by Rhawn Joseph, Ph.D.
Rhawn Joseph, Ph.D.
BrainMind.com
The core of our emotionality is mediated by the sexually differentiated limbic system. Emotional development, and in fact, sex differences in development, are mediated by the limbic system and parallels the maturation of specific diencephalic and limbic forebrain structures, notably the hypothalamus, followed by the medial amygdala and amygdala striatum, and then the septal nuclei and anterior cingulate (Joseph, 2012a, 2014) as well as the hippocampus and neocortex. The brainstem then the limbic forebrain matures in a caudal to rostral and paramedian to lateral arc followed by the maturation of the overlying six-layered neocortex (Gibson, 1991; Langworthy, 1937; Yakovlev & Lecours, 1967). Therefore, functions associated with earlier developing structures such as the brainstem, appear in advance of later maturing tissues, such as the hypothalamus, followed by the amygdala, septal nucleus, anterior cingulate and neocortex.
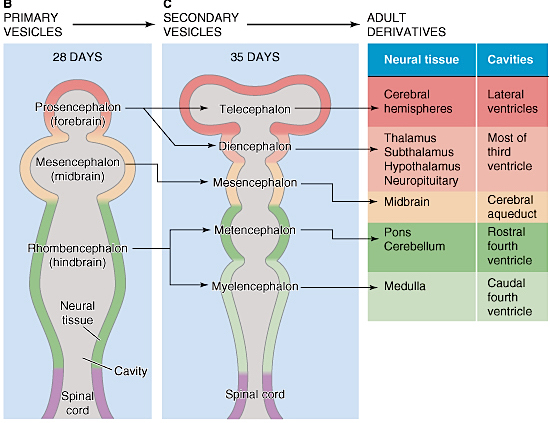
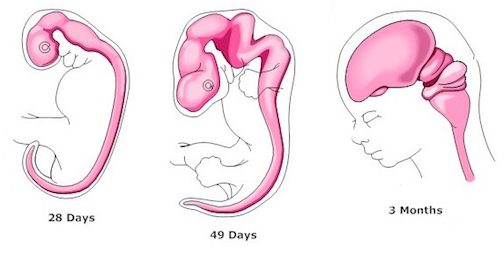
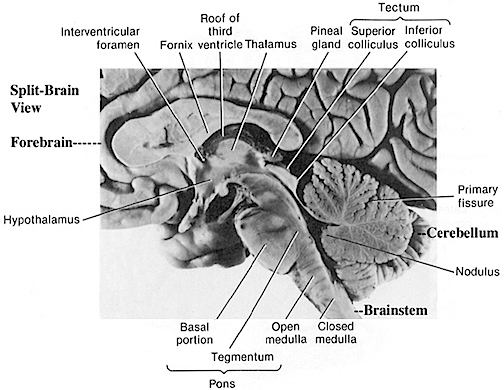
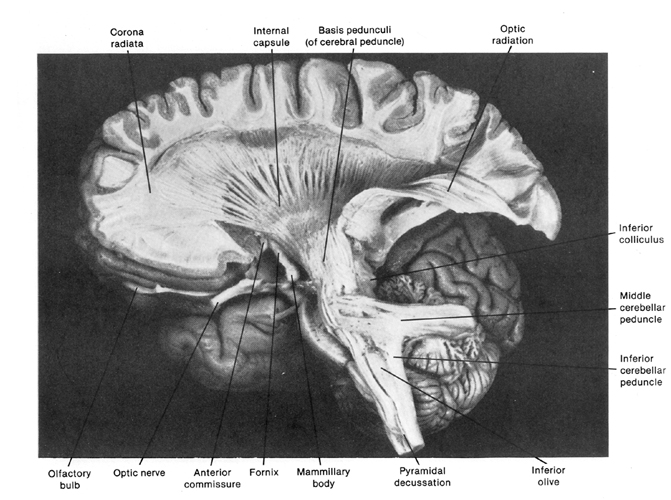
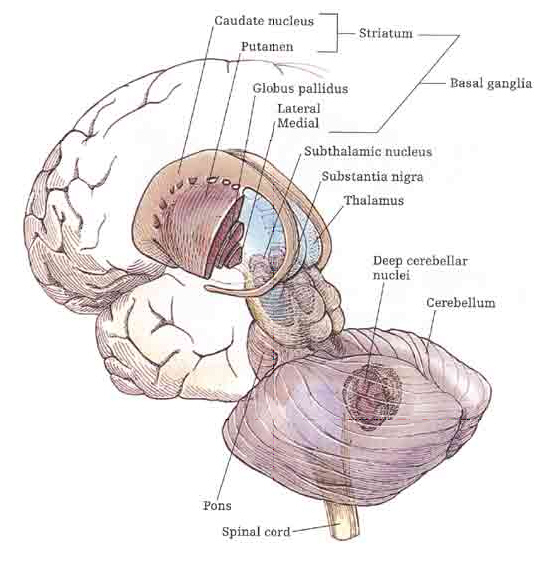
Hence (and as detailed in chapter 23), for the first several weeks of postnatal life, much of human behavior, and the first rudimentary expressions of "emotion" and "cognition" including "smiling" and vocalization, are under the reflexive control of the brainstem with still immature hypothalamus exerting increasing influences over the ensuing weeks and days. Indeed, as based on physiological indices "the general rule appears to be that phylogenetically older structures (e.g. brainstem, cerebellum, thalamus) demonstrate considerable metabolic maturity compared to telencephalic structures in the neonatal period" (Chugani, 1994, p. 155).
As noted, because the brainstem and then the forebrain matures in a caudal to rostral and paramedian to lateral arc (Barkovich et al., 2018; Brody et al., 2014; Gibson, 1991; Harbord, et al., 1990; Holland, et al., 1986; Lee et al., 1986), functions associated with the hypothalamus appear in advance of those associated with the amygdala which begin to emerge in advance of those associated with the septal nucleus and anterior cingulate, which appear in advance of those associated with the neocortex.
These differential maturational gradients are evident not only neuroanatomically and physiologically, but behaviorally and functionally and also account for the emergence of sex differences even at this tender age. That is, the overlapping progressive maturation of these limbic structures corresponds to and parallels the development of complex emotions and affective behaviors, i.e., pleasure, joy, wariness, fear, separation, anxiety, including the establishment of loving attachments and social-emotional speech.
Moreover, because these brain structures are sexually differentiated, as they mature, sex differences in affective and social behavior becomes more pronounced. In addition, because these structures mature at different, albeit at overlapping rates (Benes, 1994; Yakovlev & Lecours, 1967), the ability to perceive and express various social-emotional nuances also emerge and develop at different ages.
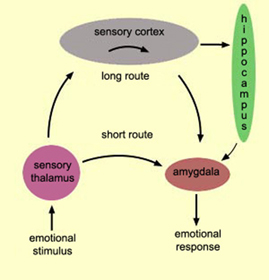
Consider for example, fear, an emotion associated with the amygdala (Davis, Walker, & Lee, 2017; Gloor, 2017; Halgren, 2012; LeDoux, 1996; Rosen & Schulkin, 1998). The human medial amygdala is exceedingly immature at birth and doesn't become well myelinated until around the 8th postnatal month (Gilles, Leviton, & Dooling, 1983; Langworthy, 1937; Yakovlev & Lecours, 1967)--the development of axonal myelin insulation being one of many indicators of functional maturation and synaptic viability. Likewise, the infant is generally quite fearless and appears unable to experience fear until around the 8th postnatal month; an emotion that develops after the emergence of wariness at around 6 months (which is also an amygdala mediated emotion). Hence, paralleling the maturation and myelination of the medial amygdala, at 6 months the infant first experiences and expresses wariness, and at 8-months the infant first begins to experience and express feelings of fear, and then becomes progressively more fearful, such as in response to an approaching stranger (Bronson, 1972; Emde, Gaensbauer, & Harmon, 1976; Sroufe & Waters, 1976). This initial fearlessness (and amygdala immaturity) is exceedingly adaptive, for the generation of fear would interfere with the initial establishment of intimate emotional attachments, and the infant's need for considerable social-emotional and physical stimulation, which is initially and eagerly welcomed even from strangers. However, because the amygdala is sexually differentiated (Bubenik & Brown, 1973; Nishizuka & Arai, 1981), females not only make eye contact, smile, and become increasingly social at an earlier age than males (Kagan, 1971; Lewis, et al., 1966; Wolf, 1963), but become fearful and even more fearful, at an earlier age.
Due to the immaturity of the forebrain the behavior of the fetus and neonate appears to be governed by the brainstem (chapter 23) which may also be sexually differentiated. Over the ensuing weeks after birth and due to the progressive development of the forebrain, reflexive brainstem activity comes to be influenced and supplemented by the still immature hypothalamus and thalamus (see chapter 24). It is not until around the second to third postnatal month, that the still immature but more rostrally/paramedially located (medial) amygdala (and limbic/corpus striatum) begins to increasingly contribute to the infant's emotional and affective-motor repertoire. Over the ensuing months, and due to increasing amygdala/striatal as well as septal and anterior cingulate influences, emotional perception and expression becomes increasingly complex, the infant demands considerable social and emotional stimulation, but then, around 8 to 12 months of age, begins to increasingly demonstrate fear and separation anxiety. And, these fears and feelings of anxiety at separation, are more pronounced in females. It is also at this later age that males and females will begin vocalizing a separation cry and will begin forming specific and enduring attachments.
Whereas fear is directly associated with amygdala maturation, separation anxiety, the separation cry, and the formation of specific emotional attachments are also associated with the maturation of the septal nuclei and anterior cingulate (Joseph, 2012, 2014). These structures are also sexually differentiated (chapters 12, 13).
As detailed in chapter 28, these limbic structures are also "experience-expectant" and require considerable social emotional and maternal input to develop normally. If subjected to sexual, physical, or emotional abuse, or other forms of traumatic stress, or if sufficient social, emotional, or maternal stimulation is not provided during each critical stage of structural development, the hypothalamus, amygdala, septal nuclei, or cingulate will abnormally develop and social emotional and even sexual functioning may become abnormal.
THE NEONATAL BRAINSTEM: FUNCTIONAL OVERVIEW
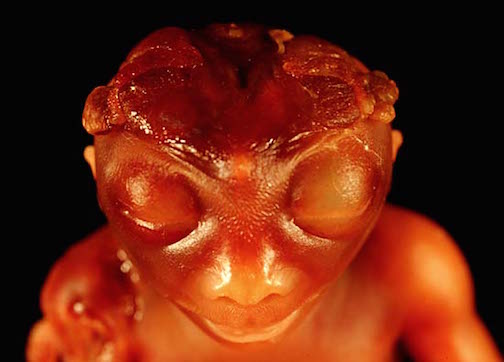
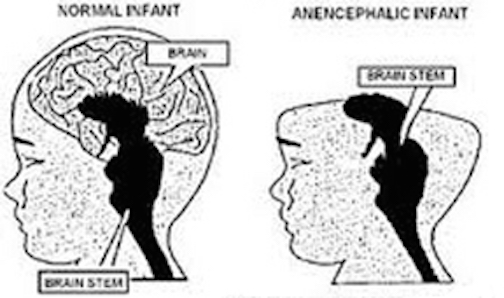
The brainstem consists of the medulla, pons, and midbrain. When specific brainstem nuclei are stimulated, involuntary, stereotyped and routine motor acts can be produced such as groping, grasping, opening and closing the mouth, touching the mouth or wiping the face with the hand, as well as visual tracking, head elevation, head turning, sucking, chewing, swallowing, screaming, and crying (Aitken, 1986; Blessing, 2017; Cohen et al., 2018, Cowie et al., 1994; Davidson & Bender, 1991; Larson et al., 1994; Skinner & Garcia-Rill, 1990; Weinstein & Bender, 1943; Zhang et al., 1994). Identical behaviors are demonstrated by the neonate (Capute, 1986; Debakan, 1970; McGraw, 1969; Piper & Darrah, 1994; Piaget, 1952). Moreover, these same behaviors, including screaming, crying, rudimentary smiling, and involuntary grasping and groping, can be triggered in the complete absence of the forebrain, and are demonstrated by some anencepahlics (Emde et al., 1976; Lemire et al., 1978; Monnier, 1956). Given the immaturity of the forebrain and the fact that there are no myelinated fibers beyond the neonatal brainstem (Yakovlev & Lecours, 1967), it can thus be concluded that these behaviors are under the reflexive control of the brainstem.
For example, because brainstem structures such as the midbrain inferior (auditory) colliculi can perceive and discriminate between a wide variety of acoustic parameters, and can trigger head turning and eye movement (Aitkin, 1986; Merzenich & Reid, 1974), the neonate is able to discriminate phonetic sounds within days after birth (Eimas, 1985) as well as turn the head and orient with the eyes or react with whole body movements to sudden or loud sounds; i.e. the Moro reflex (Capute, et al 1986; Debakan, 1970; McGraw, 1969; Piper & Darrah, 1994). Similar behaviors are typical of some anencephalics (Lemire et al., 1978) and are thus brainstem reflexes. Likewise, because the superior (visual) colliculus is sensitive to and can trigger eye and head movements in reaction to visual motion but not stationary stimuli (Davidson & Bender, 1991), for the first several postnatal days the infant is almost completely unresponsive to stationary stimuli but may fixate on and briefly follow moving objects (Bronson, 1974; Piper & Darrah, 1994).
The neonate as well as anencephalics, however, are capable of screaming and crying, as well as turning up the corners of their mouths as if "smiling." (Lemire et al., 1978; Monnier, 1956). However, these too are brainstem reflexes. Indeed, when directly stimulated, brainstem nuclei and associated oral-facial cranial nerves can produce a wide range of facial expressions such as smiling (Weinstein & Bender, 1943), as well as crying and screaming --vocalizations which are produced by the midbrain periaqueductal gray. The midbrain periaqueductal gray controls the oral-laryngeal musculature (Jurgens, 1994; Larson et al., 1994; Zhang et al., 1994) and via lower brainstem nuclei, can induce inspiration and respiration thus producing a variety of loud sounds including grunting, crying, and screaming.
In the child and adult, brainstem nuclei, including the periaqueductal gray are subject to hierarchical forebrain and neocortical control. Hence, through forebrain influences, individuals can purposeful smile, laugh, crying, and so on, or at least pretend to do so. However, forebrain influences are only brought to bear later in development. Again, as there is no evidence of myelinated fibers anterior to the midbrain during the first few days and weeks after birth (Langworthy, 1937; Yakovlev & Lecours, 1967). Given these other indices which indicate little or no forebrain activity (Chugani, 1994; Chugani, Phelps, & Mazziotta, 2014; Kellaway, 2006; Langworthy, 1937; Ohtahara, 1981; McGraw, 1969), it appears that these behaviors are also innate brainstem reflexes. This is why some anencephalics--babies born without a forebrain--can also smile, grunt and cry out (Lemire et al., 1978; Monnier, 1956). Although conscious cognitive and meaningful emotional activity is implied, these vocalizations and facial expressions are brainstem reflexes.
