Rhawn Gabriel Joseph, Ph.D.
BrainMind.com
Human sexual behavior, including gender differences in cognitive and emotional functioning are influenced by a number of hormonal, neurological, as well as environmental variables (Beach, 2004; Beatty, 2002; Grumbach, 2005; Harris, 1978; Harmson, 1990; Money & Ehrhard, 1972). However, as is apparent by the sexual activities of reptiles, amphibians, and fish, the basic rudiments of sexual activity, including sexual orientation, is genetically determined and under the neurological and hormonal control of the limbic system, specifically, the interactions of the amygdala, hypothalamus, and septal nuclei (Joseph, 1990, 2002a, 2003; Maclean, 1990). Early experience, exposure to certain role models, parental attitudes, and so on, are almost wholly irrelevant to non-mammalian species insofar as sexual activity is concerned. Indeed, as is well known, the infants of many of these creatures shun parental contact so as to avoid being cannibalized (Joseph, 2003; Maclean, 1990).
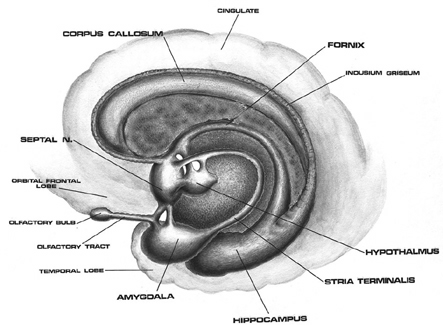
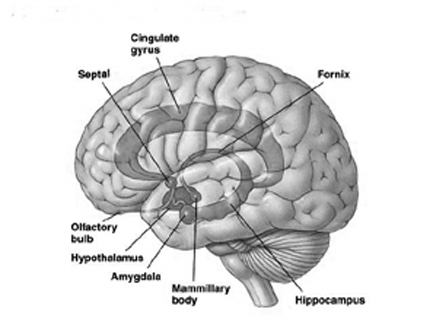
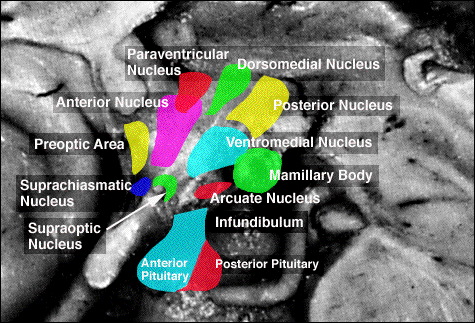
For example, electrical stimulation of the preoptic and anterior region of the hypothalamus in primates and rodents increases sexual behavior in males including the frequency of erection, copulation, and continued stimulation can induce not only pelvic thrusting in the absence of a partner, but an explosive discharge of semen. Conversely destruction of the preoptic area can completely eliminate sexual behavior or interest, and will induce gonadal atrophy (Barfield & Chen, 1977; Davis, McEwen & Pfaff, 2011; Joseph, 1990, 2002a, 1999; Maclean, 1969, 1973, 1990, Lisk, 1967, 1971).
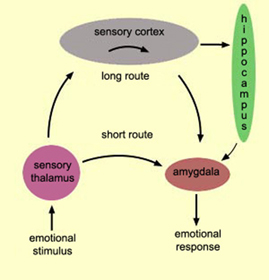
Similarly, electrical activation of the amygdala can induce ovulation, uterine contractions, lactogenetic responses, and in males, penile erection (Joseph, 1990, 2002a; Maclean, 1973; Robinson & Mishkin, 2008; Shealy & Peel, 2007). Moreover, abnormal activity in this region, such as due to seizures, not uncommonly greatly alters and often increases sexual interest in humans, and similar increases in sexuality has been reported following seizure activity within the frontal and inferior temporal lobes which are known to maintain extensive interconnections with the hypothalamus and amygdala (Bear, 2011, Currier, Little, Suess & Andy, 1971; Freemon & Nevis, 1969; Joseph, 1988; 1990, 2002a, Money & Hosta, 1967; Remilard et al. 2005; Ruff, 1980).
