Rhawn Gabriel Joseph, Ph.D.
Brain Research Laboratory
BrainMind.com
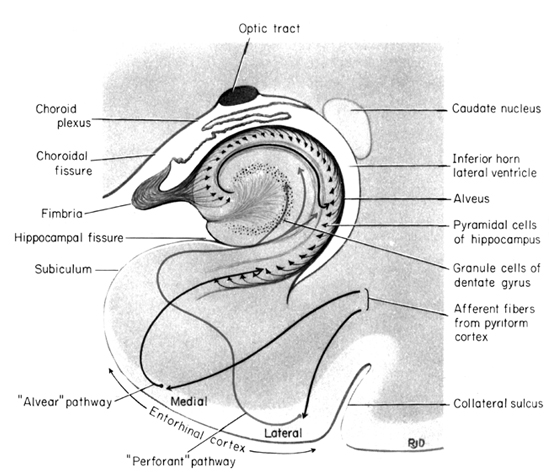
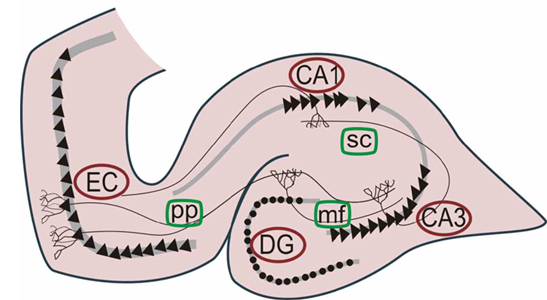
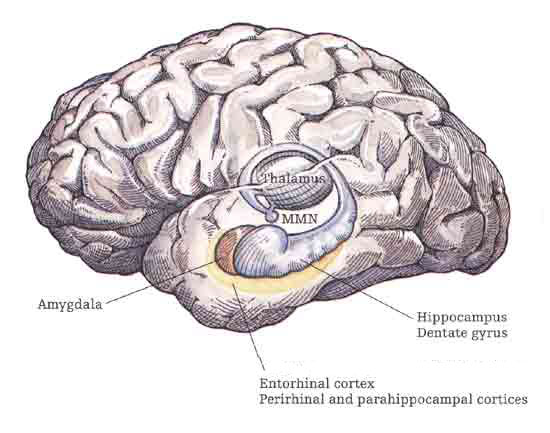
The hippocampus is unique in that unlike the amygdala and other structures, almost all of its input from the neocortex is relayed via the overlying entorhinal cortex--a five layered mesocortex. As is well known, the hippocampus is exceedingly important in memory, acting to place various short-term memories into long-term storage.
Presumably the hippocampus encodes new information during the storage and consolidation (long-term storage) phase, and assists in the gating of afferent streams of information destined for the neocortex by filtering or suppressing irrelevant sense data which may interfere with memory consolidation. Moreover, it is believed that via the development of long-term potentiation the hippocampus is able to track information as it is stored in the neocortex, and to form conjunctions between synapses and different brain regions which process and store associated memories.

HIPPOCAMPUS
Memory & Attention
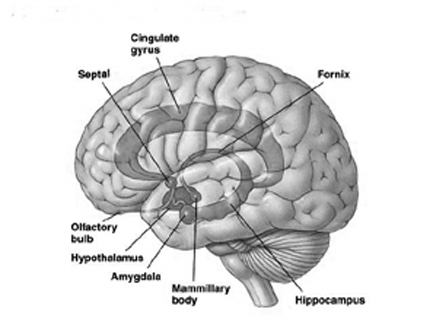
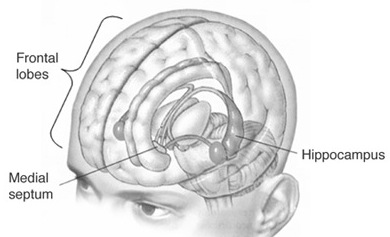
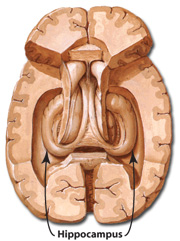
The hippocampus (Ammon's Horn" or the "sea horse") is an elongated structure located within the inferior medial wall of the temporal lobe (posterior to the amygdala) and surrounds, in part, the lateral ventricle. In humans it consists of an anterior and posterior region and depending on the angle at which it is viewed, could be construed as shaped somewhat like an old fashion telephone receiver, or a "sea horse."