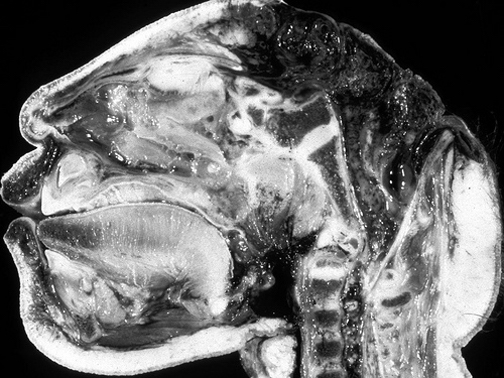
LIMBIC SYSTEM SEXUAL DIFFERENTIATION
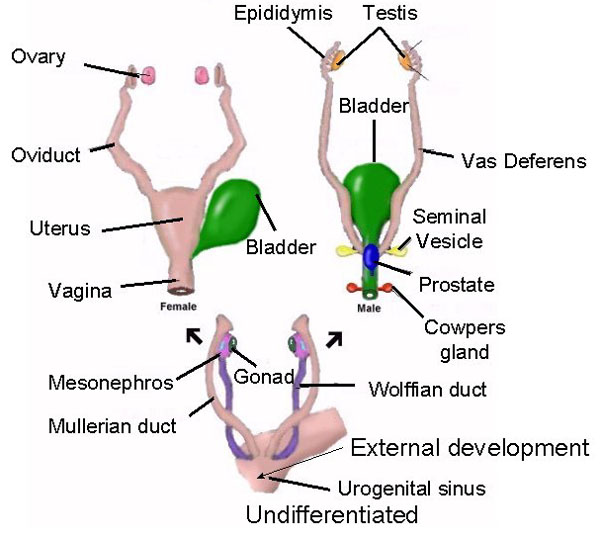
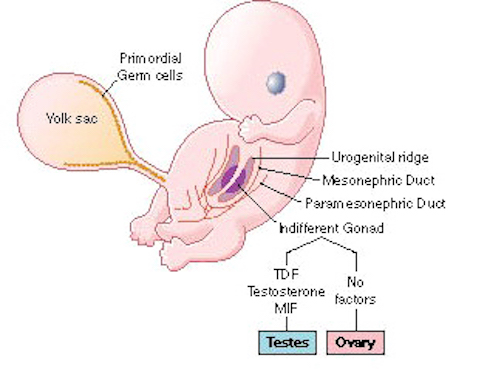
In humans the body and the brain first become sexually differentiated at about the third fetal month, and female gonads (which are very ribbon-like) and male gonads are evident, with the male sex-organs emerging in advance of the female. However, prior to this age, although genetically male or female, the fetus is physically/sexually-neutral. With the formation of the testes, and the secretion of testicular androgen, target tissues in the spinal cord, brainstem, and cerebrum become activated and transformed into "male" neurons, and form "male" patterns of neural development and neural circuitry.
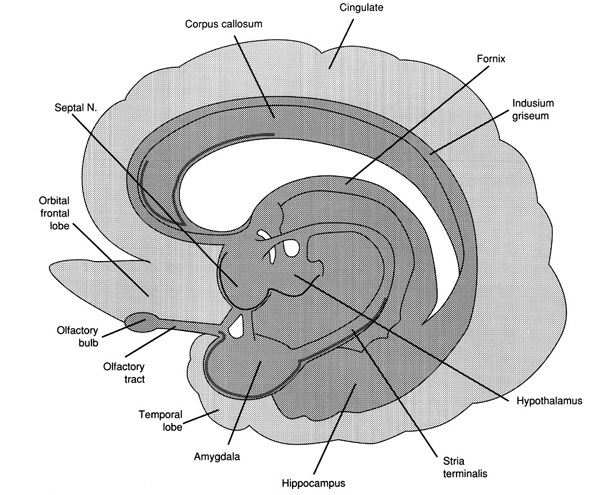
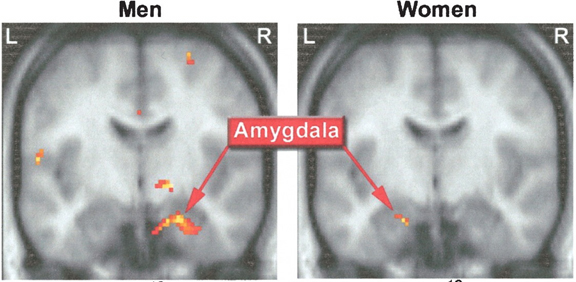
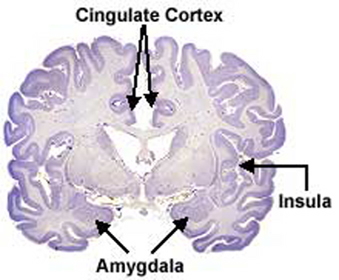
In fact, it is well established that the presence of testosterone, during critical periods of neurological development, directly effect neural growth, neural density, and the pattern of dendritic interconnections between target tissues in the hypothalamus, amygdala, hippocampus, cingulate gyrus, and the bed nucleus of the stria terminals and thus the septal nuclei as well as the amygdala (Bleier et al. 1982; Bubenik & Brown, 1973; Dorner, 1976; Gorski et al. 1978; Nishizuka & Arai, 1981; Rainbow et al. 1982; Raisman & Field, 1971, 1973). It is also well established that the presence (or absence) of testosterone directly effects and determines sexual and cognitive sex differences in mammals including humans (Barnett & Meck, 1990; Beatty, 2012; Collaer & Hines 2014; Dawson et al. 1975; Joseph et al., 1978; Masica et al., 1969; Resnick & Berenbaum 1982).
Presumably, these effects are initially triggered through the differential action of testosterone within target brain areas thus altering neuronal genomic expression ( Breedlove 2012; McCarthy 1994 for review). Testicular androgens are the primary catalyst for the male pattern of development and masculine sexual differentiation (Breedlove 2012). Specifically, by acting directly on target cell DNA, testosterone (or lack therefore) directly effects neural migration and proliferation vs programmed cell death, and thus cell growth, differentiation, density and myelination (Breedlove 2012; McCarthy 1994; Tobet & Fox 2012). In consequence, a "male" rather than a "female" brain is produced.
However, androgens appear to be modulated by a variety of genetic mechanisms which modulate receptor topography and the density of receptor populations which differ during different stages of development and as a function of gender. For example, although the adrenal cortex of both sexes and the ovaries produce some small amounts of testosterone, the genetic sex of the target tissue appears to effect receptor affinity for steroid binding, thus protecting the female from becoming "masculinized" by these androgen secretions.
It is noteworthy, however, that although testosterone, including fetal androgens are converted into dihydrotestosterone, which acts selectively on testosterone receptors, testosterone is also converted into estradiol (an estrogen), by an enzyme referred to as aromatase. Aromatase enables fetal androgens to bind to estrogen as well as to testosterone receptors (McCarthy 1994). That is, in part, males become "males" through the effects of a female hormone.
Females, however, are protected from becoming masculinized by their own estrogens or the estrogen secretions of their mothers by a suppressor protein which prevents DNA activation; i.e. (AFP) alpha-fetoprotein (Raynaud, et al. 2016). AFP binds to all circulating estrogens and thus changes its external chemical configuration so that it cannot be recognized by neural receptors (Toran-Allerand 1986). Males, however, appear to lack AFP, and there is no interference once testosterone if aromatized into estradiol.
Estrogen, however, not only effect male sexual differentiation, but female sexual differentiation. As noted, the ovaries also begin secreting estrogens during fetal development, and female hormones appear to act directly on estrogen and progesterone neural-receptors and directly influence and effect neurological differentiation (Dohler 1991; Toran-Allerand, 2012), including the sexual differentiation of the corpus callosum which is larger in male rats (Fitch & Denenberg, 2017) and larger in female humans (Holloway et al., 1994). Thus the female pattern of physical/neurological and sexual development is not merely due to default--that is, due to a lack of sufficient circulating androgens. In fact, the presence of these female hormones appear to also contribute to sex differences in behavior, including those which become manifest during infancy and childhood and certainly those which become manifest during adolescence. As detailed in chapter 8, females begin behaving in a sexually "precocious" fashion at a much earlier age than males, and in some cultures they willingly seek sex partners as young as age 8 (Ford & Beach, 1951); and estrogen directly effect sexual behavior and sexual receptivity.
Fetal Sex Differences
Anecdotally, it has been repeatedly reported that the late term male fetus is more active than the female fetus. That is, as based on anecdotal reports, the male fetus kicks, punches, and is more restless than the female fetus--which in turn provides mothers, midwives and physicians with some clue as to the sex of the baby (that is, before the invention of ultra-sound and other measures). Hence, as based on anecdotal reporting, sex differences in behavior may be in evidence just prior to birth.
Neonatal Sex Differences
Anecdotally, and as based on behavior observation, male neonates and infants are more active, squirm, and kick more than females. They are also more resistant to being held and cuddled and make less eye contact--that is, as compared to females (Freedman, 2003). These same sex differences become more apparent over the course of development (Freedman, 2003) and have also been reported in other species. Female humans, chimps, baboons and rhesus macaques cuddle more and more closely, and are cuddled more by their sisters, mothers and other females (Jensen, et al. 1968a, Hansen, 1966; Mitchell, 1968; Goodall, 1986).
Infant human or primates males are much more resistant to being held, and will kick and fuss, and actively attempt to escape their mothers arms much more so than females (Freedman, 2003; Mitchell, 2006). Males are also less likely to cuddle, and this remains a male characteristic throughout adulthood, and be it a male dog, chimpanzee, baboon, or child, they are far more likely than females to resist attempts to hold or pick them up, and may even respond as if they find it aversive.
Although these sex differences may be due to the presence of circulating androgens, they may also reflect the sexual differentiation of the brain and spinal cord, including the brainstem, hypothalamus, and amygdala. It would be the sexual differentiation of these latter structures, rather than testosterone per se, which would explain why females also smile and make direct eye contact at an earlier age and for a longer period of time and are thus more social than males (Kagan, 1971; Lewis, et al., 1966; Wolf, 1963; Spitz & Wolf, 1978). The sexually differentiated amygdala (Bubenik & Brown, 1973; Nishizuka & Arai, 1981), for example, is directly responsive to facial stimuli (Hasselmo et al., 1989, Heit et al., 2018; Morris et al., 1996; Rolls, 1984, 2012), and becomes activated when the subject is simply being gazed at (Kawashima et al., 2009). It is also exceedingly responsive to touch (see chapter 13) and to restraint (Henke, 2012). The amygdala and other limbic structures, therefore, may well play a major role in the emergence and development of these and other sex differences, as will be further discussed below.
SHAM EMOTIONS AND THE NEONATAL "SMILE"
Infants born with only a brainstem, i.e. anencephalics, are capable of crying, screaming, grunting, and make other sounds typical of neonates (Lemire et al., 1978). Anencephalics can also "smile" and their "smile" is indistinguishable from that of a "normal" neonate (Monnier, 1956). Likewise, stimulation of the brainstem can trigger a variety of facial expressions, including a wide grinned smile (Weinstein & Bender, 1943). However, these "smiles" are devoid of emotion, as they are brainstem reflexes (see chapter 24)
Like the anencephalic, the neonate can also "smile" (Izard, 1991; Sroufe, 1996). And, females begin to "smile" in advance of males. However, rather than a "true smile" the neonate and week-old infant (like the anencephalic), slightly lifts the corners of its mouth upward. And like the anencephalic, there is no evidence that these "smiles" represent true emotions (Spitz, Emde, & Metcalf, 1970; Sroufe, 1996; Wolff, 1963), as they instead appear to be brainstem reflexes (see also Lemire et al., 1978; McGraw, 1967; Monnier, 1956; Weinstein & Bender, 1943). Some investigators, such as Izard (1991), disagree with this view, and argue instead that the corresponding emotion is part of the infant's emotional repertoire regardless of the context in which the "smile" is produced. Thus, the infant "smiles" because it's happy and desirous of communicating these feelings. Izard's view, however, is rather untenable, as it is the forebrain which experiences happiness or pleasure (see chapters 10, 12, 13, 19, 21), and the forebrain of the week-old infant is exceedingly immature (Blinkov & Glezer, 1968; Conel, 1937) and there is almost no evidence of forebrain metabolic activity (Chugani et al., 2014). It is not until about one year of age that neocortical glucose activity begins to significantly increase (Chugani et al., 2014). Again, anencephalics are capable of "smiling" (Monnier, 1956). However, anencephalics do not possess an "emotional repertoire" as they do not possess a forebrain (Lemire et al., 1978). Hence, perhaps these "smiling" reactions should be considered "sham" emotions.
In fact, rather than indicating happiness, excitement or pleasure, neonatal smiles are often a prelude to immediately falling asleep and occur when the infant is tired and drowsy (Sroufe, 1996; Wolff, 1963). For example, although smiling can be triggered by feeding, as well as through vigorous visual and auditory stimulation, including the repetitive honking of a horn (Sroufe, 1996; Wolff, 1963), the infant will also immediately fall asleep and demonstrate REM-rapid eye movements (Emde & Koenig, 1969; Tennes, et al 1972). Likewise, by the end of the first month smiling can be triggered by a nodding head or mother's voice (Wolff, 1963). Again, however, the infant not only "smiles" but it may immediately fall asleep and display REM (Emde & Koenig, 1969; Emde, Gaensbauer, & Harmon, 1976; Tennes et al., 1972); and REM as well as reflexive "smiling" are brainstem mediated activities (Steriade & McCarley, 1990; Weinstein & Bender, 1943). Moreover, for the first several months of life most "smiling" occurs during REM sleep (Emde et al., 1976; Sroufe & Waters, 1976). In addition, for the first two months, physical stimulation, such as jiggling the infant or blowing on the skin, can trigger a "smile" even while the infant is sleeping (Emde & Koenig, 1969).
If the infant were truly happy, these same smiles should occur even more frequently when alert and awake. However, it's not until the infant reaches about 3-months of age that smiles can be easily triggered when the infant is fully alert (Wolff, 1963); a function of increasingy forebrain maturity (e.g. Hallett & Proctor, 1996; Herschkowitz et al., 2017).
Therefore, for the first several days and weeks, rather than feeling "happy" when the "smile" is produced, it appears the brainstem is merely aroused and triggering reflexive facial movements. That is, the brainstem activates the oral and facial musculature thus producing a "smiling" face in response to generalized and nonspecific tactile, visual and auditory stimulation and arousal, and while asleep and in REM. Thus, the brainstem appears to maintain the neural-musculature motor-memories for producing smiles, and these motor components are produced during the first few hours and days after birth.
Over the ensuing months, not only does true smiling gradually emerge, but reflexive smiling (as well as crying) declines (Herschkowitz et al., 2017); findings which suggest increasing forebrain control over the brainstem.
HYPOTHALAMUS, PERIAQUEDUCTAL GRAY, VOCALIZATION, QUIESCENCE, DISTRESS
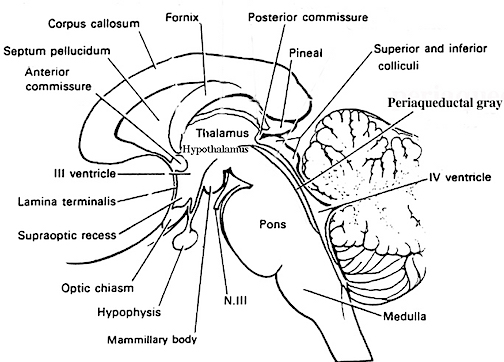
For the first several postnatal weeks, other than quiescence, the infant displays only one behavioral state suggestive of emotion: displeasure --demonstrated by crying and screaming (McGraw, 1969; Piaget, 1952; Spitz & Wolf, 1946). As based on neuroanatomical, metabolic, and physiological indices, these particular behaviors appear to be triggered by the still immature hypothalamus in conjunction with the midbrain periaqueductal gray which controls the oral-laryngeal musculature. When activated the periaqueductal gray acts on and coordinates the activity of the laryngeal, oral-facial, and principal and accessory muscles of respiration and inspiration and can thus vocalize a variety of sounds, including grunting, screaming, yelling, as well as crying in reaction to noxious stimuli (Jurgens, 1994; Larson et al., 2014; Zhang et al., 2004).