EXPERIENTIAL & SEX DIFFERENCES IN NEURONAL ORGANIZATION & COGNITION
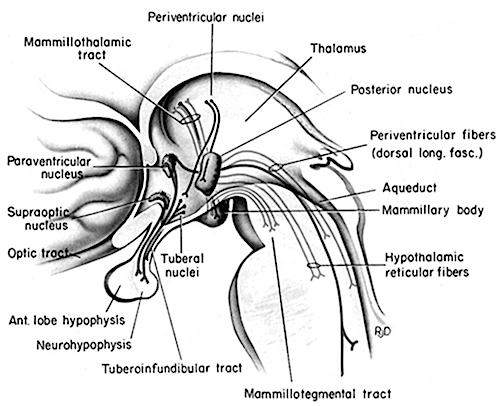
It has now been well established that the amygdala and the anterior-preoptic, ventromedial and suprachiasmatic nucleus of the hypothalamus are sexually differentiated and have sex specific patterns of neuronal and dendritic development, a consequence of the presence or absence of testosterone during fetal development in humans, or soon after birth in some species such as rodents (Allen, Hines, Shryne & Gorski, 1989; Blier, Byne & Siggelkow, 1982; Gorski, Gordon, Shryne, & Southam, 1978; Joseph, Hess & Birecree, 1978; Rainbow, Parson & McEwen, 1982; Raisman & field, 1971, 1973; Swaab & fliers, 1985). The presence or absence of testosterone during these early critical periods appear to also be responsible for neurological alterations which greatly effect sex differences in cognitive functioning (Beatty, 2002; Dawson et al. 1975; Harris, 1978; Joseph, 1990, 2002ab, 2003; Joseph, et al. 1978; Stewart et al, 2005; Williams et al. 1990).
For example, it is well known that among humans and rodents, that males demonstrate superior spatial-perceptual and maze learning capabilities as compared to females. Similarly, it has been found that heterosexual males demonstrate superior spatial perceptual capabilities as compared to homosexual males (Gladue et al. 1990; Sanders & Ross-Field, 2004; Wilmot & Brierley, 1984).
When infant female rodents are exposed to testosterone or male rodents are castrated or injected with cyproterone acetate (an anti-testosterone hormone) soon after birth, these sex related differences are completely reversed (Dawson, et al. 1975; Joseph, et al. 1978; Stewart et al. 1975). However, adult testosterone levels have little or no influence on spatial performance (Joseph et al. 1978). Similarly, human males whose brains were not sufficiently exposed to testosterone during fetal development demonstrate inferior spatial capabilities (Dawson, et al. 1975).
Nevertheless, sexual behavior and gender related differences in cognition, are also greatly influenced by early environmental influences as well and in fact can be reversed depending on rearing experience. For example, females reared in an enriched environment demonstrate superior spatial maze learning capabilities as compared to males reared in an deprived environment who in turn continue to outperform deprived females. However, enriched males continue to outperform all groups. (Joseph, 2011; Joseph & Gallagher, 1980). Indeed, early experience not only alters cognitive functioning, but can drastically influence social-emotional development in humans and primates as well as neuronal development and perceptual functioning in rats and prosimians (Casagrande & Joseph, 1978, 1980; Harlow, 1962; Joseph, 2011; Joseph & Gallagher, 1980; Joseph & Casagrande, 1980; Joseph, 1982, 2002b; Langmeir & Magejcek, 1975).
The structural and functional foundations of limbic system functioning appear to be very sensitive to early environmental and particularly early hormonal influences. For example, by preventing androgenization of the brain during early critical periods of neurological development, limbic system nuclei will assume the female pattern of neuronal development (Goy & McEwen, 1980; Raisman & Field, 1973; Whalen, 1980). Adult male rats who were subject to these conditions during infancy will assume female sexual postures when stimulated. Adult females who were exposed to testosterone during these same critical periods will in turn display male mounting behaviors (Goy & McEwen, 1980; Whalen, 1980).
HUMAN SEXUAL ORIENTATION & EARLY EXPERIENCE
Although there is considerable evidence that human sexual orientation is fairly well established by early childhood, it is not at all clear, however, as to the role of early hormonal influences on these inclinations (Byne & Parson, 2002; Goy & McEwen, 1980; Meyer-Bahlburg, 1984; Downey, Ehrhardt, Schiffman Dyrenfujth & Becker, 2007Money & Ehrhard, 1972). However, it is clear that hormonal treatment, behavioral modification or psychotherapy during adulthood, has little or no effect on reversing or altering hetero or homosexual inclinations (Money & Ehrhard, 1972; Meyer-Bahlburg, 1984; Gartrell, 1982; Bell, Weinberg & Hammersmith, 1981), although there is anecdotal evidence that homosexual promiscuity can be partly curtailed by long term psychotherapy. Similarly, although testosterone levels have been shown to fluctuate in regard to aggressive or success oriented experience (Mazur & Lamb, 1980), studies of testosterone levels in adult hetero or homosexuals have been equivocal and have shown little difference between these groups (Meyer-Bahlburg, 1984).