HIPPOCAMPAL AROUSAL, ATTENTION & INHIBITORY INFLUENCES
Various authors have assigned the hippocampus a major role in information processing, including memory, new learning, cognitive mapping of the environment, voluntary movement toward a goal, as well as attention, behavioral arousal, and orienting reactions (Douglas, 1967; Eichenbaum et al. 2014; Enbert & Bonhoeffer, 2009; Frisk & Milner, 1990; Grastayan et al., 1959; Green & Arduini, 1954; Isaacson, 1982; Milner, 1966, 1970, 1971; Nishitani, et al., 2009; Olton et al. 1978; Routtenberg, 1968; Squire, 1992; Victor & Agamanolis, 1990; Xu et al., 2012). For example, hippocampal cells greatly alter their activity in response to certain spatial correlates, particularly as an animal moves about in its environment (Nadel, 1991; O'Keefe, 1976; Olton et al., 1978; Wilson & McNaughton, 2013). It also developes slow wave theta activity during arousal (Green & Arduini, 1954) or when presented with noxious or novel stimuli (Adey et al.1960)--at least in non-humans.
However, few studies have implicated this nucleus as important in emotional functioning per se, although responses such as "anxiety" or "bewilderment" have been observed when directly electrically stimulated (Kaada et al. 1953). Indeed, in response to persistent and repeated instances of stress and unpleasant emotional arousal, the hippocampus appears to cease to participate in cognitive, emotional, or memory processing (chapter 30). Thus the role of the hippocampus in emotion is quite minimal.
AROUSAL
Hippocampal-neocortical interactions. Desynchronization of the cortical EEG is associated with high levels of arousal and information input. As the level of input increases, the greater is the level of cortical arousal (Como et al. 1979; Joseph et al. 1981; Joseph, 2012b, 2009d). However, when arousal levels become too great, efficienty in information processing, memory, new learning, and attention become compromised as the brain becomes overwhelmed (Joseph, 2012b, 2009d; Joseph et al., 1981; Lupien & McEwen, 2010; Sapolsky, 2012).
When the neocortex becomes desynchronised (indicating cortical arousal), the hippocampus often (but not always) developes slow wave theta activity (Grastyan et al., 1959; Green & Arduni, 1954) such that it appears to be functioning at a much lower level of arousal--as demonstrated in non-humans. Conversely, when cortical arousal is reduced to a low level (indicated by EEG synchrony), the hippocampal EEG often becomes desynchronized.
These findings suggest when the neocortex is highly stimulated the hippocampus, in order to monitor what is being received and processed, functions at a level much lower in order not to become overwhelmed. When the neocortex is not highly aroused, the hippocampus presumably compensates by increasing its own level of arousal so as to tune in to information that is being processed at a low level of intensity.
Hence, in situations where both the cortex and the hippocampus become desynchronized, there results distractability and hyperresponsiveness such that the subject becomes overwhelmed, confused, and may orient to and approach several stimuli (Grastyan et al., 1959). Attention, learning, and memory functioning are decreased. Situations such as this sometimes also occur when individuals are highly anxious or repetitively emotionally or physically traumatized (see chapter 30).
There is also evidence to suggest that the hippocampus may act so as to reduce extremes in cortical arousal. For example, whereas stimulation of the reticular activating system augments cortical arousal and EEG evoked potentials, hippocampal stimulation reduces or inhibits these potentials such that cortical responsiveness and arousal is dampened (Feldman, 1962; Redding, 1967). On the otherhard, if cortical arousal is at a low level, hippocampal stimulation often results in an augmentation of the cortical evoked potential (Redding, 1967).
It is thus likely that the hippocampus may act to influence information reception and storage at the neocortical level as well as possibly reduce extremes in cortical arousal (be they too low or high) perhaps by activating inhibitory circuits in the dorsal medial nucleus, thus ensuring that the neocortex is not over or underwhelmed when engaged in the reception and processing of information. This is an important attribute since very high or very low states of excitation are incompatible with alertness and selective attention as well as the ability to learn and retain information (Joseph et al. 1981; Lupien & McEwen, 2010; Sapolsky, 2012).

In many ways, the hippocampus appears to act in concert with the medial hypothalamus and septal nuclei (with which it maintains rich interconnections) so as to also prevent extremes in emotional arousal and thus maintain a state of quiet alertness (or quiescence). Moreover, similar to the results following stimulation of the medial hypothalamus, it has been reported that the subjective components of aversive emotion in humans is correlated with electrophysiological alternations in the hippocampus and septal area (Heath, 1976).