Specifically, the "motor engrams" for vocalization appear to be pre-programmed into the midbrain periaqueductal gray (Jurgens, 1994; Larson et al., 1994; Zhang et al., 1994). Hence, if the periaqueductal gray is disconnected from the limbic system and neocortex (such as by a midbrain transection), although language can no longer be produced, stimulation of this nuclei, or the immediately adjacent diencephalon (Davison & Kelman, 1939; Ironside, 1956; Martin, 1950) will continue to evoke vocalizations that are little different from a howling infant. This is why babies born without a forebrain can also grunt and cry out (Lemire et al., 1978; Monnier, 1956). Although conscious cognitive activity is implied, these vocalizations are brainstem reflexes.
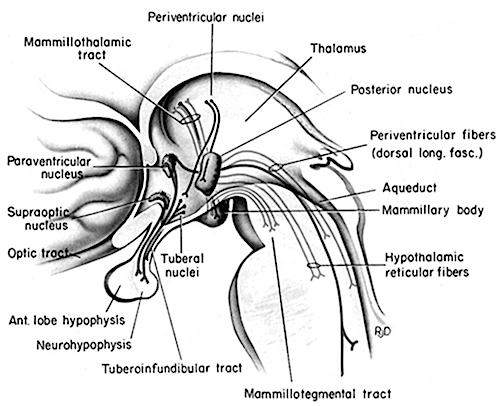
The hypothalamus, which monitors hunger and thirst, and governs internal homeostasis, is immediately anterior to the brainstem, and is richly connected with the periaqueductal gray. Given that the medial hypothalamus begins to mature and has established its brainstem connections at birth (Gibson, 1991; Langworthy, 1937), this structure can also stimulate crying and screaming if the infant is experiencing hunger, thirst, or other internal noxious sensations (see chapter 24).
As noted, the brainstem generally matures in a caudal to rostral arc, with the hypothalamus (and thalamus) beginning its cycle of maturation and myelination following the brainstem. Indeed, the normal pattern of maturational development--as is evident behaviorally and as based on myelination-- is that the brainstem and cerebellum begin to develop in advance of the forebrain, which in turn matures in a rostral arc, i.e. diencephalon, limbic system, striatum, neocortex (Barkovich et al., 2018; Brody et al., 2014; Gibson, 1991; Harbord, et al., 1990; Holland, et al., 1986; Lee et al., 1986). And these maturational events continue well into late childhood, adolescence and adulthood (e.g., Pfefferebaum, et al., 1994; Jernigan, et al., 1991; Kinney et al., 2018).
Nevertheless, at birth, the fiber pathways linking the hypothalamus and the brainstem are well established (Debakan, 1970; Langworthy, 1937) and appear to be fully, or at least, semi-functional (Joseph, 2012a). Since the hypothalamus is directly implicated in the generation of noxious and unpleasant mood states (MacLean, 1990; Olds & Forbes, 1981) it can be assumed that the hypothalamus contributes and may be responsible for the infant's initial tendency to frequently cry as well as display displeasure. However, like the brainstem, although capable of learning and demonstrating synaptic plasticity (Nakamura & Ono, 1986), the hypothalamus responds in a rather reflexive and immediate fashion when activated (see chapter 13).
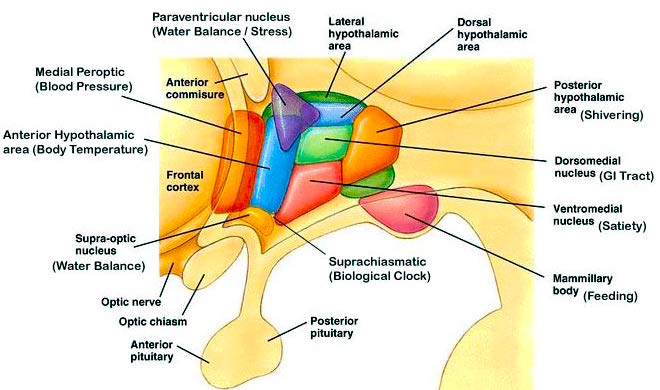
Because the forebrain generally matures in a paramedian to lateral direction (Dekaban, 1970; Yakovlev & Lecours, 1967), the medial hypothalamus matures in advance of the lateral hypothalamus (Joseph, 2012a); though in fact the hypothalamus as a whole continues to grow in volume well into puberty (Jernigan et al., 1991). It is the medial nucleus which generates negative and unpleasant emotions and mood states as well as quiescence. Depth electrode activation of the medial hypothalamus is so aversive subjects will work to reduce it (Olds & Forbes, 1981). Hence, at birth the medial hypothalamus appears fully capable of acting on the brainstem so as to reflexively trigger crying and distress, particularly in reaction to hunger and thirst as the hypothalamus also monitors internal homeostasis. And, it also appears to be responsible for generating quiescence such as following feeding (chapter 13).
The medial hypothalamus regulates the parasympathetic nervous system (e.g. reduction in heart rate, increased peripheral circulation) and exerts a dampening effect on certain forms of emotional/motivational arousal thereby producing quiescence. The parasympathetic nervous system also matures in advance of the (SNS) sympathetic nervous system (reviewed in Schore, 1994) which controls and promotes extremes in emotionality including positive emotions. However, the SNS is governed by the later to mature lateral hypothalamus (MacLean, 1990; Smith, DeVito, Astley,1990).
Quiescence and distress, the two predominate behavioral states demonstrated by the infant (McGraw, 1969; Piaget, 1952; Spitz & Wolff, 1946), is thus associated with medial hypothalamic activity. Therefore, for the first several postnatal weeks it appears that the medial hypothalamus is responsible for and is able to reflexively activate the infant's brainstem so as to produce crying in response to hunger, or quiescence following feeding.
Over the ensuing weeks and months, the medial followed by the lateral hypothalamus continues to mature--a developmental process which continues until puberty (Yakovlev & Lecours, 1967). However, in contrast to the medial hypothalamus, the lateral nucleus is implicated in positive mood states (Olds, 1956; Olds & Forbes, 1981). Therefore, as the lateral hypothalamus matures, the infant begins to display genuine feelings of pleasure.
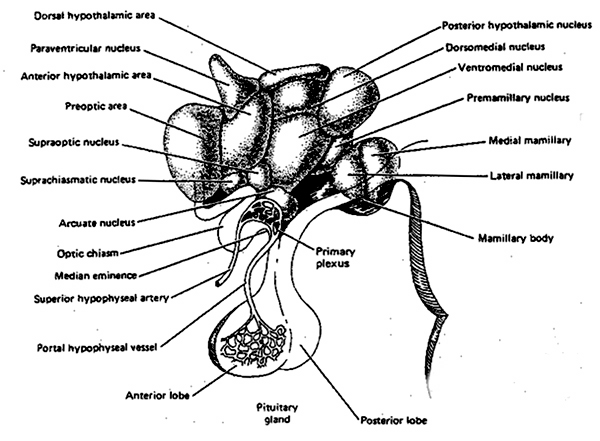
THE LATERAL HYPOTHALAMUS AND THE PLEASURE PRINCIPLE
Feelings of pleasure (as demonstrated via depth electrode self-stimulation) have been obtained following excitation of a number of diverse limbic areas including the olfactory bulbs, amygdala, hypothalamus, cingulate gyrus, and the medial forebrain bundle (MacLean, 1990; Olds & Forbes, 1981). The greatest area of concentration of reward sites, and the highest rates of self-stimulatory activity occur in the lateral hypothalamus. According to Olds (1956), animals "would continue to stimulate as rapidly as possible until physical fatigue forced them to slow or to sleep." By contrast, if the lateral region is destroyed the experience of pleasure and emotional responsiveness is almost completely attenuated (Marshall & Teitelbaum, 1974).
Thus, whereas the medial hypothalamus produces distress and quiescence, the later to mature lateral hypothalamus produces feelings of pleasure; and in this regard, it could be said that the hypothalamus mediates the pleasure principle (Joseph, 2012a). And yet, these reactions are only maintained so long as the hypothalamus is activated, for the moment the stimulation is removed, hypothalamic activity ceases, and all corresponding behavioral expressions of emotion disappears (MacLean, 1990; Olds & Forbes, 1981). Thus, much of the emotional activity associated with the hypothalamus is reflexive--as is the behavior of the infant for the first several weeks after birth. .
Nevertheless, although innately organized to react in an immediate and reflexive fashion, the hypothalamus displays synaptic plasticity in response to positive vs negative experience and is thus capable of learning (Nakamura & Ono, 1986). Thus the hypothalamus is also "experience-expectant" and its functional organization and its reactivity to positive vs negative experiences can also be altered, learned, and modified.
THE AMYGDALA AND SOCIAL EMOTIONAL DEVELOPMENT
By 3-months of age infants will vigorously smile with an open mouth, laugh, coo, express pleasure, and crinkle their eyes, and these smiles can be easily triggered when the infant is fully alert (Sroufe, 1996; Wolff, 1963); affective behaviors associated with the amygdala and lateral hypothalamus, and which are indicative of increasingly forebrain maturity (e.g. Hallett & Proctor, 1996; Herschkowitz et al., 2017). Correspondingly, it is not until after 3-months that drowsy and REM-sleep-induced smiling begins to disappear (Emde et al., 1976; Sroufe & Waters, 1976). It is also around 3-months that infants begin to smile at stationary stimuli (Zelazo, 1972), such as mother's smiling face, or stimuli which can put into motion by the infant (Wolff, 1963; Spitz et al., 1970). In contrast to the initial reflexive smiles, these latter "smiles" often occur only after prolonged or repeated presentations of an engaging stimulus. Therefore, and as also argued by Sroufe (1996), it is not until 3-months that the infant displays "true emotions," and a true smile --a time period which corresponds to increased forebrain functional activity as demonstrated by changes in the EEG (Spitz et al., 1970), which are still actually rather random in organization (Dekaban, 1970; Kellaway, 2009; Ohtahara, 2011).
The development of these more complex emotional reactions implicates the amygdala and striatum, as together these structures begin to mature around 2-3 months of age, and produce complex affective motor movements, vocalizations, and facial displays of emotion including social smiling (Joseph, 2014). Indeed, as the amygdala, striatum, and other limbic forebrain nuclei mature, displeasure, pleasure and brainstem-hypothalamic reflexive behaviors including rudimentary "smiling" become differentiated into more complex emotions and affective-motor reactions, such as wariness, joy, laughter, and fear.
Because the forebrain myelinates and matures in a caudal to rostral, paramedian to lateral arc (Gibson, 1991; Langworthy, 1937; Yakovlev & Lecours, 1967), the paramedially located amygdala begins to functionally mature and myelinate in advance of anterior-medially located forebrain structures such as the septal nuclei and anterior cingulate (Joseph, 2012a, 2014). Hence, given the role of the amygdala in generating, perceiving, and expressing a variety of complex emotions, including the experience and expression of fear, joy, and the desire for social emotional contact (Davis, Walker & Lee, 2017; Gloor, 2012, 2017; Halgren, 2012; Kling & Brothers 2012; LeDoux, 1996; Morris et al., 1996; Rolls, 2012; Rosen & Schulkin, 1998), and as the amygdala is also responsive to facial stimuli, including direction of gaze and eye-to-eye contact (Hasselmo, Rolls, & Baylis, 1989, Heit et al., 2018; Kawashima et al., 1999 Morris et al., 1996; Rolls, 1984, 2012), the infant's emotional, vocal, and affective-motor repertoire becomes increasingly complex, and spends more time gazing as faces and making eye-to-eye contact, as the amygdala matures.
Specifically, through its rich interconnections with various subcortical and neocortical regions including the brainstem, thalamus, and frontal, temporal, and parietal lobes (Amaral, Price, Pitkanen, & Thomas, 2012; Mesulam & Mufson, 1982; O'Keefe & Bouma, 1969), the amygdala receives converging auditory, visual, somesthetic, and gustatory sensory associations and is able to detect, monitor, sample and scrutinize this information for motivational and emotional significance. This includes the ability to discern, experience and express (via the striatum and brainstem) even subtle social-emotional nuances such as friendliness, threat, fear, love, affection, wariness, anger, etc., and at a more basic level, determine if something might be good to eat (Fukuda, Ono, & Nakamura, 2014; Gloor, 2012; Halgren, 2012; Kling & Brothers, 2012; LeDoux 1996; Morris, Frith, Perett, Rowland, Young, Calder, & Colan, 1996; O'Keefe & Bouma, 1969; Rolls 2012; Rosen & Schulkin, 1998). The amygdala is exceedingly important in regard to most aspects of social and emotional functioning, including the the maintenance of auditory and visual attention (see below).
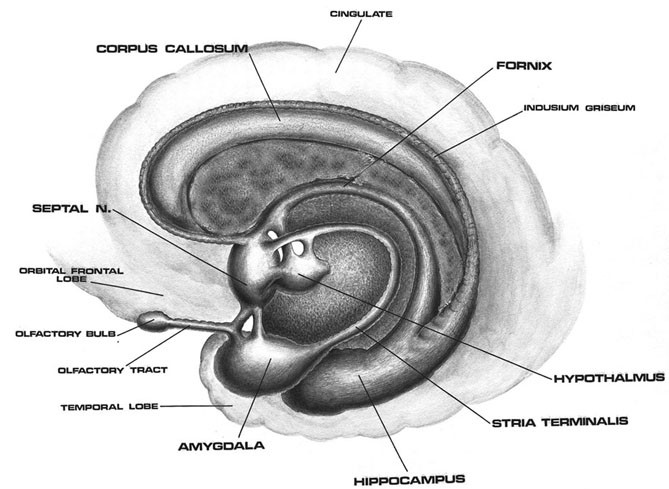
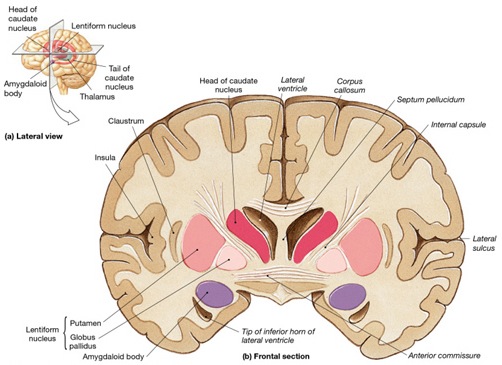
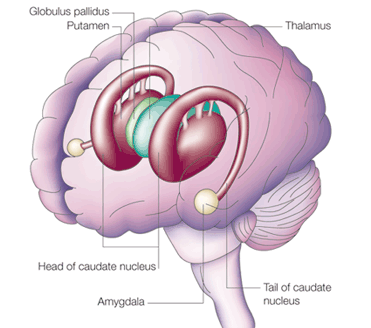
The amygdala also responds to and in turn, exerts controlling influences on the striatum, brainstem and hypothalamus, and thus emotional behavior, via the tail of the caudate nucleus and through the amygdalofugal pathways and a rope of fibers called the stria terminalis (Davis et al., 2017; Joseph, 2012a; LeDoux, 1996). The stria terminalis is a bidirectional pathway which interlinks the medial and lateral amygdala with the hypothalamus, as well as with the septal nuclei and brainstem including the periaqueductal gray (Krettek & Price, 1978). Hence, activation of the amygdala can produce complex affective-motor displays (see below), social-emotional vocalizations (Jurgens, 1990; Robinson, 1967, 1972), including laughter, or conversely a dense depression, coupled with sadness and crying (e.g., Chen & Forster, 1973; Offen, Davidoff, Troost, & Richey, 1976; Sethi & Rao, 1976).