As noted, childhood rearing experiences can exert drastic influences on emotional, social, sexual, and neurological development. Nevertheless, there is no convincing evidence that the manner in which children are reared significantly contributes to the development of homosexuality in the general population. Although there are several studies which indicates that as children many male homosexuals tend to shun sports and avoid fights, prefer female childhood companions, have a fear of physical injury, and tend to have over protective mothers and distant fathers (Bell, Winberg & Hammersmith, 1981; Bieber et al. 1962; Van Den Aardweg, 1984), there is little evidence that improper or absent role modeling plays a significant role in determining orientation, although certainly it exerts significant influences on one's social-sexual interest and emotional development (Brooks-Gunn & Matthews, 2011; de Beavior, 1961; Harlow, 1962; Joseph, 2002b, Money & Ehrhard, 1972).
For example, there have been a number of anthropological reports of cultures and societies where young males are sodomized by adults or encouraged and expected to orally copulate older males for much of their childhood (Ford & Beach, 1951; Money & ehrhard, 1972). As noted by Ford and Beach (1951) in describing the Karaki of New Guinea, "...in the course of his puberty rites each boy is initiated into anal intercourse by the older males. After his first year of playing the passive role he spends the rest of his bachelorhood sodomizing the newly initiated" (p. 132). However, in these and other cultures, once they reach adulthood males are expected to marry and cease to engage in such acts.
It is noteworthy that although ostensibly these acts are homosexual and involve ejaculation, they are a function of the social hierarchy and represent the establishment of dominance and submissive relationships (Ford & Beach, 1951). It is for these reasons that upon reaching late adolescence or adulthood, that males either cease to engage in these acts or reverse position and assume dominance over those who must now orally copulate them.
Moreover, it is important to note that adults who engage in such practices with male children, are pedophiles. In this regard, although pedophilia may have a variety of etiologies, several studies have indicated that the arousal experienced by these individuals is facilitated by the helplessness, vulnerability, and submissiveness of their victims (Eibl-Eibesfeldt, 1990; Howells, 19; Langevin, 2005).
HOMOSEXUAL DOMINANCE & SUBMISSION
Similar dominance and submissive relationships are formed in prisons and during war time with invading and conquering armies sometimes sodomizing inferiorally ranked males and/or castrating the vanquished (Brownmiller, 1975; Eibl-Eisbesfeldt, 1990). However, these "dominant" males do not usually view themselves as homosexuals although they certainly feel they have taken away the masculinity of those they have violated (Brownmiller, 1975; Eibl-Eibesfeldt, 1990; Lockwood, 1980; Scacco, 1975; Sheilds & Sheilds, 2005). Heterosexual males in fact frequently utilize sex to diminish, ridicule, demean, other men as well as women, and have engaged in the rape of both sexes to achieve these ends (Brownmiller, 1975; Eibl-Eibesfeldt, 1990; Ellis, 1989; Joseph, 2002b, 2003; Lockwood, 1980; Scacco, 1975). Indeed, males who are raped often refuse to report these experiences for fear of being viewed as homosexual or feminized.
Women, of course, are also subject to rape during war time, which in turn is often a consequence of a desire to defile the victim and to humiliate their men. In fact, when men or women are raped, bottles, sticks, guns, pipes and other objects are frequently used so as to exacerbate the victims sense of being dehumanized and totally dominated (Scacco, 1975; Shields & Shields, 2005). Findings such as these have led to the conviction among some social scientists and political groups that rape is not truly a sex act but is a function of a males desire to violently control and dominate their victims through sex (Brownmiller, 1975; Scacco, 1975).
In fact, males in this culture, particularly those involved in blue collar and the construction trades, often indulge in verbalized forms of sexual dominance with new or younger workers, and may tease or "jokingly" verbally threaten or belittle them sexually (Joseph, 2002b, 2003). Sexual threats, with direct exposure of an erect penis is often employed by primates in their dominance displays. As pointed out by Wickler and Eibl-Eisbesfeldt, not only due a variety of monkeys often stand guard by exposing their genitals, but phallic displays are a "universal" expression among human societies (Eibl-Eibesfeldt, 1975; Wickler, 1973).
Indeed, phallic aggression has even crept into the vocabulary. When someone is really "pissed off" we know he is angry and we should watch out. If someone says "fuck you!" we know they are not inviting us to have sex, and if someone has been "fucked" or "screwed" it is understood that someone else has diminished or taken advantage of them in some manner.