The hippocampus also appears to be heavily involved in the modulation of reactions to frustrations or mild punishment (Gray, 1970, 1990), particularly in regard to single trial but not multiple trial learning. For example, the hippocampus responds with trains of slow theta waves when presented with noxious stimuli but habituates or ceases to respond with repeated presentation. It is likely, however, that these physiological responses are secondary to activity within the amygdala and hypothalamus which then effects hippocampal functioning.
ATTENTION & INHIBITION
The hippocampus participates in the elicitation of orienting reactions and the maintainance of an aroused state of attention (Foreman & Stevens, 2007; Grastayan et al., 1959; Green & Arduini, 1954; Nishitani, et al., 2009; Routtenberg, 1968). When exposed to novel stimuli or when engaged in active searching of the environment, hippocampal theta appears (Adey, et al. 1960). However, with repeated presentations of a novel stimulus the hippocampus habituates and theta disappears (Adey et al. 1960). Thus, as information is attended to, recognized, and presumably learned and/or stored in memory, hippocampal participation diminishes. Theta also appears during the early stages of learning as well as when engaged in selective attention and the making of discriminant responses (Grastyan et al. 1959).
When the hippocampus is damaged or destroyed, animals have great difficulty inhibiting behavioral responsiveness or shifting attention. For example, Clark and Issacson (1965) found that animals with hippocampal lesions could not learn to wait 20 seconds between bar presses if first trained to respond to a continous schedule. There is an inability to switch from a continous to a discontinous pattern, such that a marked degree of perseveration and inability to change sets or inhibit a pattern of behavior once initiated occurs (Douglas, 1967; Ellen, et al. 1964). Habituation is largely abolished and the ability to think or respond divergently is disrupted. Disinhibition due to hippocampal damage can even prevent the learning of a passive avoidance task, such as simple ceasing to move (Kimura, 1958).
Hence, when coupled with the evidence presented above, it appears that the hippocampus acts to possibly selectively enhance or diminish areas of neural excitation which in turn allows for differential selective attention and differential responding, as well as the storage and consolidation of information into long term memory. When damaged, the ability to shift from one set of perceptions to another, or to change behavioral patterns is disrupted and the organism becomes overwhelmed by a particular mode of input. Learning, memory, as well as attention, are greatly compromised.
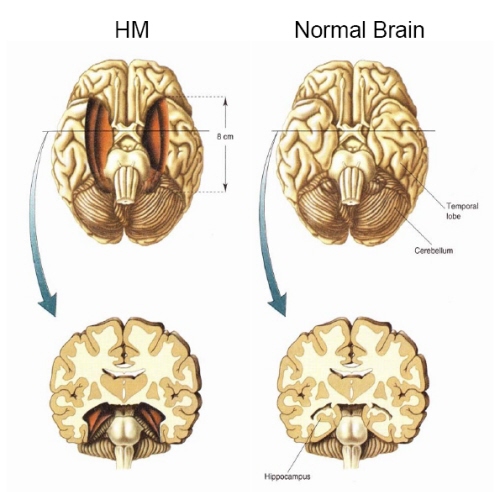
H.M., although without memory for new (non-motor) information, has adequate intelligence, is painfully aware of his deficit and constantly apologizes for his problem. "Right now, I'm wondering" he once said, "Have I done or said anything amiss?" You see, at this moment everything looks clear to me, but what happened just before? That's what worries me. It's like waking from a dream. I just don't remember...Every day is alone in itself, whatever enjoyment I've had, and whatever sorrow I've had...I just don't remember" (Blakemore, 1977, p.96).
Presumably the hippocampus acts to protect memory and the encoding of new information during the storage and consolidation phase via the gating of afferent streams of information and the filtering/exclusion (or dampening) of irrelevant and interfering stimuli. When the hippocampus is damaged there results input overload, the neuroaxis is overwhelmed by neural noise, and the consolidation phase of memory is disrupted such that relevant information is not properly stored or even attented to. Consequently, the ability to form associations (e.g. between stimulus and response) or to alter preexisting schemas (such as occurs during learning) is attenuated (Douglas, 1967).