However, initially the amygdala is so immature that its influences are negligible. The neonate initially behaves as if it has been amygdalectomized (Joseph, 2012a, 2014). The newborn does not fixate, does not maintain attention, does not respond to visual threat, and appears incapable of experiencing fear, wariness, or anxiety--emotions and motoric reactions stereotypically associated with the amygdala (Davis et al., 2017; Gloor, 2012; Halgren, 2012; Kling & Brothers 2012; LeDoux 1996; Rolls 2012; Rosen & Schulkin, 1998). In fact, these emotional reactions and almost all aspects of social and emotional behavior are generally abolished following bilateral amygdalectomy (Kluver & Bucy, 1939; Lilly, Cummings, Benson, & Frankel, 1983; Marlowe, Mancall, Thomas,1975; Terzian & Ore, 1955; Weiskrantz, 1956).
Although some amygdala neurons may well become fully functional soon after birth, in general, this structure (i.e. the medial/uncal region) does not begin to myelinate until around the second postnatal month (Yakovlev & Lecours, 1967). Over the ensuing weeks and months, the medial amygdala continues to rapidly mature, reaching an advance stage of myelination by the end of the first year (Yakovlev & Lecours, 1967) and therefore increasingly influences affective behavior including vocalization (chapter 15). The expression of these complex affective behaviors is accomplished via the rapidly maturing (e.g. Humphrey, 1972; Yakovlev & Lecours, 1967) amygdala-hypothalamic (stria terminalis), amygdala-brainstem (amygdalofugal) and amygdala-striatal fiber pathways.
THE MEDIAL AND LATERAL AMYGDALA AND THE HYPOTHALAMUS
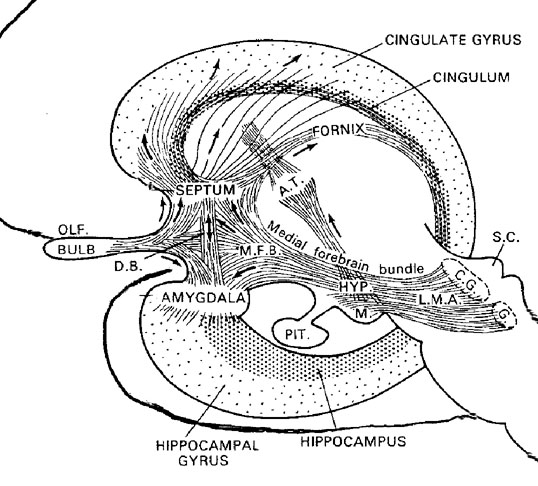
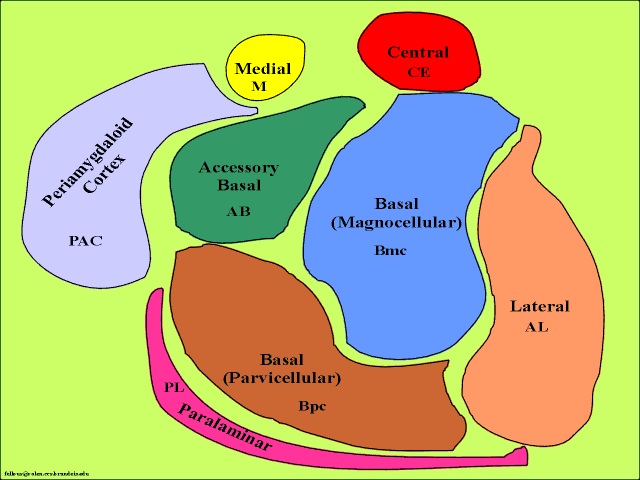
The medial amygdala is an exceedingly ancient structure that is concerned with influencing the most basic and rudimentary aspects of emotion and motivational functioning, including the motoric components of feeding, fighting, fleeing, and sexual behavior (chapter 13). By contrast, the lateral amygdala is a more recent evolutionary acquisition, is most highly developed in primates, takes longer to mature (Humphrey, 1972), and (in conjunction with the central nucleus) is concerned with highly complex affective states, including the generation of wariness, fear, emotional and sexual ideas, and anxiety (Davis et al., 2017; Gloor, 2012, 2017; Halgren, 2012; LeDoux, 1996; Rosen & Schulkin, 1998). The lateral amygdala is also implicated in the experience and expression of extreme pleasure (Olds & Forbes, 1981), as opiate receptors can be found throughout the amygdala (Atweh & Kuhar, 1977; Uhl, Kuhar, & Snyder, 1978). It also receives and contributes fibers to the medial forebrain bundle (MFB) which in turn has its site of origin in the lateral hypothalamus (Mehler, 2003) and then projects to the brainstem.
Through the MFB, amygdalofugal pathways, and stria terminalis, the lateral and medial amygdala responds to and exerts considerable excitatory and inhibitory influences on the hypothalamus, as well as act at the behest of hypothalamically induced drives (Joseph, 2012a). For example, if certain nutritional requirements need to be meet, the hypothalamus signals the amygdala which surveys the external environment for something good to eat or drink. If the amygdala were to detect a potentially threatening stimulus, it acts to excite and drive the hypothalamus (as well as the brainstem and striatum) so that the organism is mobilized to take appropriate action. Hence, when the hypothalamus is activated and driven by the amygdala, instead of responding in an on/off manner, cellular activity continues for an appreciably longer time period (Dreifuss, Murphy, & Gloor, 1968; Rolls 2012). The amygdala can also tap into the reservoir of emotional energy mediated by the hypothalamus as well as act independently of this structure so that certain emotional, sexual, or motivational needs may be attained.
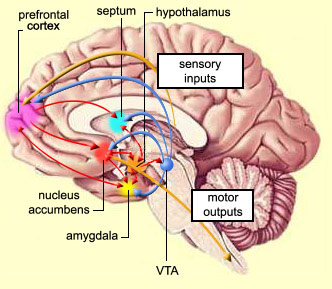
INFANT ATTENTION AND AMYGDALA MATURATION
The amygdala engages in environmental surveillance and can trigger orienting responses as well as mediate the maintenance of attention if something of interest or importance were to appear (Gloor, 2012; Rolls 2012; Ursin & Kaada, 1960). In part, these behaviors are made possible through interconnections with the thalamus, striatum, frontal and temporal lobes, and the amygdalfugal brainstem pathway which enables the amygdala to influence arousal as well as attention and the orienting response. Hence, with depth electrode amygdala stimulation the EEG becomes desynchronized (indicating arousal), heart rate becomes depressed, respiration patterns change, and the galvanic skin response significantly alters (Bagshaw & Benzies, 1968; Kapp, Supple, & Whalen,1994; Ursin & Kaada, 1960) --reactions which characteristically accompany the orienting response of most species.
Initially, due to amygdala (and forebrain) immaturity, although the neonate may become highly aroused (mediated by the brainstem) it is unable to focus or maintain attention. In fact, newborn infants often fall asleep when highly aroused (Tennes et al., 1972). However, by the end of the first month and over the ensuing weeks there is a significant increase in the amount of time the infant appears to be awake and responsive. By two to three months the infant's attention can be captured by a variety of engaging stimuli, in which case the infant will orient and cease to move (Sroufe, 1996). This latter reaction is basically identical to the orienting and "freezing" response triggered by direct amygdala stimulation (Gloor, 1960; Kapp et al., 2012) and is related to amygdala-striatal (chapter 16) as well as amygdala-brainstem activation (Davis et al., 2017; LeDoux, 1996; Rosen & Schulkin, 1998).
By the second month, the infant will orient, but may also smile, coo, and display periodic motor activity as they regard the stimulus (Tennes et al., 1972). Cooing, as well as early babbling, is directly associated with the initial stages of amygdala maturation as these sounds and oral behaviors can be triggered by amygdala activation (chapter 15). That is, the immature amygdala (as well as the "frightened" amygdala) can produce (as well as perceive, Halgren, 2012; Heit, Smith, & Halgren, 2018) emotional vocalizations, and if highly aroused it can induce a "teeth chattering" mandibular reaction, which when coupled with vocalization, results in babbling. As detailed in chapter 15, it is the maturation of the amygdala which is associated with "early babbling" and which vocalizes a variety of emotional sounds including cooing and laughter.
Therefore, by 3- to 4-months the amygdala begins to mature, the range of prosodic emotional vocalizations becomes more elaborate, and infants increasingly engage in environmental surveillance, interact with items of interest, maintain attention for prolonged time periods as they scrutinize whatever has captured their attention, and display pleasure as represented by the social smile and even laughter. However, the maturation of the amygdala continues throughout the first year and beyond, developmental events which are also paralleled by the emergence of wariness and then around 8 months, fear--emotions stereotypically associated with the amygdala.
THE AMYGDALA, STRIATUM, AND AFFECTIVE-MOTOR BEHAVIOR
The amygdala is a major nucleus of the basal ganglia and is intimately linked with the corpus striatum (caudate, putamen) and limbic striatum (nucleus accumbens, olfactory tubercle, substantia innominata/inferior globus pallidus). And, like the amygdala, the striatum, and the nigorstriatal system is sexually differentiated and differentially sensitive to male vs female hormones (Becker & Ramirez 1981; Camp, Robinson and Becker, 1984).
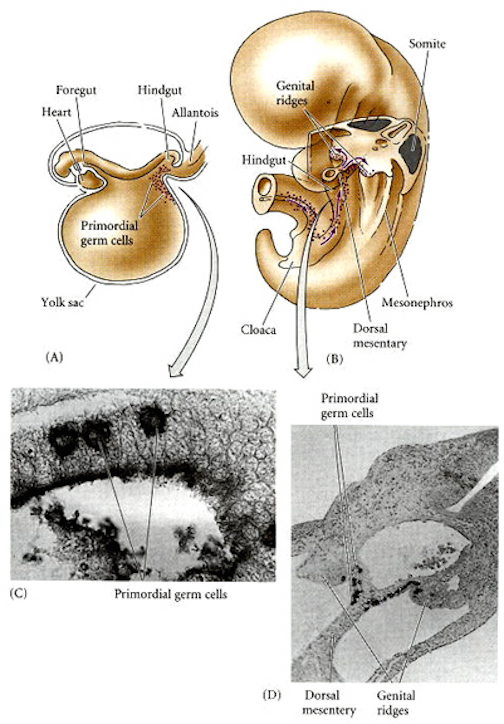
The amygdala forms the bulbous tail of the corpus striatum, whereas the limbic striatum is considered part of the "extended amygdala" (Heimer & Alheid, 1991). Specifically, the striatum and amygdala are derived from the arc shaped "striatal ridge," the caudal portion giving rise to the primordial amgydala at about the 6th week of gestation, and the anterior-basal portion later giving rise to the primordial striatum which initially overlies and is contiguous with the amygdala (Gilles et al., 1983; Humphrey, 1968). Thus the amygdala begins to develop before and then continues to develop simultaneously with the more rostrally situated striatum and both remain intimately interconnected (Gilles et al., 1983).
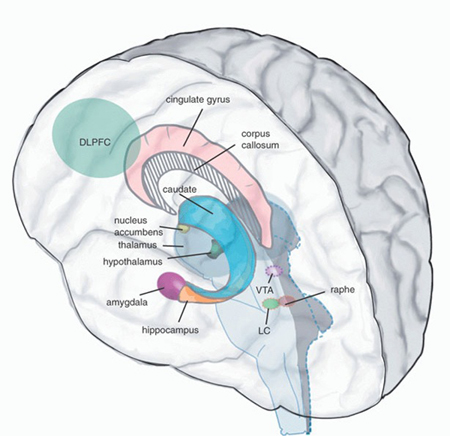
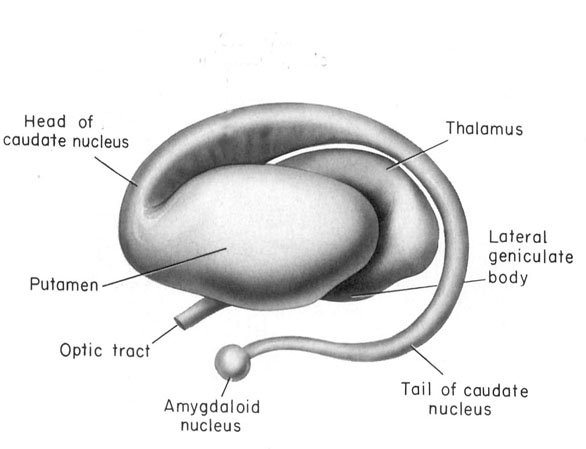
The amygdala and corpus and limbic striatum constitute a functional unit and interact in producing emotional facial expressions and coordinating gross, or whole body motor activity in reaction to emotional concerns (Everitt & Robbins, 2012; Heimer & Alheid, 1991; MacLean, 1990; Mogenson & Yang, 1991; Royce, 2014). If activated by the amygdala, the striatum (and subthalamus) may induce smiling, frowning, as well as a variety of stereotyped and ballistic motor actions such as running, kicking, and punching, or conversely "freezing" in reaction to extreme fear (chapter 16). Likewise, because all humans possess a striatum and limbic system that evolved, develops, and is organized in an identical manner, when happy, sad, angry, frightened, and so on, the facial and body musculature assumes the same readily identifiable emotional postures and expressions regardless of culture or racial orgins (Ekman 1993; Eible-Ebesfedlt, 1990; Joseph, 1993).
Like the amygdala, the striatum begins to myelinate and glucose metabolism begins to increase around the 2nd to 3rd postnatal month (Chugani, 1994; Yakovlev & Lecours, 1967), the age at which infant's first begin to display "true emotions" and true smiles (Sroufe, 1996). However, the striatum does not approximate adult levels of myelination until around 2-4 years of age (Gibson, 1991; Yakovlev & LeCours, 1967), and does not complete this developmental cycle until 8 years of age (Yakovlev & LeCours, 1967). It is after this later age that the striatum begins to diminish in volume (Jernigan et al., 1991)
Presumably, because the striatum (and subthalamus) are associated with ballistic motor movements of the arms and legs, including stereotyped perseverative motor activities (chapter 16), as these structures begin to mature, the infant begins to display rhythmical and perseverative motor stereotypies ("secondary circular reactions," Piaget, 1952), such as bouncing, swaying, kicking, waving, and banging (Thelen, 1982). Specifically, rhythmical stereotypies tend to emerge around 10-12 weeks of age, and then peak in intensity and frequency between 26-42 weeks of age (Thelen, 1982), thus paralleling striatal maturation (see also Chugani, 1994). According to Thelen (1982, p. 241), "at 6 months the infant may respond to many stimuli with this limited stereotyped motor output.... the infant uses rhythmical kicking both to greet the mother and to protest the removal of a toy. At 12 months, those same situations may be met... perhaps by crawling or walking," behaviors which are under the control of the neocortex and telencephalon.
Therefore, as the striatum (and amygdala) reach advanced levels of maturation around one year (and as the frontal motor areas also begin to mature at this later time period), likewise, these motor stereotypies decrease in frequency, particularly those which are triggered by kinesthetic stimulation or when the infant is in a non-alert state (Thelen, 1982).
THE AMYGDALA, THE SOCIAL SMILE, AND THE HUMAN FACE
The amygdala is exceedingly responsive to social and emotional stimuli as conveyed vocally, through touch, and via the face and eyes (Gloor, 2012; Halgren, 2012; Kawashima et al., 1999; Kling & Brothers 2012; Morris et al., 1996; Rolls, 1984, 2012). In fact, the amygdala, as well as the overlying (and partly coextensive) temporal lobe, contains neurons which respond selectively to smiles and to the eyes, and which can determine the direction of another persons gaze and/or become activated in response to direct eye contact (Kawashima et al., 1999) and which differentiate between male and female faces and the emotions they convey (Hasselmo, Rolls, & Baylis, 1989, Heit et al., 2018; Rolls, 1984). For example, the normal human amygdala responds to frightened faces by increasing its activity (Morris et al., 1996), whereas injury to the amygdala disrupts the ability to recognize faces (Young, Aggleton, & Hellawell,2014).