Moreover, among monkeys, apes as well as other species, such as canines and wolves, what ostensibly appears to be homosexual behavior among males is often a non-sexual means of achieving dominance, demonstrating submission (Eibl-Eibesfeldt, 1975; Wickler, 1973), or as a form of appeasement (at least in pygmy chimpanzees) following aggressive altercations. That is, dominant males not uncommonly will mount a more submissive male and even make pelvic thrusting movements, albeit, without penetration. In these instances, just as the rape of women or men is more often a function of aggression than sex, similar dominance-submissive displays among otherwise heterosexual males is also aggressive and a function of dominance seeking. Such interactions not uncommonly result in violence or are a consequence of threat.
Among humans, apes, monkeys, and dogs, although one male may attempt to mount another male so as to display dominance, this is not always achieved as resistance may be offered such that instead a fight may ensue. One need not be a prisoner in jail or an anthropologist to witness such actions, for such mounting attempts and fights are not infrequently observed by owners of male dogs.
DOMINANCE, SUBMISSION, VIOLENCE & HOMO-PHOBIA
Like homosexuality, what has been referred to as "homo-phobia" is often expressed cross culturally, predominantly by males. In this country and others, male homosexual are not uncommonly the victims of violence. Wide spread executions of homo sexual males recently took place in Iran following the Islamic revolution in that country. Throughout history homosexual men have been the subject of incredible violence and prejudice. In fact, over 90& of homosexuals claim they have been the targets of violent verbal abuse or threats, and almost 25% claim they have been physically attacked due to their sexual orientation (Herek, 1989).
Some political groups claim that "homo-phobia" is simply a cultural prejudice and is a form of discrimination, and have claimed that in other cultures and societies homosexual behavior is widely accepted. For example, like the dominance/submission relationships between men and boys in certain societies mentioned above, it is often claimed that the ancient Greeks and Romans not uncommonly formed homosexual relationships. However, although for various time periods such relationships were temporarily tolerated, they were certainly not widespread, and generally consisted of older, aristocratic men taking on a sexualized parental/teacher role with a younger male who served in a submissive capacity (Durant, 1939; Lewis & Reinhold, 1966). Although such behavior is homosexual, it is also a function of pedophilia. This was also true of some ancient Greek warriors, such as Achilles, who had several young male lovers, all of whom served in a submissive capacity (Homer, 1939). However, Achilles was also very fond of women and in fact briefly refused to participate further in the attack on Troy due to a dispute with Kind Aggammenon over a woman (Homer, 1939). It is also noteworthy that ancient Roman law made homosexuality punishable by death (Durant, 1939; Lewis & Reinhold, 1966)
As noted above, "homosexual" actions among monkeys, apes, and dogs, also often result or are a consequence of violence and are a function of attempts to achieve dominance rather than sex. Similarly, male homosexual overtures, although perceived as sexual by fellow homosexuals, are often not responded to as such by heterosexual males, many of whom react aversively or aggressively when subject to such attentions. In this regard, it is likely that homosexual overtures are often not perceived by heterosexual males as sexual per se, but as an attempts to achieve dominance or to behave submissively. That is, such behavior may well be perceived as an aggressive attempt to demean and humiliate, or as an invitation to assume dominance over the homosexual via sexual mounting or violent aggressiveness in which case the homosexual may be attacked (Joseph, 2003). As such, homosexual actions sometimes invite attack, a consequence, perhaps, of not only the dominance submissive aspect of the relationship, but the sexual discomfort some heterosexual males may experience when solicited.
One might assume that lesbian acts are not as upsetting as male homosexual actions because females do not pose the potential threat of dominance. In fact, this is possibly why many women do not find male homosexuality as aversive or threatening as do heterosexual males, and why women can hug, kiss, and even sleep together without anyone thinking anything is amiss (Joseph, 2003). In contrast, men not only avoid such contact but tend to perceive the simple act of staring as aggressive and a possible indication of homosexual intent (Joseph, 1985).
It is not uncommon for male homosexual to be subject to violent retribution by presumably heterosexual males who in fact search them out and/or attack them without provocation. There are several explanations for such actions, including the desire to establish one's own masculinity and thus dominance over a male who advertises their willingness to assume the submissive posture. However, many heterosexual males engage in what has been described as "gay bashing" because they are in fact beating upon their own unknown face and are responding to their fears regarding their own sexuality and inadequacy (Joseph, 2002b). In large part, however, as based on the dominance and submissive dimensions of what ostensibly appears to be homosexuality among otherwise heterosexual humans and animals, and the fact that such conduct is often aggressive in nature, it certainly could be argued that in their competitive drive for dominance (Joseph, 2003) adult heterosexual males are biologically predisposed to respond aversively or aggressively to homosexual overtures and that this is not a learned prejudice.