HIPPOCAMPAL & SEPTAL INTERACTIONS
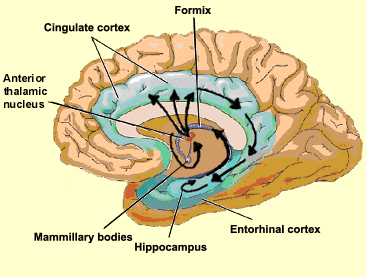
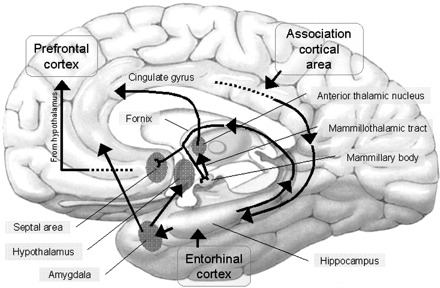
The septal nuclei consists of medial and lateral nuclei, and can be further subdivided into several nuclear components (Ariens Kappers et al., 1936; Swanson & Cowan, 1979), such as the nucleus of the diagonal band of Broca. The septal nuclei is an evolutionary derivative of the hippocampus and the hypothalamus, and in the human brain is richly interconnected with both structures including the amygdala, and the substantia innomminata (SI) which is a major memory center, and which manufactures ACh--a transmitter directly implicated in memory (Gage et al., 1983; Olton, 1990). Andy and Stephan (1968) and Swanson and Cowan (1979) considered the bed nucleus of the stria terminals (which gives rise to a major pathway linking the septal nuclei, and amygdala and hypothalamus) as part of the septal nuclei, whereas others (Gloor, 2010) consider it to be part of the "extended amygdala." Likewise, some consider the nucleus accumbens as part of the septal nuclei, and others consider it part of the "extended amygdala;" i.e. the limbic striatum.
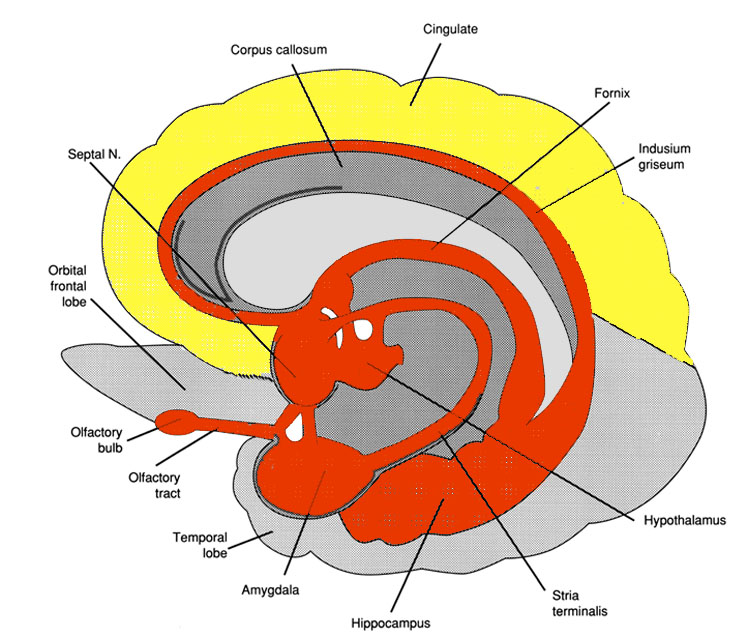


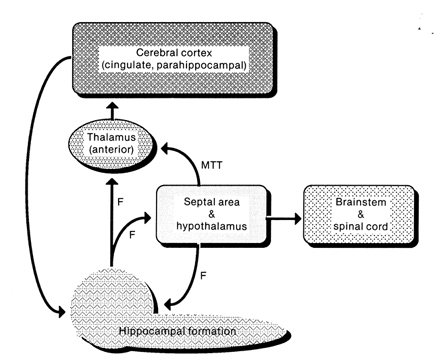
The septal nucleus in part regulate hippocampal memory-related activity not only by stimulating ACh and other neurotransmitter production (Gage et al., 1983, 1986), but as it provides excitatory input and inhibitory-GABAnergic-- especially from the medial septal nuclei which in general exerts inhibitory influences not only on the hippocampus but the amygdala and hypothalamus. In general, it is supposed that the excitatory-inhibitory influences on the hippocampus (like those on the amygdala and hypothalamus) serve to modulate activity and prevent extremes in arousal (Joseph, 1992a, 2012b, 2009d). This is accomplished in part not only through the interconnections maintained with the amygdala, hypothalamus and entorhinal cortex, but the brainstem reticular formation (Petsche et al., 1965)--with which the hippocampus is also connected directly and via the entorhinal cortex.