As the medial-uncal regions of the temporal lobe and medial and lateral amygdala mature, a broad range of feature-detecting neurons become functional and activated by specific stimuli, such as the human face (chapter 15, 28). In fact, the human (female) infant will display semi-orienting responses toward faces, and even the outlines of the human face, as early as 9 minutes post partum--that is, so long as it contains all facial features. If the features are deleted this waxing and waning tendency disappears (Goren et al., 1975). These findings also indicate that the brain is genetically predetermined to respond to facial stimuli.
That these responses are mediated by the amygdala and not the visual cortex is also apparent based on the lack of responsiveness of the visual cortex to facial stimuli, and the fact that it is not until around the 4th month of age that the neurons and connections in the neocortex necessary for form perception begin to develop (Brukhalter et al., 1993). Hence, these behaviors appear to be largely mediated by the amygdala.
As the infant approaches 2-months of age it will orient toward a familiar face (Carpenter, 1974) and begins to display definite preferences for facial stimuli. By 6-months it can discriminate between male and female faces, and by 9 months it can easily discriminate between different facial expressions (Caron, Caron & Myers, 1985; Sroufe, 1996)--functions associated with the temporal lobe and amygdala.
In fact, as these structures and their facial-feature-detecting neurons mature, infants increasingly attend to and demonstrate an almost irresistible interest in facial stimuli (Bronson, 1972). If presented with a strange face, although they may look away they will also quickly look back, "and will make eye-to-eye contact as though drawn by a magnet" (Sroufe, 1996, p. 103).
The maturation of temporal lobe-amygdala neurons which are selectively sensitive and drawn to human faces is an exceedingly adaptive development as it promotes social emotional, face-to-face interaction and the formation of emotional attachments. Moreover, these eye-to-eye and facial-detecting-neurons are richly interconnected with yet other amygdala-temporal lobe and striatal neurons concerned with social and emotional functioning, including those which can trigger a smiling, laughing, and even a crying and sobbing response (Chen & Forster, 1973; Offen et al., 1976; Sethi & Rao, 1976). Therefore, activation of these tissues can also trigger smiling and social behavior. However, as these neurons are "experience-expectant" and require social-emotional stimulation in order to develop, establish neural pathways, and thrive, if this form of input is insufficient or abnormal, these neurons are likely to die (see below).
Hence, as the infant matures and its ability to attend to and perceive specific social emotional stimuli becomes more pronounced, so too does its ability to produce a "social" smile (Joseph, 2014)--a capacity that first appears around 3-months of age (Sroufe, 1996) and which is likely produced by amygdaloid influences on the striatum and brainstem. Over the next three months these smiles become increasing social as they are produced most frequently during greeting and when making face-to-face and eye-to-eye contact, especially with their mothers (Ainsworth, 1973); perceptions and behaviors which activate the amygdala. Moreover, these smiles may be accompanied by genuine laughter--vocal expressions associated with the amygdala (as well as the hypothalamus).
By 4-months, laughter similar to adult laughter can be reliably evoked (Sroufe, 1996), and from 4- to 6- months of age, it takes progressively less vigorous stimulation to produce laughter which in turn is evoked by a broader range of auditory and visual stimuli (Sroufe, 1996). Therefore by 3-4 months the infant appears fully capable of experiencing as well as expressing pleasure as demonstrated through smiling and laughing--that is, so long as it is provided sufficient social-emotional stimulation.
Laughter, and the social smile plays an exceedingly important role in promoting social interaction and in communicating and forming an emotional attachment to the mother. That is, the mother is rewarded by the infant's smile and laughter which thus promotes maternal attention and affection. These interactions also serve to provide increasing social and emotional stimulation to the "experience-expectant" and still maturing amygdala and limbic forebrain. Again, if normal input is not provided, the amygdala, as well as other forebrain nuclei, might atrophy, form abnormal interconnections, and develop seizure-like activity, such that in consequence, all aspects of social and emotional behavior (including emotional memory) become abnormal (chapter 28).
THE AMYGDALA AND SEX DIFFERENCES IN SMILING
The female and male primate amygdala are sexually differentiated and have their own unique patterns of dendritic growth and organization (Bubenik & Brown, 1973; Nishizuka & Arai, 1981). In humans the male amygdala is 16% larger (Filipek, et al., 1994)., and in male rats the medial amygdala is 65% larger than the female amygdala (Breedlove & Cooke, 1999). By contrast, female amygdala neurons are smaller and more numerous, and densely packed than those of the male (Bubenik & Brown, 1973; Nishizuka & Arai, 1981), which in turn would effect frequency and ease of firing. In addition, the striatum and nigro-striatal pathways are sexually differentiated and respond selectively to male vs female hormones (Becker & Ramirez 1981; Camp et al., 1984) )
These sex differences not only appear to be directly due to the effects of fetal androgens on gene expression and thus neural differentiation, but the male amygdala grows or shrinks in the presence of testosterone (Breedlove & Cooke, 1999). By contrast, dendritic spine density in the female rat hippocampus also increases and decreases by as much as 30% during each estrus cycle (Woolley, et al., 1990), and the amygdala and hippocampus are not only adjacent, but intimately interconnected and functionally interact. The hippocampus, for example, also contains neurons which respond to faces, as does the overlying temporal lobe (Hasselmo et al., 1989, 2018; Rolls, 1984).
In addition, the human anterior commissure which connects the right and left amygdala/temporal lobe is sexually differentiated (Allen et al. 1989), as is primate/mammalian hypothalamus (Bleier et al. 1982; Dorner, 1976; Gorski et al. 1978; Rainbow et al. 1982; Raisman & Field, 1971, 1973)--with which the amygdala is intimately interconnected. Likewise, the bed nucleus of the stria terminalis is sexually differentiated, and this pathway links the amygdala with the septal nuclei.
Activation of these structures induces obvious sex differences in behavior, including, such as sex mounting vs the lordosis (sexually receptive) posture (Hart et al., 1985; Lisk, 1967, 1971; Maclean, 1973; Robinson and Mishkin, 1968; Stoffels et al., 2003). However, in females, activation of these structures can also trigger affiliative social-emotional behaviors including the desire to cuddle and make eye-to-eye and face to face contact as is stereotypical between mother and infant (Numan, 1985).
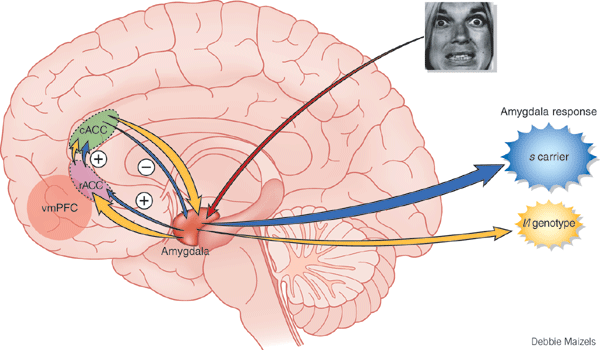
As noted, the amygdala becomes activated when the subject is simply being gazed at (Kawashima et al., 1999). Females stereotypically find face to face and eye to eye contact to be relatively rewarding, that is, as compared to males who view direct eye contact and the baring of teeth as a challenge and a threat (Eibl-Eibesfelt, 2014; Joseph, 1993). As per females, this is exceedingly adaptive, as women have babies, and as face-to-face and eye-to-eye contact is important in social-emotional development (see chapter 28).
Females begin smiling soon after birth and at an earlier age than males (Wolf, 1963) and thus mothers spend more time making eye-to-eye and face-to-face contact with their daughters who reciprocate by smiling. Females smile on average about 30% more than males and are far more likely to maintain sustained and direct eye contact with a woman than a man is with a man (Freedman, 2003). Even across cultures females smile more than males and at a higher rate as compared to men (Freedman, 2003; Eibl-Eibesfelt, 2014). However, whereas among males the smiling response can be viewed as an indication of threat, or appeasement, females employ smiles to indicate friendliness and appeasement (Eibl-Eibesfelt, 2014). When the baby girl begins smiling, she does so not only as a neurologically hard-wired response, in preparation for the maternal role she will someday play, but also as a signal which states: Don't hurt me. I am submissive. I am friendly. Because of the "submissive" (and aggressive) connotations, males are far more likely to smile at a female stranger, than at another male. Males avoid providing appeasement gestures except when under duress and are more leery of smiling as it may be perceived as aggressive or submissive by other males who in turn are likely to demand, "What are you smiling about?" In that a smile can involve the bearing of teeth, it is thus not surprising that a grin might be seen as antagonistic, particularly since males tend to perceive or suspect belligerence even when there is no aggressive intent.
When females smile at males, they also tend to make fleeting eye contact coupled with head bows, nods, and other appeasement signals (Eibl-Eibesfelt, 2014)--which are mediated by the amygdala-striatum (see chapter 16). Similarly when female and subordinate male chimps approach dominant males, they will smile, bow, and bob their heads. The smile and bowing indicates friendliness or submission which serves to reduce social tension and threat, which is why females smile more than males. These are common appeasement gestures utilized not only by humans, monkeys, and apes, but wolves, dogs, and similar creatures (Eibl-Eibesfelt, 2014), and these sex differences, which appear soon after birth, appear to be a direct consequence of the sexual differentiation of the limbic system, including the striatum and the amygdala in particular.
THE EVIL EYE
Insofar as a smile may be mistaken for appeasement (or conversely as aggressive), men are less likely to employ a smile even when making direct eye contact. They are in fact even less likely to make direct eye contact with other males (vs females) due to the propensity of some males to aggressively demand, "What are you looking at?" Males often perceive direct eye contact as a threat and are thus less willing to provide it (Eibl-Eibesfelt, 2014). Many male animals, including apes, monkeys, and even dogs, also avoid direct eye contact except to indicate aggressive intent (Eibl-Eisbesfelt, 2014, Lorenz, 1966). It is for this reason that among many cultures direct eye contact can even connote evil and the casting of spell; i.e. the "evil eye." Hence, males tend to avoid staring at others.
In consequence, even among married couples women often complain that their husbands or sons fail to look at them when they are talking, as they instead tend to stare at a newspaper or the television. Male often fail to provide sustained eye contact even with his dates or girlfriends unless he is feeling aggressive or sexually aroused. As such, the woman is likely to feel he is not paying attention or taking her seriously, and may even feel threatened by what she believes is his "roving eye."
Again, these sex differences are apparent soon after birth (Kagan, 1971; Lewis, et al., 1966). Females make direct eye contact soon after birth, make more direct eye contact, and as they age, more prolonged eye contact, whereas infants males will spend as much time gazing at a bull's eye target as a human face (Kagan, 1971; Lewis, et al., 1966; Wolf, 1963; Spitz & Wolf, 19). Again, this sex difference can also be attributed to the amygdala, for as this structure matures, these sex differences become more pronounced and is even evident in the different types of toys boys vs girls prefer. That is, females are more drawn to toys that have an easily identifiable and human-like face, such as teddy bears and dolls and play games where there is maximal face to face contact (Ahlgren & Johnson, 2006; Olds & Shaver, 2003; Duda, 1983; Fagot, 1978).
In contrast, boys like face-less toys, such as guns, trucks, blocks, hammers, military toys, vehicles, machines, sports equipment (Rhengold & Cook, 1975; Fagot, 1974; Smith & Daglish, 1977; Gaylin, 1993, Ross, 1993), including action figures where all essential humanness is deemphasized; e.g. Ninja Turtles. Hence, whereas male toys emphasize aggression, killing, conquering, and destruction, female toys and games tend to stimulate social cooperation and face to face interaction. This is because females are more geared toward receiving and providing social and face to face stimulation.
Again, even during early infancy females are more socially inclined and responsive to faces than are males, and can differentiate adult male from female faces and voices, and are more interested in faces and will stare at faces longer than boys (Kagan, 1971; Lewis, et al., 1966) who in turn are as likely to look at a bull's eye as a face (Lewis, et al. 1966).
WARINESS, STRANGER FEAR, AND THE AMYGDALA
For the first several weeks infants express distress in reaction to internal noxious stimulation, such as hunger and thirst, but remain largely unresponsive to noxious external stimuli (Wolff, 1969). Although the hypothalamus triggers distress in response to hunger and thirst, these initial, emotionally limited reactions are indications of forebrain-telencephalic immaturity. Indeed, the baseline EEG of a 3-month and even 6-month old infant consists of random, poorly organized low voltage slow waves, and it is not until 6-8 months that a transient regularity in EEG activity becomes apparent (Dekaban, 1970; Emde et al., 1976; Kellaway, 2006; Ohtahara, 1981). This diffuse and random slow wave neocortical activity appears to be a direct reflection of random and immature neural activity, and thus the diffusely overabundant, random, largely unmyelinated synaptic organization of the telencephalon and neocortex (Rakic, Bourgeois, Echenhoff, Zaecevic, & Goldman-Rakic, 1986) --much of which continues to mature well beyond the first decade (Blinkov & Glezer, 1968; Conel, 1937-1967; Flechsig, 1901; Holland et al., 1986; Huttenlocher, 1990; Yakovlev & Lecours, 1967).
Likewise, due to forebrain-amygdala immaturity, for the first several postnatal months the infant does not display any signs of wariness or fear. Wariness and fear, however, are associated with the functional integrity of the amygdala which does not reach adult levels of myelination until the end of the first year (Yakovlev & Lecours, 1967).
As noted, the medial and lateral amygdala mature at different rates and are concerned with somewhat different and more primitive vs more advanced emotional functions. Likewise, emotional reactions, such as pleasure, wariness and fear appear at different ages; which is exceedingly adaptive. As fear reinforces selective- as well as inhibits indiscriminate-social contact seeking, feelings of fear emerge much later in development; otherwise fear might interfere with the experience-expectant emotional needs of the developing brain by preventing or interfering with the reception of social stimulation. Thus initially the infant is fearless, and later, around 4 months they demonstrate wariness, which is followed, around 8-9 months of age, by true fear (Emde et al., 1976; Sroufe, Waters, & Matas, 1974).
At this later age, infants may orient toward a novel, strange, or threatening stimulus, and then immediately express considerable fear and negativity coupled with heart rate deceleration (Emde et al., 1976; Sroufe et al., 1974). By contrast, at 4-months of age it takes up to 30 seconds before a wariness and a distress reaction can be triggered (Bronson, 1972).
Hence the wariness versus the later appearing fear response can be taken as indications of increasing amygdala maturity, and both reactions can be evoked following depth electrode activation of the amygdala. Indeed, fear is the most common emotional reaction elicited from direct amygdala stimulation (Chapman, 1960; Davis et al., 2017; Gloor, 2012; Halgren, 2012; Rosen & Schulkin, 1998; Ursin & Kaada, 1960). The EEG becomes desynchronized, heart rate decellerates, respiration patterns change, the galvanic skin response significantly alters, the pupils dilate, the face contorts and will display extreme fear, and the subject will cringe, withdraw, and cower (Bagshaw & Benzies, 1968; Davis et al., 2017; Kapp et al., 1994; Ursin & Kaada, 1960). This cowering reaction in turn may give way to panic and the subject may attempt to take flight via amygdaloid influences over the brainstem and striatum.
Likewise, the human amygdala becomes activated when experiencing fear (Halgren, 2012; Rauch, van der Kolk, Fisler, Alpert, Orr, Savage, Fischman, Jenike, & Pitman, 1996) and abnormal activity in the amygdala or overlying temporal lobe can evoke overwhelming, terrifying feelings of death-like "nightmarish" fear (Herman & Chambria, 2003; Strauss, Risser, & Jones, 1982; Weil, 1956). Hence, the fear response which appears around 8 months of age, is an obvious indication of an advanced level of amygdala maturation, whereas the infant's initial fearlessness is an indication of amygdala immaturity.