It is noteworthy, however, that males who engage in "gay bashing" sometimes vent their anger on their victims genitals or sometimes force their victims to perform fellatio. And yet, like the conquering warrior or a hardened prisoner, those who seek out homosexuals or forcibly sodomize or force other males to perform fellatio, generally do not view themselves as homosexuals (Lockwood, 1980; Scacco, 1975). This is perhaps not entirely surprisingly as even among scholars and scientists there does not appear to be a commonly accepted definition of what causes or constitutes homosexuality.
THE LIMBIC FOUNDATIONS OF SEXUAL DESIRE & ORIENTATION
In a recent series of MRI studies, it was demonstrated that the orbital frontal lobes of heterosexual males reacts to female faces, where the homosexual and female orbital frontal lobes react to male faces.
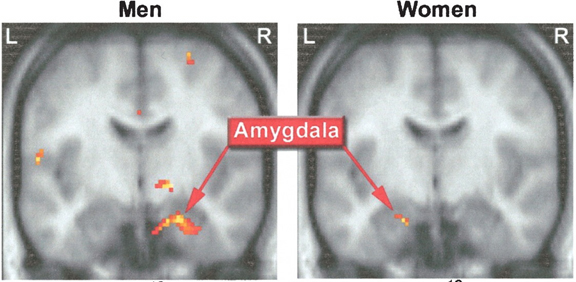
Although the etiology of homosexuality remains in question, recently it has been shown that the ventromedial and anterior hypothalamic nuclei of male homosexuals demonstrate the female pattern of development (Levay, 1991; Swaab, 1990). Moreover, differences in the size of the anterior commissure (AC), the axonal fiber pathways that link that amygdaloid nuclei of the right and left cerebral hemisphere, are also different in heterosexual male, vs female, vs male homosexuals (Allen & Gorski, 2002). Specifically, it has been found that the female AC is 18% larger than in the male, and that the homosexual AC is 35% larger than in heterosexual males, and 17% larger than in the female.
It is noteworthy that the cells of origin for the AC are found in the amygdala, which, as noted, is also sexually differentiated. Like the corpus callosum, the AC is responsible for information transfer as well as inhibition within the limbic system (Joseph, 1990, 2002a, 1999). The amygdala (in conjunction with the hypothalamus) also provides the neuronal foundation for sexual attraction and desire (Joseph, 1990, 2002ab, 2003, in press), including sex differences in emotional expression, perception and speech (Joseph, 2003, 1999).
When the bed nuclei for the AC and the amygdala of both cerebral hemispheres are damaged, hyperactivated, or completely inhibited (such as following bilateral amygdalectomy), a striking disturbance in sexual and social behavior is evident (Brown & Schaffer, 1888; Gloor, 1960; Joseph, 1990, 2002ab, 2003, in press; Kluver & Bucy, 1939; Terzian & Ore, 1955; Schriner & Kling, 1953). Specifically, humans, non-human primates, and felines who have been subject to these procedures often engage in prolonged, repeated, and inappropriate sexual behavior, including numerous episodes of masturbation as well as repeated sexual acts with members of either sex or even members of different species.
Given the minimal role of early experience in determining sexual orientation and the dominant role of the limbic system in all aspects of sexual, social, and aggressive behavior, including the generation of sexual imagery as well as the capacity to experience orgasm, it might well be suspected that these neurological differences in the structure of the hypothalamus and amygdala are in large part responsible for sex differences in human emotionality as well as sexual behavior, including orientation.
For example, the amygdala engages in environmental surveillance so as to detect stimuli, events, or individuals which are emotionally or motivationally significant (Joseph, 1990, 2002ab, 2003, 2000). Moreover, it contains facial recognition neurons which are capable of determining the sex of the individual being viewed. In this regard, it is possible that the amygdala also acts not only to discern the emotional characteristics of the environment, but to detect potential sexual partners and then to motivate sex-appropriate behavior.
