases only when undamaged neurons return to a functionally active state (Burke et al., 2011) rather than acting to rejuvinate injured tissue. In fact, hyperperfusion may endanger neuronal recovery (Mies et al. 2009). On the otherhand oxygen metabolism seems to correlates better with clinical status and functional recovery than does blood flow (Wise et al., 2009).
Recovery is often greatest during the first 30 days after stroke (Dombovy, et al., 2011; Lind, 1982), but continues up to 6 months in some patients (Wade & Hewer, 2011). It has been estimated that although about 60% of stroke patients are able to achieve total independence in activities of daily living (Meier & Strauman 2014; Wade & Hewer, 2011) only approximately 10 to 30% of initial survivors return to their jobs without gross or obvious disability (Bekson & Cummings 2014). Depending on the nature of the stroke, about 40% demonstate mild disability, 40% are severely disabled, and 10% require institutionalization (Stallones et al, 1972; Absher & Tool, 1996).
SUMMARY
Stroke is the third most common cause of death (after heart disease and cancer) in the U.S. and Europe. Thrombosis and embolism account for approximately 75% of all strokes, whereas about 20% are due to hemorrhage. Up to 70% of all major stroke victims are usually permanently and significantly disabled (Bekson & Cummings 2014), with subarachnoid hemorrhages often resulting in sudden death (Schievink et al. 2015). Of those who survive, the five year accumulative risk of repeated stroke is about 40% in men and 25% in women, with recent evidence indicating that women are increasingly at risk for stroke and repeated stroke (secondary to heart disease) due in part to the increased incidence of female smoking.