Moreover, unlike hypothalamic on/off emotions, amygdala-fear reactions can last up to several minutes after the stimulation is withdrawn. Amygdala pathways may also remain potentiated for minutes, hours, and even days following fear induced stimulation (Clugnet & LeDoux, 1990; Rosen & Schulkin, 1998) such that it appears to contine to process--in the abstract--information that is no longer observable (O'Keefe & Bouma, 1969). Hence, the child or the adult may remain fearful long after the threat has been removed. Unfortunately, if the child or adult is repeatedly frightened, this will accentuate and promote the development of those neural pathways which mediate the fear response--a function of fear induced synaptic plasticity (e.g. Clugnet & LeDoux, 1990; LeDoux, 1996). In consequence, the child/adult who is repeatedly traumatized, later becomes easily startled, frightened and fearful and thus displays many of the classic signs of PTSD.
However, if both the right and left amygdala are completely destroyed, this results in a loss of fear, reduced aggressiveness, and docility even among purportedly ferocious creatures such as the agoutie and lynxe (Schreiner, & Kling, 1956; Weiskrantz, 1956; Vochteloo & Koolhaas, 2014). Likewise, adult humans who have undergone bilateral amygdala destruction become emotionally unresponsive and lose the ability to demonstrate or recognize vocally or facially conveyed fear or other emotions (LeDoux, 1996; Lilly et al., 1983; Marlowe et al., 1975; Ramamurthi, 2018; Scott et al., 2017; Terzian & Ore, 1955 Young et al., 2014). On the other hand, if sufficiently aroused or irritated, even the most placid of amygdalectomized animals can be induced to flee or fiercely fight (Fuller, Rosvold, & Pribram, 1957). However, these flight and aggressive responses are very short-lived and appear to be reflexively mediated by the hypothalamus (Joseph, 2012a; Wasman & Flynn, 1962).
THE DEVELOPMENT OF SOCIAL BEHAVIOR AND STRANGER-FEAR
Prior to developing fear, infants become increasingly socially oriented--an amygdala (and cingulate) mediated activity. Until 6-7 months of age the infant will smile at the approach of anyone, even complete strangers. The infant will also vigorously protest any form of separation from strangers (e.g. if they leave the room). It is the intense need for social and emotional stimulation which in part explains the infants indiscriminate tendency to welcome contact even from strangers--activities which maximizes opportunities for physical-social interaction and which provides the experience-expectant stimulation that the limbic system requires in order to develop and function in a normal manner. In fact, so intense is the need for physical and social contact that young animals raised in social isolation will form attachments to bare wire frames, to television sets, to dogs that might maul them, to creatures that might eat them, and among humans and non-human primates, to mothers that might abuse and even kill them (Cairn, 1966; Harlow & Harlow 1965a,b; Joseph, 2012a, 2014).
It is not until about 7-months of age that infants become more discriminant in their interactions and it is during this time period that a very real and specific attachment is formed; for example, to one's mother --an attachment which normally becomes progressively more intense and stable. After these specific attachments have been formed, children increasingly display wariness, and then fear and even flight reactions at the approach of a stranger (Spitz & Wolff, 1946). By 9-months, 70% of children respond aversively to strangers, whereas by 10-months they might cry out if a stranger were to approach (Schaffer, 1966; Waters, Matas, & Sroufe, 1975). By one year 90% of children respond aversively to strangers (Schaffer, 1966). The fear reaction, of course, is also exceedingly adaptive as strange people, animals, or objects, represent potential danger. Moreover, amygdala generated feelings of stranger-fear reinforce and promote the establishment of a safe and secure emotional attachment with the primary caretaker; e.g. the mother.
Indiscriminate contact seeking, followed by the establishment of specific attachments, and then the fear response are not only linearly linked but are manifestations of maturational and developmental changes occurring in the amygdala, followed by the septal nuclei and cingulate (Joseph, 2012a, 2014), and (around the first year of life) the frontal lobes (e.g., Schore, 1994; Steklis & Kling, 1985).
SEX DIFFERENCES IN FEAR AND STRANGER WARINESS
For most of the first year of life, infants are fairly indiscriminate in regard to contact seeking and show little fear of strangers. It is not until humans reach 8-11 months of age, that wariness and fear of strangers has become fairly apparent. At this age, girls and boys seek the proximity and the arms of their mothers when in the presence of strangers. However, girls will more quickly retreat and seek comfort in their mother's arms (Freedman, 2003), whereas boys will more quickly disengage from their moms, and will become less fearful of strangers at a much earlier age than females (Freedman, 2003), and this is also true of other mammals (Fedigan, 2012; Jensen, et al. 1968a, Hansen, 1966; Mitchell, 1968c; Goodall, 1986). Be it little girls, or adult women, females in general are much more fearful than males and this is also evident in the different manner in which boys and girls interact with strangers and thus express fear of strangers.
For example, boys between the ages of 3 to 5 will avoid making full facial contact with strangers and will tend to stand at an angle with their heads turned to the side (Steren & Bender, 19 ). As noted, males find direct facial and eye contact aversive and aggressive, and young males are wary of provoking attack. However, when in the presence of strangers young males tend to place their hands behind their back which is a form of genital display and a type of threat (Wickler, 1973; Eibl-Ebesfeldt, 2014). Hence, young males tend to assume postures that are intermediate between fear and threat. This enables them to mask their true feelings whatever they may be.
Females, in contrast, will fully face the stranger and smile or hide their face with their hands. They are also more likely to make hand-to-face gestures, such as touching their hair, head, or eyes or lips, and to hold their hands in front of their bodies when facing strange adults or when feeling fear or anxiety (Stern & Bender, 19). Placing the hands in front of the face is a common defensive posture used across cultures and is employed during times of surprise, shock, and embarrassment (Eibl-Eibesfeldt, 2014).
Not just girls but young women tend to show apprehensive smiles and mouthing behavior, such as biting or sucking their lips in, holding them tightly together and smiling, particularly when faced with strangers or novel situations. These are smiles of fear (Eibl-Eibesfeldt, 2014) and are used to signal appeasement. Female baboons, chimpanzees, and macaques also use the fear grin to ward off attack (DeVore, 1965; Kummer, 1966; Goodall, 1986; Fedigan, 2012; Eibl-Eibesfeldt, 2014; Wickler, 1973).
Girls, however, sometimes avoid making direct eye contact during these times and may stand as if frozen, or assume a hunched protective posture without moving. They may also fail to smile. However, often they do smile which makes them appear to be acting coyly when in fact they are experiencing low level fear. As noted, in contrast to males, young females are also more likely to retreat and withdraw from situations involving strange adults. Boys are not only less fearful in general, but by age 10 they are more likely to stand closer to someone they fear, whereas females are more likely to stand closer to someone they like (Guardo, 19).
Young females also tend to be very fearful of strange, complex or novel situations or environments even when no strangers are near whereas young boys have no difficulty playing under these conditions (Lewis & Weinraub, 19; LeMasters 1975; Rosenblum, 19; Stern & Bender, 19;Hannerz, 1970 Haugh et al. 2003; Rubin et al. 1983). Even during play in a familiar environment when no strangers are present females are far more likely than males to gather close to their mothers and teachers (Fagot, 1978; Omark & Edelman, 1976; Freedman, 1974; Rubin, et al. 1976Bott, 1971; Komarovsky, 1964; Young & Wilmot, 1957), and the same is true of female primates.
Girls will also play longer when in the company of their mothers vs strangers or even their fathers in which case they may withdraw or sit quietly, whereas boys show no difference. If given a choice between remaining near their mother or exploring a complex or strange environment, males are far more likely to explore and to greatly decrease maternal contact whereas females will increase maternal contact even though it is only toys and novel objects that confront them and no stranger is present (Rosenblum).
Females, in fact, are not only less self-confident and more fearful than males (Lenney, 1977; Dweck & Bush, 1976; Dweck et al. 1978) but they are far more likely to develop phobias and pathological fearfulness as adults (see chapter 31). These findings hold true across cultures be it the Japanese, Balinese, Chinese, or even Australian Aborigines, and is also the case for boys and girls blind from birth.
STRANGER WARINESS AMONG FEMALE PRIMATES
Beginning before the first year of life and continuing throughout childhood girls remain more fearful of strangers or novel environments or situations, and are more attached to their mothers whom they seek for reassurance. This is not a function of differential parenting or sexist training, however, for these sex differences hold true even among opposite sex twins as well as among all social female primates ( Mason, et al. 1959; Brooks & Lewis, 19; Goldberg & lewis, 19; Mitchell, 1973; Goodall, 1986; Fedigan, 2012). These sex differences are innate and appear to be directly attributable to sex differences in the limbic system, the amygdala in particular.
Like their human counterparts, female primates are much more wary of strangers than males (Mason, et al. 1959; Goodall, 1986). Similarly during the first two years of life female primates prefer to remain closer to their mothers and will run to her when frightened (Mitchell, 2006; Fedigan, 2012; Goodall, 1986). For example, even when there is no stranger present four month old females macaque monkeys spend 75% of their time with their mother, whereas males are as likely to wander away as to maintain contact. Similarly, when a stranger is present, infant female macaques are significantly more likely to seek their mother's, whereas males do not. In fact, female primates spend almost twice as much time with their mother whereas males spend as much time with either the stranger or mom or engaged in some other activity (Fedigan, 2012). Moreover, if the mother is absent females will avoid the stranger 10 times more so than males and will withdraw and sit in a far corner or as far away as possible. It is not until much later in age that females begin to show a decrease in their wariness and are willing to approach strange females (but not males) as much as their mothers (Rosenblum, 19).
Hence, again, these sex differences appear to be innate, as they appear not only across cultures, but among different species, including human and non-human primates. These sex differences appear to be directly attributable to sex differences in the structure and functioning of the amygdala and other limbic tissues, and are probably directly influenced by the presence of absence of circulating testosterone.
SEPTAL NUCLEI, AMYGDALA, SOCIAL-EMOTIONAL DEVELOPMENT & ATTACHMENT
That the amygdala promotes social emotional behavior and attachment is evident from the consequences of amygdala destruction. Social interest is abolished, animals that were formerly dominate become subordinate or social outcasts, and humans no longer react in an emotionally and socially appropriate manner and lose interest or no longer seem to recognize friends, relatives, and loved ones including their own offspring and parents (Dicks et al., 1969; Jonason & Enloe, 1971; Kling, 1972; Kling & Brothers 2012; Lilly et al., 1983; Marlowe et al., 1975; Terzian & Ore, 1955). Although they may behave in a sexually inappropriate fashion and masturbate and expose themselves to others, they also become socially unresponsive, and ignore, avoid, fail to recognize, or respond cruelly to others, and prefer to sit in isolation.
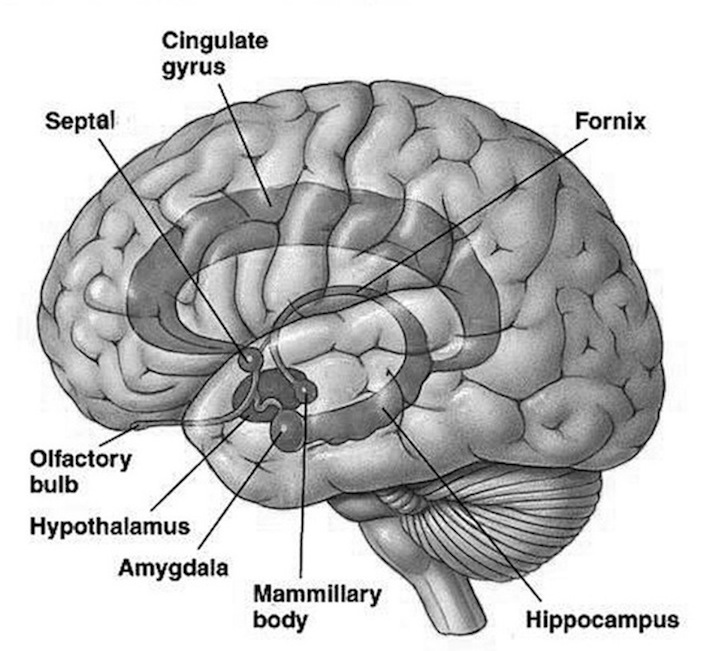
These disturbances, particularly those secondary to abuse or insufficient or abnormal early stimulation, is also related to environmental lesions which affect other limbic structures, including the septal nuclei and the anterior cingulate (Joseph, 2012a, 2014). The amygdala maintains a mutually influential and counterbalancing relationship with the septal nuclei as both are richly interconnected via the stria terminalis. In general, these structures exert mutually inhibitory and excitatory influences on each other, with septal influences tending toward the inhibitory and emotionally dampening (chapter 13, 15). For example, when the septal nucleus is electrically stimulated for brief periods, animals generally reduce their activity, and may lay down as if resting, and may even become unresponsive or oblivious to threat or other disturbances in the environment (MacLean, 1990). If the amygdala is destroyed, the septal nucleus becomes disinhibited, which in turn results in increased septal inhibitory and modulating influences on emotion and behavior.
However, if septal stimulation is prolonged and at high voltages, such that seizure-like spiking develops in the EEG, animals or humans may respond with pleasure (Heath, 1976; Olds & Forbes, 1981), and display increased sexual feelings coupled with erection or clitoral engorgement and genital manipulation, or conversely with rage, anger, and oppositional feelings of negativity (MacLean, 1990). Presumably, these latter behaviors are due to disruption in septal inhibitory functions, and are thus secondary to activation of the amygdala and hypothalamus. However, as noted, if the amygdala is destroyed, the septal nucleus may also become disinhibited and over active, which may result in severe disturbances in social as well as sexual behavior. This may explain why with amygdala lesions, although the desire for social contact is abolished, animals and humans may continue to engage in sexual behavior and may even attempt to have sex with members of their own sex or animals of other species (Kluver & Bucy, 1937).
For much of the first year, septal influences are minimal as this nucleus functionally matures at a much later age than the amygdala (Brown, 1983; Joseph, 2012a, 2014; Yakovlev & Lecours, 1967). In fact, the maturation of this structure appears to be rather prolonged as septal volume continues to increase well into late childhood and early adulthood (Jernigan et al., 1990), which may reflect the effects of pubertal hormones. Hence, infants behave in a socially indiscriminate manner as the developing amygdala is not subjected to septal inhibitory influences until later in development. As the septal nucleus matures, these indiscriminate socializing tendencies are restricted, which assists in the strengthening of specific attachments, such as to one's mother and family. This also insures that the infant or "toddler" does not wonder off with strangers. Thus, the latter maturation of the septal nuclei is exceedingly adaptive.
In fact, the initial development of the septal nuclei is influenced if not triggered by the extended amygdala, the tuberculum olfactorium (Humphrey, 1967), and later, it is only upon the receipt of amygdala afferent fibers that the septal nuclei begins to differentiate (Brown, 1983). Hence, in part, the maturation of the amygdala influences and promotes the later maturation of the septal nucleus which begin to myelinate until around the 4th fetal month; a process which is "extraordinarily protracted" and which can take well over 3 years to reach advanced stages of development (Yakovlev & Lecours, 1967), with the septal nuclei, including the hypothalamus and pituitary increasing in volume until well into puberty (Jernigan, et al., 1990).