Septal influences on hippocampal/entorhinal arousal is also indicated by fluctuations in rhythmic slow activity (theta), which is generated by both the hippocampus and entorhinal cortex (Alonso & Garcia-Austt, 2007). As detailed in chapter 14, theta is an indication of hippocampal arousal (Green & Arduini, 1954; Petsche et al., 1965; Vanderwolf, 1992) and is associated with learning and memory (O'Keefe & Nadel, 1978). Theta is a robust electrophysiological phenomenon which has been found in the hippocampus of most species studied, including monkeys (Stewart & Fxx, 1990) and humans (Sano et al., 1970); though in primates it seems to differ from the theta rhythm of non-primates (see Gloor, 2010).
O'Keefe and Nadel (1978) believe that theta plays an important role in creating the spatial maps that are maintained by hippocampal "place" neurons; i.e. pyramidal neurons which are attuned to specific environmental features and landmarks and the animals place in that environment as they move about. Moreover, long term potentiation (LTP) which is associated with learning and memory, is generated in those neurons demonstrating theta or activity that is at the "theta frequency" (Staubli & Lynch, 2007).
Neurons of the septal nucleus which innervate the hippocampus fluctuate in activity in parallel with changes in the theta rhythm (Petsche et al., 1965), whereas septal lesions abolish hippocampal theta (Green & Arduini, 1954). It has long been believed that septal neurons act as an interface between the reticular formation and the hippocampus (Petsche et al., 1965) and in conjunction with its connections with the amygdala and hypothalamus, therefore modulate hippocampal arousal as well as learning and memory.
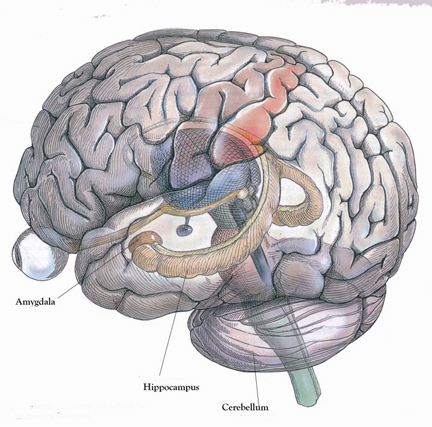
HIPPOCAMPAL & AMYGDALOID INTERACTIONS: MEMORY
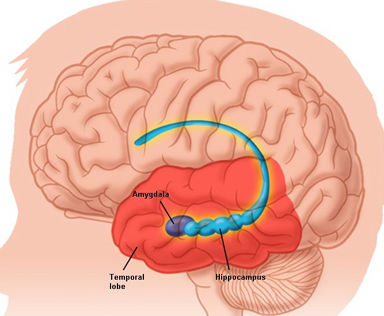
Moreover, there is considerable evidence which strongly suggests that the hippocampus plays an interdependent role with the amygdala in regard to memory (Gloor 1992, 2010; Halgren 1992; Kesner & Andrus, 1982; Mishkin, 1978; Murray 1992; Sarter & Markowitsch, 2005); particularly in that they are richly interconnected, merge at the uncus, and exert mutual excitatory influences on one another. For example, it appears that the amygdala is responsible for storing the emotional aspects and personal reactions to events in memory, whereas the hippocampus acts to store the cognitive, visual, and contextual variables (chapter 14) whereas that the amygdala activates the hippocampus by providing excitatory input (Gloor, 1955, 2010).
Specifically, the amygdala plays a particularly important role in memory and learning when activities are related to reward and emotional arousal (Gaffan 1992; Gloor 1992, 2010; Halgren 1992; LeDoux 1992, 2012; Kesner 1992; Rolls 1992; Sarter & Markowitsch, 2005). Thus, if some event is associated with positive or negative emotional states it is more likely to be learned and remembered.
The amygdala becomes particularly active when recalling personal and emotional memories (Halgren, 1992; Heath, 1964; Penfield & Perot, 1963), and in response to cognitive and context determined stimuli regardless of their specific emotional qualities (Halgren, 1992). However, once these emotional memories are formed, it sometimes requires the specific emotional or associated visual context to trigger their recall (Rolls, 1992; Halgren, 1992). If those cues are not provided or ceased to be available, the original memory may not be triggered and may appear to be forgotten or repressed. However, even emotional context can trigger memory (see also Halgren, 1992) in the absence of specific cognitive cues.