MALE VS FEMALE VS HOMOSEXUAL SEXUAL DESIRE AND BEHAVIOR
Hetero and homosexual male and female sexual behavior and desire, which is mediated by the limbic nuclei, are obviously striking different. It is well known that once a male ejaculates he goes through a refractory period where he is physiologically and psychologically disinclined to engage in further sexual acts (Brecher & Brecher, 2006). In contrast, females are capable of engaging in repeated sex acts, with the same or different partners, and can enjoy multiple organisms which become increasing intense following each immediately subsequent sexual episode (Brecher & Brecher, 1966). This capacity has given rise to the myth and fear of the insatiable female.
Nevertheless, although the human female is certainly capable of engaging in and enjoying repeated sexual contacts with little or no intermission, it is well known that males are more promiscuous and more likely to seek repeated sexual contacts with a number of partners and are much less discriminating than women (Daly & Wilson, 2005; Trivers, 1985). In contrast, women are much less indiscriminate and tend to prefer mates who they perceive to be of high social or economic status, or males who tend to provide emotional stimulation, be it positive or negative, that is similar to their own childhood rearing experiences (Betzig, 1985; Buss, 2007; Joseph, 2002b; Townsend, 1989).
However, it has also been shown that the more a women enjoys and engages in sex with her mate or partner, the more likely she is to engage in affairs outside the relationship (Bellis & Baker, 1990). Moreover, women who engage in such affairs tend to do so during that part of their menstrual cycle when they are most fertile (Bellis & Baker, 1990). In fact it has been argued by Ford and Beach (1951) that in societies where women need not hide their sexuality, women engage in extramarital affairs as frequently as do men. Moreover, among chimpanzees (creatures who share 99% of our gene structure), once a female becomes sexually receptive, she may engage in continuous sex and will copulate with every member of her troop repeatedly (Gooodall, 1973).
Nevertheless, recent surveys of female sexuality indicate that although perhaps as many as 50% of married American women have sex outside of marriage that males remain far more promiscuous (Wolfe, 1981; Hite, 1981). It is likely that these gender differences, although subject to societal/parental influences, at least among humans, and are also mediated by differences in the structure and activity of the male vs female limbic system.
It is also well known that male homosexuals tend to be extremely promiscuous, with reports of males seeking up to fifty sexual contacts a day (Aaron, 1973; Symons 2011). These activities were apparently quite common in homosexual "bath houses" such as were found in San Francisco until, despite the protests of the local homosexual community, they were closed down for health reasons. Moreover, according to Symons (2011), homosexual males not infrequently attempt to form harems of willing male partners, the sole purpose of which is for continuous loveless sex.
It thus appears, given these differences and similarities between the homosexual male and heterosexual female vs the heterosexual males limbic system, that these orientation differences in sexual practice and promiscuity may be similarly limbically based. That is, just as heterosexual functioning is governed by the limbic system, so to is the sexual activity of homosexuals. Indeed, it is the interaction of the amygdala, septal nuclei, cingulate gyrus and hypothalamus which enables human beings and other higher animals to form attachments and to experience not only sexual desire, but feelings of love (Joseph, 1990, 2002ab, 2003).
Given the above, there is thus a strong possibility that the female pattern of dendritic growth in the hypothalamus and the larger AC of the amygdala of the male homosexual gives rise to a biological drive to respond to males in a sexual manner and to derive sexual satisfaction from such contact much in the manner in which a heterosexual female derives enjoyment from male partners. It is also possible that the capacity of females and the desire of male homosexuals to engage in repeated sexual acts is due to these same differences, particularly those found in the AC of the amygdala. It is also likely that these same limbic differences in sexual organization account for the similarities between homosexual males and heterosexual females in regard to spatial ability, emotionality, and even the emotional-melodic aspects of speech.
Hence, in summary, being in possession of a female hypothalamus, female amygdala, and a larger than normal heterosexual AC, and as demonstrated in recent studies demonstrating that the "homosexual" and female orbital frontal lobes responds similarly to "male" faces, then homosexual males not only respond in a female manner to male cues, but in a female manner in regard to emotionality, spatial ability, and the capacity to engage in repeated, if not constant sexual activity, which would explain the propensity of some males homosexuals to seek 25 to 50 or more contacts a day. Given the natural promiscuity of males in general (regardless of orientation) such tendencies would be subject to few inhibitions. As such, although these sex practices are obviously unhealthy and self-destructive, such behaviors among select groups of male homosexuals may not be a function of choice per se. Rather such proclivities may be more of a compulsion, and are not due to deficits in morality, as some groups have claimed, but are a consequence of diminished restraint, and are biologically determined. It is perhaps for these reasons that male homosexual behavior is found not only across cultures but among various animals, including geese, dolphins and apes.