In fact, as the septal nuclei is also implicated in sexual, aggressive as well as oppositional behavior (see below) the later maturation of this structure (including the frontal lobes, Schore, 1994) likely contributes to the oppositional and defiant childish attitude that emerges around age two: the so called "terrible twos," as well as the tendency of some youngsters to begin exploring their genitals at this age. Moreover, in response to the increased secretion of gonadal hormones, this structure likely plays a significant role in the sexual behavior of adolescents and adults.
In this regard, the development of the septal nuclei, in part is coordinated by the maturing amygdala, and as the infant develops, these two structures interact so as to promote selective and discriminate social, and then later, sexual behavior and the formation of specific emotional, and later, sexual attachments.
THE HIPPOCAMPUS, AMYGDALA, AND SEPTAL NUCLEI
The hippocampus is intimately associated with the septal nuclei (see chapter 14). The septal nuclei appears to directly modulate hippocampal activity and arousal levels. The septal nuclei also acts as an interface between the hippocampus and the hypothalamus, and appears to exert counterbalancing influences between the amygdala and hippocampus (see chapter 13, 14).
As noted, the bed nucleus for the stria terminals is sexually differentiated, and this is a major pathway via which the septal nuclei interacts with other limbic structures. In addition, the hippocampus is sexually differentiated (Wooley, et al., 1990), and synaptic and dendritic density fluctuates in response to fluctuating levels of estrogen.

As noted, the hippocampus is also responsive to faces, and it is exceedingly important in the formation of long-term memory as is the septal nuclei (see chapter 14). In this regard, the hippocampus (in conjunction with the amygdala and septal nuclei) is also important in the formation of attachments. In order for friendships and personal attachments cannot be established or maintained requires memory, and if the hippocampus is injured, the ability to remember not just places and things, but persons can be profoundly disrupted. Consider the famous case of H.M., who underwent the bilateral removal of the hippocampus and part of the amygdala (see chapter 14). Brenda Milner has worked with H.M. for over 30 years, yet she is an utter stranger to him.
In addition to remembering people, the hippocampus plays a significant role in establishing memories for places, i.e. spatial memory (Mishkin, et al. 1984; Nunn et al., 1999; Weiskrantz, 2014). In fact, the capacity to cognitively map, or visualize one's position and the position of other objects and individuals in visual-space is dependent on the hippocampus (Nadel, 1991; O'Keefe, 1976; Wilson and McNaughton, 1993). The hippocampus contains "place" neurons which are able to encode one's position and movements in space and which are sensitive to particular spatial coordinates and the location of objects in visual space. The hippocampus appear to create a spatial map of the environment, and a map that is very plastic. For example, as a subject moves about in the environment, entire populations of cells will fire, but only when in a particular spot, whereas other cells would fire when in a different location or when moving about or turning in one direction or another. Moreover, some cells are responsive to the movements of other people in that environment and will fire as that person is observed to move about (Nadel, 1991; O'Keefe, 1976; Wilson and McNaughton, 1993).
The hippocampus, therefore, can create a cognitive map of an individual's environment and their movements within it and the movement and location of other people in that environment. Again, as noted, the hippocampus is sexually differentiated, and females display decidely inferior visual-spatial capabilities as compared to males (see chapter 8). And, these sex differences in spatial ability are apparent even in early infancy and have been documented among other species. Hence, these sex differences are innate, and appear to be directly attributable to sex differences in the hippocampus and other limbic structures--the hippocampus becoming structurally mature by 15 months of age (Kretschmann, et al. 1986).
Hence, the fact that these sex differences emerge in infancy and early childhood (e.g., Harris, 1978; Joseph, 1999e; Petersen & Crokett, 1985) appears to be attributable to the early onset of hippocampal maturation--a process, however, that is exceedingly prolonged, taking decades to complete (Benes et al., 1994; Yakovlev & Lecours, 1968).
SEX DIFFERENCES EXPLORATION, DEPENDENCY & ATTACHMENT
Sex differences in emotionality, sociability, dependency, aggression, and visual spatial skills are apparent in early infancy and become more pronounced during early and then late childhood, and even more so during early and then late adolescence and early adulthood (see chapter 8)--thus paralleling the maturation of the limbic system and telencephalon. For example, whereas young females are playing with dolls and already display an almost intrusive interest in babies--even approaching little boys and girls in playgrounds and providing "maternal" care-- little boys are waving and throwing sticks, throwing rocks, acting aggressively, and wondering away from their mothers--"skills" and tendencies associated with hunting and the dispatching of prey, e.g. throwing spears, shooting arrows, stalking game (Joseph, 1993). Indeed, whereas little girls will fondle, play with, and "baby" her dolls, when dolls are given to a one year old boy he tends to break or take them apart, and this includes even boys who are blind from birth (Freedman, 2003). As boys age they continue to demonstrate a propensity for breaking and taking things apart whereas girls have little interest in engaging in these actions (Freedman, 2003.).
One year old human males are also more active, independent, exploratory, and spend more time away from their mothers as compared to females (Freedman, 2003; Goldberg & Lewis, 1969; Kagan, 1971). Young males are not only more aggressive and demonstrate superior visual-spatial perceptual abilities, they are also more likely than females to explore their physical environment in an assertive and challenging manner and to wander further from home or the home base. This is the case be it male chimpanzee, baboon, wolf, dog or cat (Manning, 19; ; Jolly, 1985; Devore, 1966; Kummer, 1966; Fedigan, 2012). However, "masculinized" girls with visual-spatial capabilities equal to that of most males tend to range as far as boys (Munroe & Munroe, 1971) as do "masculinized" and aggressive female chimpanzees (Goodall,1986, 1990), and estrus females--who are in search of a sex partner. Indeed, fluctuations in spatial ability are seen across the menstrual cycle, and appear to correlate with changes in estrogen level (Hampson1990; Hampson & Kimura 2018).
Hence, these sex differences are not a function of environmental influence or childhood rearing practices as much as it is a reflection of differences in innate capabilities directly attributable to the presence of absence of testosterone, estrogen levels, and the sexual differentiation of the brain, including the amygdala, septal nuclei, hypothalamus, and hippocampus.
In their wanderings, infant males are also more likely to aggressively climb and crawl over obstructions. For example, if a toy is placed behind a barrier, little boys will reach around for it, whereas girls will simply sit and watch and wait for it to reappear (Kagan, 1971), or they may just simply cry helplessly. When children crawl away so as to explore a strange environment and a barrier is then set up blocking their passage back to their mothers, again girls tend to sit and cry helplessly, whereas little boys attack and will beat upon the barrier (Goldberg & Lewis, 1969).
Similarly, normally reared male rhesus macaque monkeys and chimpanzees, like their human counterparts, are significantly more active and exploratory, and are more likely to touch and explore novel objects than females, though their tendency to break and take things apart is somewhat similar (Sacket, 19; Goodall, 1986, 1990). Male infant and juvenile chimps and rhesus macaques are also more independent of their mothers and soon seek out the company of older males (Fedigan, 2012, Goodall, 1986, 1990). Male primates, like male humans, also engage in more rough and tumble play and aggressive behavior, and will swagger about and even engage in belligerent displays toward their mothers and other females, but not adult males (Mitchell, 2006; Fedigan, 2012; Goodall, 1986, 1990). They also engage in threat and will attack others at an earlier age than females.
In contrast, female chimps, baboons, rhesus monkeys and humans are not only more emotional and less likely to wander away, they are more social and more interested in forming emotional attachments, and this is evident beginning in infancy. Hence, just as there is a trade off in visual-spatial skills (male) vs emotional memory (female), there is a trade off between aggression and independence (male) vs social-emotional attachment (female). And these sex differences appear to be directly attributable to the sexual differentiation of the brain, the amygdala, hippocampus, and hypothalamus in particular.
THE ANTERIOR CINGULATE GYRUS
As the forebrain matures in a caudal to rostral, paramedial to medial/lateral arc, paramedian structures such as the amygdala begin to mature in advance of the medially located septal nuclei and the medially situated anterior cingulate; developmental patterns which are reflected behaviorally and emotionally. Like the amygdala, the anterior cingulate is exceedingly important in affective-motor activities, including those involving the hands (e.g. grooming, cuddling), and the oral-laryngeal musculature; i.e. vocalization (Joseph, 1993, 2014). The anterior cingulate is also associated with emotional learning, attention and concentration, the development of play, fantasy, and affectionate behavior, and the formation of long-term attachments and infant-maternal interactional behavior including separation anxiety (Devinsky, Morrell, & Vogt, 2014; Joseph, 2014; MacLean, 1990; Slotnick, 1967; Smith, 1945, Stamm, 1955; Ward, 1948). In fact, electrical stimulation of the anterior cingulate can produce a separation cry identical to that of a frightened infant (MacLean 1990; Robinson, 1967).
In this regard, capacities associated with the anterior cingulate (e.g. play, separation anxiety) also emerge later in development and thus parallel the functional maturation of this structure. Specifically, although the posterior cingulate begins to myelinate around the second to third postnatal month, the anterior cingulate begins to myelinate and mature around the fourth postnatal month and then reaches an advanced stage of myelination around the end of the first year (Benes, 1994; Brody et al., 2014; Yakovlev & Lecours, 1967)--the age at which attachment behaviors, and separation anxiety and stranger fear become most pronounced (Schaffer, 1966). However, like other limbic structures, myelination of the cingulate continues for several years (Brody et al., 2014; Yakovlev & Lecours, 1967).
Thus the maturation and myelination of the anterior cingulate parallels and appears to contribute to the development of those social-emotions which emerge during the latter half of the first year; i.e. separation anxiety, stranger fear, the development of playfulness and affectionate behavior, and the formation of long term emotional attachment. Moreover, the maturation of the anterior cingulate also parallels and promotes the development of complex social emotional vocalizations, including "late" and canonical babbling (see chapter 15), including the emerging ability to engage in sound play and to produce sounds which do not correspond to the infant's (or adult's) true mood state.
Indeed, the cingulate is capable of producing exceedingly complex social emotional vocalizations which sometimes have no bearing on the organism's mood or true emotional state (Jurgens, 1990; Jurgens & Muller-Preuss, 1977; Meyer et al., 1973), and completely different emotional calls can be elicited from electrodes which are immediately adjacent (Jurgens, 1990). Thus the cingulate is capable of considerable vocal flexibility and enables an individual to modulate, disguise or enphasize the emotional, prosodic, melodic components of speech so that one's true feelings can be disguised or emphasized in order to produce sounds suggestive of, for example, incredulity, hilarity, empathy, maternal concern, or separation anxiety.
As noted, it has been demonstrated that the anterior cingulate can generate a separation cry which is identical to that produced by a frightened infant (MacLean 1990; Robinson, 1967), including those vocalizations which infants produce in accompaniment to those produced by their mothers (Bayart, Hayashi, Faull, Barchas, & Levine, 1990; Jurgens, 1990; Wiener, Bayart, Faull, & Levine, 1990). As argued by MacLean (1990), the cingulate produces these infantile/maternal vocalizations and associated emotions so as to maintain maternal-offspring contact and maternal care. When mothers and infants mutually vocalize, these interactions reinforce and promote attachment behaviors, and contribute to the development of language. Again, however, just as the cingulate of the infant appears to be responsible for producing infantile sounds and behaviors, including the separation cry, these maternal vocalizations also appear to be produced by the anterior cingulate (and right frontal lobe), as it is this structure which enables a speaker, including a mother to vary and emphasize prosodic vocalizations--a characteristic of mothers when interacting with their infants (Fernald, 1985, 1991, 2012). In this regard it appears that the cingulate and limbic system of the mother is essentially communicating with the cingulate and limbic system of the infant, thereby promoting maternal-infant attachment as well as the development of human speech (chapter 15).
The importance of the cingulate in emotional vocalization and affectionate, playful behavior becomes most evident following injury to this region of the brain. For example, adult, non-human primates who have suffered cingulate destruction cease to display acts of affection, cease to vocalize, engage in abnormal maternal behavior, and will even walk and step on their offspring "as though they were inanimate objects" (Ward, 1948). Likewise, human adults who have experienced cingulate destruction become mute, inattentive, and socially and emotionally unresponsive and will ignore loved one's including their children (Barris & Schuman, 1953; Devinksy et al., 2014; Laplane, Degos, Baulac, & Gray, 1981; Tow & Whitty, 1953). Similarly, infants with anterior cingulate destruction cease to engage in affectionate behavior, no longer play with siblings or conspecies, and lose all interest in establishing or maintaining affiliative behavior, and generally become mute and no longer vocalize (MacLean, 1990).
Thus, the maturation of the anterior cingulate promotes the formation and strengthening of emotional attachments, including various aspects of speech, as well as those infantile behaviors which stimulate maternal behavior which in turn are also, in part, mediated by the cingulate. Hence, this structures in parts serves as a mother-infant interface, and in fact, it appears that the evolution of this structure, some 200 million years ago, coincided with and ushered in the development of long term maternal behavior and the establishment of the first families with infants remaining attached until reaching the juvenile period.
Of course, the anterior cingulate has also continued to evolve, culminating in complexity with the emergence of primates and modern humans. Moreover, as it evolved, it appears to have contributed to the evolution of the medial frontal neocortex (which is also involved in vocalization), as well as the lateral frontal neocortex including Broca's area and the emotional-melodic speech area in the right frontal lobe with which it maintains extensive interconnections (chapters 15, 19). In fact, it is via these extensive neocortical-anterior cingulate (as well as amygdala) interconnections that enables a child or adult to hierarchically subserve and express, as well as punctuate limbic vocalizations so as to produce word-rich grammatically complex human language.
It is not until around one year that the immature neocortical language areas begin to exert hierarchical influences over the cingulate, and midbrain vocalizations centers, as reflected by the development of jargon babbling (chapter 15). That is, jargon babbling appears to be a function of the immature neocortical speech areas slowly gaining control over the cingulate, amygdala, and periaqueductal gray. However, as the neocortex slowly matures not only does the infant begin to vocalize its first words (see chapter 15) but emotional behavior also begins to become increasingly under neocortical control.
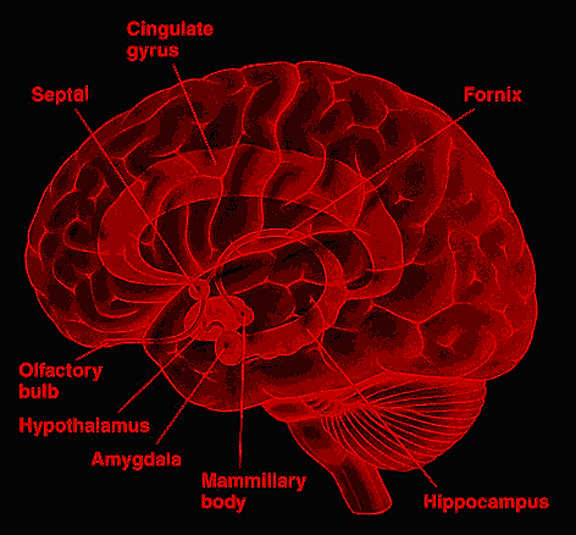
THE CINGULATE AND SEX DIFFERENCES IN PLAY BEHAVIOR
The mammalian cingulate gyrus is sexually differentiated and differentially responds to steroids and male vs female hormones (MacLusky, Clark, Naftolin & Goldman-Rakic 2014; MacLusky, Walters, Clark & Toran-Allerand 1994). Hence, as the cingulate matures, cingulate related sex differences also emerge at a young age. For example, in addition to being more socially oriented and making more eye contact, females begin babbling, make more eye contact while jargon babbling, begin speaking their first words at an earlier age than males (see above and chapters 6,7,15), play differently than males and engage in play behaviors that involve "mothering" even before age 3. By age 3 most girls increasing ask for and indicate a preference for dolls and cradles (Liss, 1981; Conner & Serbin, 1977). As noted, boys prefer weapons and toys that are dehumanized and begin demonstrating these preference as early as age two; and these sex differences in toy preferences have been demonstrated across time (Parten, 1933) and culture (Zitkala-Sa, 19; Eastman, 19 ).