Similarly, it is also possible for emotional and non-emotional memories to be activated in the absence of active search and retrieval, and thus without hippocampal or frontal lobe participation. Recognition memory which may be triggered by contextual or emotional cues. Indeed, there are a small group of neurons in the amygdala, as well as a larger group in the inferior temporal lobe which are involved in recognition memory (Murray, 1992; Rolls, 1992). Because of amygdaloid sensitivity to visual and emotional cues, even long forgotten memories may be evoked via recognition, even when search and retrieval repeatedly fail to activate the relevant memory store.
According to Gloor (1992), "a perceptual experience similar to a previous one can through activation of the isocortical population involved in the original experience recreate the entire matrix which corresponds to it and call forth the memory of the original event and an appropriate affective response through the activation of amygdaloid neurons." This can occur "at a relatively non-cognitive (affective) level, and thus lead to full or partial recall of the original perceptual message associated with the appropriate affect."
In this regard, it appears that the amygdala is responsible for emotional memory formation whereas the hippocampus is concerned with storing verbal-visual-spatial and contextual details in memory. Thus, in rats and primates damage to the hippocampus can impair retention of context, and contextual fear conditioning, but it has no effect on the retention of the fear itself or the fear reaction to the original cue (Kim & Fanselow 1992; Phillips & LeDoux 1992, 2012; Rudy & Morledge 2014). In these instances, fear-memory is retained due to preservation of the amygdala. However, when both the amygdala and hippocampus are damaged, striking and profound disturbances in memory functioning result (Kesner & Andrus, 1982; Mishkin, 1978).
Therefore, the role of the amygdala in memory and learning seems to involve activities related to reward, orientation, and attention, as well as emotional arousal and social-emotional recognition (Gloor, 1992, 2010; Rolls, 1992; Sarter & Markowitsch, 2005). If some event is associated with positive or negative emotional states it is more likely to be learned and remembered. That is, reward increases the probability of attention being paid to a particular stimulus or consequence as a function of its association with reinforcement (Gaffan 1992; Douglas, 1967; Kesner & Andrus, 1982).
Moreover, the amygdala appears to reinforce and maintain hippocampal activity via the identification of motivationally significant information and the generation of pleasurable rewards (through action on the lateral hypothalamus). However, the amygdala and hippocampus act differentially in regard to the effects of positive vs. negative reinforcement on learning and memory, particularly when highly stressed or repetitively aroused in a negative fashion. For example, whereas the hippocampus produces theta in response to noxious stimuli the amygdala increases its activity following the reception of a reward (Norton, 1970).
In contract, right temporal destruction typically produces deficits involving visual memory, such as the learning and recall of geometic patterns, visual or tactile mazes, locations, objects, emotional sounds, or human faces (Corkin, 1965; Milner, 1965; Nunn et al., 2009; Kimura, 1963). Right sided damage also disrupts the ability to recognize (via recall) olfactory stimuli (Rausch et al. 1977), or recall emotional passages or personal memories (Cimino et al., 1991; Wechsler, 1973).

AMYGDALA & PLEASURE
The amygdala maintains a functionally interdependent relationship with the hypothalamus in regard to emotional, sexual, autonomic, consumatory and motivational concerns. It is able to modulate and even control rudimentary emotional forces governed by the hypothalamic nucleus. However, the amygdala also acts at the behest of hypothalamically induced drives. For example, if certain nutritional requirements need to be meet, the hypothalamus signals the amygdala which then surveys the external environment for something good to eat (Joseph, 1982, 1992a). On the other hard, if the amygdala via environmental surveillance were to discover a potentially threatening stimulus, it acts to excite and drive the hypothalamus as well as the basal ganglia so that the organism is mobilized to take appropriate action.
When the hypothalamus is activated by the amygdala, instead of responding in an on/off manner, cellular activity continues for an appreciably longer time period (Dreifuss et. al., 1968). The amygdala can tap into the reservoir of emotional energy mediated by the hypothalamus so that certain ends may be attained (Joseph, 1982, 1992a)
HALLUCINATIONS
The amygdal-hippocampal complex, particularly that of the right hemisphere, is very important in the production and recollection of non-linguistic and verbal-emotional images associated with past experience. In fact direct electrical stimulation of the temporal lobes, hippocampus and particularly the amygdala (Gloor, 1990, 2010) not only results in the recollection of images, but in the creation of fully formed visual and auditory hallucinations (Gloor 1992, 2010; Halgren 1992; Halgren et al., 1978; Horowitz et al., 1968; Malh et al., 1964; Penfield & Perot, 1963), as well as feelings of familiarity (e.g. deja vus).
Indeed, it has long been know that tumors invading specific regions of the brain can trigger the formation of hallucinations which range from the simple (flashing lights) to the complex. The most complex forms of hallucination, however, are associated with tumors within the most anterior portion of the temporal lobe (Critchley, 1939; Gibbs, 1951; Gloor 1992, 2010; Halgren 1992; Horowitz et al. 1968; Tarachow, 1941); i.e. the region containing the amygdala and anterior hippocampus.
Similarly, electrical stimulation of the anterior lateral temporal cortical surface results in visual hallucinations of people, objects, faces, and various sounds (Gloor 1992, 2010; Halgren 1992; Horowitz et al., 1968)--particularly the right temporal lobe (Halgren et al. 1978). Depth electrode stimulation and thus direct activation of the amygdala and/or hippocampus is especially effective.
For example, stimulation of the right amygdala produces complex visual hallucinations, body sensations, deja vu, illusions, as well as gustatory and alimentary experiences (Weingarten et al. 1977), whereas Freeman and Williams (1963) have reported that the surgical removal of the right amygdala in one patient abolished hallucinations. Stimulation of the right hippocampus has also been associated with the production of memory- and dream-like hallucinations (Halgren et al. 1978; Horowitz et al. 1968).
The amygdala also becomes activated in response to bizarre stimuli (Halgren, 1992). Conversely, if activated to an abnormal degree, it may in turn produce bizarre memories and abnormal perceptual experiences. In fact, the amygdala contributes in large part to the production of very sexual as well as bizarre, unusual and fearful memories and mental phenomenon including dissociative states, feelings of depersonalization, and hallucinogenic and dream-like recollections (Bear, 1979; Gloor, 1986, 1992, 2010; Horowitz et al. 1968; Mesulam, 1981; Penfield & Perot, 1963; Weingarten et al. 1977; Williams, 1956). In addition, sexual feelings and related activity and behavior are often evoked by amygdala stimulation and temporal lobe seizures (Halgren, 1992; Jacome, et al. 1980; Gloor, 1986, 2010; Remillard, et al. 1983; Robinson & Mishkin, 1968; Shealy & Peele, 1957), including memories of sexual intercourse (Gloor 1990) or severe emotional trauma and abuse (Gloor, 2010).
Moreover, intense activation of the temporal lobe and amygdala has been reported to give rise to a host of sexual, religious and spiritual experiences; and chronic hyperstimulation (i.e. seizure activity) can induce some individuals to become hyper-religious or visualize and experience ghosts, demons, angels, and even God, as well as claim demonic and angelic possession or the sensation of having left their body (Bear 1979; Gloor 1986, 1992; Horowitz, Adams & Rutkin 1968; MacLean 1990; Mesulam 1981; Penfield & Perot 1963; Schenk, & Bear 1981; Weingarten, et al. 1977; Williams 1956).
LSD.
As is well known, LSD can elicit profound hallucinations involving all spheres of experience. Following the administration of LSD high amplitude slow waves (theta) and bursts of paroxysmal spike discharges occurs in the hippocampus and amygdala (Chapman & Walter, 1965; Chapman et al. 1963), but with little cortical abnormal activity. In both humans and chimps, when the temporal lobes, amygdala and hippocampus are removed, LSD ceased to produce hallucinatory phenomena (Baldwin et al. 1959; Serafintides, 1965). Moreover, LSD induced hallucinations are significantly reduced when the right vs. left temporal lobe has been surgically ablated (Serafintides, 1965).
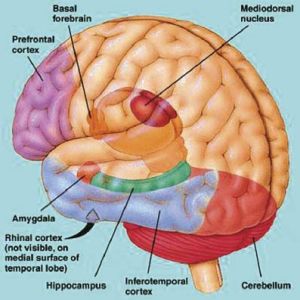
Overall, it appears that the amygdala, hippocampus, and the neocortex of the temporal lobe are highly interactionally involved in the production of hallucinatory experiences. Presumably, it is the neocortex of the temporal lobe which acts to interpret this material (Penfield & Perot, 1963) as perceptual phenomena. Indeed, it is the interrelated activity of the temporal lobes, hippocampus and amygdala which not only produce memories and hallucinations, but dreams. In fact, the amygdalas involvement in all aspects of emotion and sexual functioning, including associated memories, the production of overwhelming fear as well as bizarre and dream-like mental phenomenon, may well account for why this type of unusual stimuli, including personal and innocuous memories also appears in dreams.
The Right Hemisphere & Dreams.
There have been reports of patients with right cerebral damage, hypoplasia and abnormalities in the corpus callosum who have ceased dreaming altogether, suffer a loss of hypnogic imagery or tend to dream only in words (Botez et al. 2005; Humphrey & Zangwill, 1951; Kerr & Foulkes, 1981; Murri et al. 1984). However, there have also been some report that when the left hemisphere has been damaged, particularly the posterior portions (i.e. aphasic patients), the ability to verbally report and recall dreams also is greatly attenuated (e.g., Murri et al. 1984). Of course, aphasics have difficulty describing much of anything, let alone their dreams.
Electrophysiologically the right hemisphere also becomes highly active during REM, whereas, conversely, the left brain becomes more active during N-REM (Goldstein et al. 1972; Hodoba, 1986). Similarly, measurements of cerebral blood flow have shown an increase in the right temporal regions during REM sleep and in subjects who upon wakening report visual, hypnagogic, hallucinatory and auditory dreaming (Meyer et al. 2007). Interestingly, abnormal and enhanced activity in the right temporal and temporal-occipital area acts to increase dreaming and REM sleep for an atypically long time period (Hodoba, 1986). Hence, it appears that there is a specific complementary relationship between REM sleep and right temporal electrophysiological activity.
Interestingly, daydreams appear to follow the same 90-120 minute cycle that characterize the fluctuation between REM and NREM periods, as well as fluctuations in mental capabilities associated with the right and left hemisphere (Broughton, 1982; Kripke & Sonneschein 1973). That is, the cerebral hemisphere tend to oscillate in activity every 90-120 minutes -- a cycle which appears to correspond to the REM-NREM cycle and the appearance of day and night dreams.
Forgotten Dreams.
Most individuals, however, have difficulty recalling their dreams. This may seem paradoxical considering that hippocampal theta is being produced. However, this is theta punctuated by high levels of desychronized activity, which is not conducive to learning. In this regard, theta activity may represent the reverberating activity of neural circuits formed during the day, such that the residue of day time memories come to be inserted into the dream. Conversely, due to the high level of desychronization occuring in the hippocampus (as it is so highly aroused), although it contributes images and the days memories, it does not participate in storing these dream-like experiences into memory.
Consider the results from temporal lobe, amygdala, and hippocampal electrical stimulation on memory recall and the production of hallucinations. Although personal memories are often activated at low intensities of stimulation (memories which are verified not only by the patient but family), if stimulation is sufficiently intense, the memory instead will become dreamlike and populated by hallucinated and cartoon like characters (Halgren, et al. 1978). That is, at low levels of stimulation memories are triggered but these memories become increasingly dream-like with high levels of activity. Moreover, once these high levels of stimulation are terminated, patients soon become verbally amnesic and fail to verbally recall having had these experiences (Gloor, 1992; Horowitz, et al. 1968). However, these memories can be later recalled if subjects are provided with specific contextual cues (Horowitz, et al. 1968). The same can occur during the course of the day when a fragment of a conversation, or some other experience, suddenly triggers the recall of a dream from the previous night which had otherwise been completely forgotten. Presumably it had seemingly been forgotten because the hippocampus did not participate in their storage and thus could not assist in their retrieval (see chapters 29, 20).
There is also some evidence to suggest that different regions of the hippocampus show different levels of arousal during paradoxical sleep. For example, it appears that the posterior hippocampus becomes activated during paradoxical sleep and shows theta activity, whereas the more anterior portions become inhibited (Olmstead et al. 1973). As the anterior portions are more involved in new learning (at least in humans), whereas the posterior hippocampus is more concerned with old and well established memories, this would suggest that the posterior hippocampus is contributing older or already established memories to the content of the dream--which explains why theta, which is associated with learning and memory, is also produced during the dream--that is, it is replaying various fragmentary memories. Conversely, the inhibition of the anterior region would prevent this dream material from becoming re-memorized.