Young children in fact tend to have very rigid concepts as to what are appropriate gender related behaviors and sex roles (Eaton, 1983), especially for males. At an early age they know what are girl vs boy toys, clothes, and activities and even these "tomboys" increasingly develop stronger preferences for girls toys and activities as they age (Robinson & Morris, 1986; Blakemore et al. 2006). Although many a "feminist" or "lesbian" mother has attempted to thwart these behaviors, and although various sexual-political groups have campained against what believe to be "sexist" attitudes hoisted on children by their unwitting parents, in general, young children tend to reject toys as well as activities that seem gender inappropriate (Bussey & Perry, 1982; Huston & Carpenter, 1985; Carter & McCloseky, 1984). They will not play with what they do not find fun. In fact, most parents respond to the requests of their children when buying toys as they are seeking to please their kids and make them happy (Robinson & Morris, 1986), the exceptions being gender neutral toys such as art and educational items which parents generally choose.
Sex differences in play, including so called "rough and tumble" behaviors, which typically involve male humans and male apes and monkeys wrestling, pushing, shoving, and hitting one another, also become increasingly pronounced as the brain and child age. In consequence, these sex differences not only become exaggerated among older children, and then adolescents, but fathers and mother tend to repeat the same play patterns when interacting with their own children--a consequence, perhaps not only of training and experience, but sex differences in limbic system structures including the cingulate. That is, the way in which males and female played and behaved as children tend to be similar to the way they play, as adults, with their own children.
For example, fathers are more likely to initiate play and to engage in much more active, arousing, physical and vigorous games such as wrestling and rough housing, (especially with their sons), whereas mothers engage in nurturing, highly verbal, much less strenuous and non-aggressive forms of play such as patty cake (Lynn, 2006; Parke & O'Leary, 1976; Lamb, 1977; Power & Parke, 1982; MacDonald & Parke, 1986; Stevenson et al. 2018; Clarke-Stewart, 2003). In fact, fathers are more likely to physically play with their children, especially their boys whereas females are much more likely to verbalize and nurture them. Mothers are also more likely to at least attempt to emotionally dissect the behavior of their children, whereas fathers tend to have little or no interest in the emotional inner life of their children or each other. Not surprisingly, young children, especially boys, prefer their fathers as playmates (Clarke-Stewart, 2003; Lynn & Cross, 1974; Lamb, 1977).
Hence, in many ways, the style and play activities of boys and girls become their play activities when they become mothers and fathers. Indeed, for most females, mothering was a form of play when they were little girls.
MATERNAL GAMES & FANTASIES
Be it a female chimpanzee, baboon, rhesus macaque, or human, females begin to demonstrate an extraordinary interest in babies and in play-mothering during even the earliest phases of their own childhood (see chapter 7). When girls play together, much of their fantasy and conversation concerns fashion and making out and revolves around adult relationships, including the raising of a family and the behavior and misbehavior of children (their dolls). Babies are of enormous interest to females, be they human, ape or monkey, and those who have babies usually become tremendously popular and the center of attention. Mothers, grandmothers, young and adolescent females, and even women who describe themselves as "feminists" show much more interest in babies than do men, even when the baby is not their own (Berman, 1990; Nash & fledman, 1981a; Frodi & Lamb, 1978; Melson & Fogel, 1982; Azhn-Waxler et al. 1983; Berman & Goodman, 1984; Blakemore 1981, 1985, 1990). Adolescent girls spend significantly more time talking about new baby's than boys (Berman, 1990), and mothers spend more time talking about the baby with their daughters than their sons (Berman, 1990). Girls not only talk more but play and care for their infant sisters and brothers significantly more and show consider amounts of nurturant interest in the babies well being (Blakemore, 1990) even when there has been no request or pressure to do so. Indeed, girls often demonstrate an intrusive interest in babies (Berman, 1983), and will give infants much more care than they require (Ainsworth & Wittig, 1969), as if often the case with mothers (Stewart, 19).
Similarly, among non-human female primates, be it gorilla, chimpanzee, baboon, rhesus macaques, lemur, and so on, even those without infants will eagerly seek to groom, cuddle, and carry the child as much as the mother will allow (Jolly, 1972; Devore, 1964; Kummer, Suomi, 1972; Mitchell, 2006; Goodall, 1971) and may spend all day passing them back and forth. Like human females, some will even steal these infants. Those female primates who show the greatest interest, however, are young females who had not yet had babies.
Such behavior is obviously not the result of sexist training for it is typical of all primates. For example, boy chimpanzees show little interest in their younger infant siblings, whereas girl chimps become increasingly fascinated and will hold and cuddle them and will attempt to model their mother's interactions with the infant (Goodall, 1971). If a new mother dies but her baby has older male siblings, less than 25% will adopt the little orphan whereas females siblings are quite anxious and happy to take this role.
Again, these sex differences can be attributed to the sexual differentiation of the limbic system, including the cingulate, amygdala, and hypothalamus. The sexual differentiation of the brain, and not supposed societal pressures, explains why little boys have little or no interest in babies, in dolls, or emotionally dissecting the behavior of their friends, enemies or parents, and why human males and fathers rarely behave in any manner that approximates normal female maternal behavior (Belsky et al. 1984; Clarke-Stewart, 1978; Frodi, et al. 1982) as this is simply not an activity they find interesting, pleasurable or rewarding.
THE HOMOSEXUAL LIMBIC SYSTEM
As discussed in chapter 13, there is a "male" vs a "female" vs a "homosexual" limbic system. Specifically, the ventromedial and anterior nuclei of the hypothalamus of male homosexuals demonstrate the female pattern of development (Levay, 1991; Swaab, 1990). Moreover, among homosexuals, sexually differentiated structures such as the anterior commissure, not only resemble the female pattern more than the male pattern, but are suggestive of hypertrophy. The anterior commissure is 35% larger in homosexual males vs male heterosexuals (Allen & Gorski, 2012).

As also mentioned in chapter 13 and 28, there are a number of possible "causes" of homosexuality (e.g., Bailey et al., 1993; Byne & Parsons, 1993), including fetal stress (e.g., Meyer-Bahlburg, 1993; Reinisch & Sanders, 2012), or profound abusive trauma, such as as sex abuse, experienced during early childhood (Johnson and Shrier, 1985; Simari & Baskin, 2004). Finkelhor (2006) reported that males with a history of CSA are four times more likely than non-abused males to engage in homosexual activities. [-INSERT FIGURE 6 ABOUT HERE-]
Regardless of its etiology, it is clear that the limbic system plays a significant role in sexuality and emotion, and that the limbic system is sexually differentiated. Likewise, a "homosexual" pattern of limbic system development, and a pattern that is established during the fetal, or infant stage, likely accounts for why "homosexuals" begin acting like homosexuals, that is, acting more like girls than boys, at an early age, and why these differences become more pronounced as they age. This includes using female body language and behaving in an effeminate manner, employing female speech pattern and vocal tones, having a high interest in girls' toys and girls clothes or fashion, being easily frightened and upset, being especially fearful of physical injury, and avoiding sports or aggressive activities (Bell et al. 1981; Bieber et al. 1962; Saghir & Robins, 1973; Grellet et al. 2012; Green, 2014; Van Den Aardweg, 1984; Tripp, 2014), prefering instead to hang out and talk with the girls. Many also tend to maintain intense dependency relations with their mothers and to remain distant from strong male figures including their fathers (Green, 2014). From 67% to 75% of homosexuals vs 2%-3% of heterosexual males reported being "feminine" and more like girls than boys as children (Saghir & Robins, 1973; Green, 2014). Moreover, homosexual males, like heterosexual females, demonstrate comparatively inferior spatial perceptual capabilities as compared to heterosexual males (Gladue et al. 1990; Sanders & Ross-Field, 1986; Wilmot & Brierley, 1984). Homosexuals tend to perform similarly to females on these spatial tests. And, must as females demonstrate various language superiorities over males (see chapter 8), homosexual men show similar trends (Gladue et al., 1990; Wegesin, 2008).
There is, however, some resistance to the likelihood of a biological foundation to homosexual behavior--especially among certain groups of homosexuals and the professional organizations they belong to, and those political-religious organizations who view these actions in terms of "morality." Presumably, at least among some homosexuals, this orientation could be a matter of "choice," perhaps a "choice" resulting from being sexually victimized as a child. On the other hand, it is not likely that a child would chose to be "homosexual" and in my opinion, those who claim otherwise are pedophiles.
Although various political-educational groups have campaigned and have attempted to educate children to believe and think otherwise, children have stereotypical views of what is appropriate and acceptable "male" vs "female" behavior. Because boys are even more rigid than girls in these beliefs, and as males are inclined to exploit what they perceive to be weakness, they tease, threaten, ridicule, beat up, and torment those males who engage in activities or play with toys associated with the opposite sex (Green, 1977; Fagot 1977; Berndt & Heller, 1986). Both boys and girls as young as 6 years of age disapprove, ridicule and reject boys who are "sissies," or who act or behave like "girls." Even homosexually inclined "straight" acting males reject "sissies" and find their behaviors aversive (Tripp, 1985).
Unfortunately for these "sissy" males, their behaviors not only seem "inappropriate," but are indicative of appeasement, fear, and subordination. That is, "sissy" males, by displaying female behaviors, are also signaling (that is, to other males) weakness, fear, and submission, and inadvertently invite attack and ridicule from those who are alway seeking someone to dominate; i.e. males in general. Even parents (Feinman, 2004, 2007; Green,2005), especially fathers, tend to reject "sissy" sons, in part because he views the boys behavior as embarrassing.
Hence, "sissy" boys tend to be rejected and ridiculed by other children, and receive numerous negative messages and pressures from their parents, especially their fathers. Not surprisingly, they often tend to be unhappy, depressed, and maintain a negative relationship with their peers and parents (especially their fathers). In consequence, "sissified" boys are far more likely than girls to be referred for "treatment" as they are viewed as "maladjusted." Thus, the notion that a little boy would consciously choose to be treated in this fashion, and would thus choose to be a "homosexual" is not to be taken seriously.
In contrast, girls who behave like "tomboys" are readily accepted by peers and parents (Green, 1977; Fagot 1977; Berndt & Heller, 2006). Perhaps in part this is because "tomboys" in contrast to "sissies" tend to display aggressive behavior and act in a domineering fashion. Hence, their behavior does not invite attack. Hence, children and parents tend to be accepting of "tomboyish" behavior (Feinman, 1981; Green, 1975; Williams, et al. 2005), even when they might prefer that their daughters behave in a feminine fashion. However, "tomboys" are generally seen as passing through a phase that tends to disappear once they reach adolescence. In fact, the vast majority of "tomboys" later accept and identify with femininity, and prefer males for sex partners.
In contrast, parents tend to fear that if their "sissy" son does not act in a stereotypical male fashion, he is going to be harmed socially and emotionally by the reactions of others. These parents also tend to fear that this "sissy" boy may become a homosexual (Antill, 2014; Green, 1975). This may be a correct assumption for "sissified" boys do tend to engage in homosexual activities as teens and adults (Bell, et al. 1981; Saghir & Robins, 1973; Grellet et al. 3022; Green, 2014). As noted, from 67% to 75% of homosexuals vs 2%-3% of heterosexual males reported being "feminine" and more like girls than boys as children (Saghir & Robins, 1973; Green, 2014). These boys preferred girl toys, girl friends, girl clothes, and many in fact wanted to be girls--behaviors and attitudes which strongly suggest that these traits are innate, and probably secondary to the "homosexual" differentiation of the limbic system.
THE ORBITAL FRONTAL LOBES AND INHIBITORY EMOTIONAL CONTROL
Whereas the limbic system is associated with affective, sexual, and emotionally impulsive behavior, the neocortex, and the frontal lobes in particular, are associated with inhibitory "executive" control (Fuster, 2017; Joseph, 1986a, 1999a). That is, whereas the limbic system may proclaim: "I want it, now!" the frontal lobes, being concerned with consequences, planning skills, and long term goal formation, may instead proclaim: "You can't have it!" Hence, whereas the limbic system may be associated with the "Id" the frontal lobes are associated with the "super ego" or conscience--as in "let your conscience be your guide." Nevertheless, like the limbic system, the frontal lobes, are also sexually differentiated and have "male" vs "female" patterns of dendritic branching which are due to the presence of absence of gonadal hormonal (Kolb & Stewart 1991). This may explain why "abnormalities" of the frontal lobe can produce abnormal sexual behaviors.
Although the limbic system certainly has the capability of hijacking and overthrowing the more rational aspects of the cerebrum, including the frontal lobes, these structures also interact, and are richly interconnected, such that, under most conditions, limbic system impulses remain under some degree of frontal lobe inhibitory guidance. Specifically, the orbital frontal lobes regulate and inhibit limbic system activity and arousal via the the medial magnocellular dorsal medial nucleus of the thalamus (Joseph 1999a) which is a major relay nucleus which links the neocortex to the limbic system. The orbital frontal lobes also interact with the brainstem reticular formation so as to exert inhibitory control over arousal (Kuypers 1958; Rossi and Brodal 1956; Sauerland et al. 1967). Hence, via these interconnections the orbital frontal lobes can exert inhibitory influences over brainstem and limbic system arousal, including the motoric expressions of emotion.
Moreover, the orbital frontal lobes are not only richly interconnected with and exert inhibitory influences on the anterior cingulate, amygdala, septal nuclei, and hypothalamus, but exert executive control over autonomic functions as well as the bladder and anal sphincter and thus urination and defecation (Joseph, 1999a). Hence, if the orbital frontal lobes were severely injured (such as following orbital frontal lobotomy), urinary bladder control may be lost and patients may freely urinate as well as defecate on themselves (Freeman & Watts 1942; Langworthy & Richter 1939; Greenblatt 1950; Rinkel et al. 1950a,b; Rose 1950). Patients may urinate or defecate while lying in bed, sitting in front of the T.V. during dinner, or while sitting in a restaurant. One patient was observed to empty her bladder while waving goodbye to a friend. She stopped on request but completed the act as she walked to the toilet (Rose 1950).
Moreover, orbital frontal injury is also associated with the development of silly, childish, impulsive, and irresponsible behavior, personal untidiness and dirtiness, and tendencies to become easily enraged and to display a total lack of judgment and common sense (Fuster 2017; Freeman and Watts 1942; Girgis 1971; Hacaen 1964; Joseph 1986a, 2018a, 1999a; Luria 2003; Passingham 1993; Petrie 1952; Stuss 1991; Stuss and Benson 1986). Following severe injuries there may be periods of gross disinhibition which may consist of loud, boisterous, and grandiose speech, singing, yelling, and beating on trays. The destruction of furniture and the tearing of clothes is not uncommon. One patient, with a tumor involving the right frontal area, following resection, attempted to throw a fellow patient's radio through the window because he did not like the music. He also loudly sang opera in the halls. Indeed, during the course of his examination he would frequently sing his answers to various questions (Joseph 1986a).
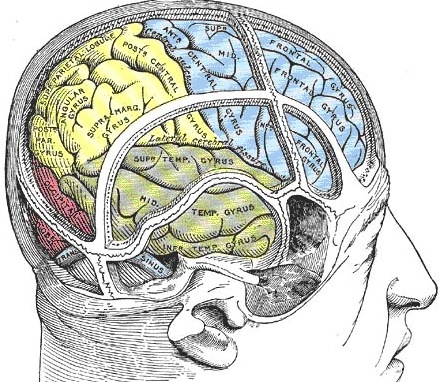
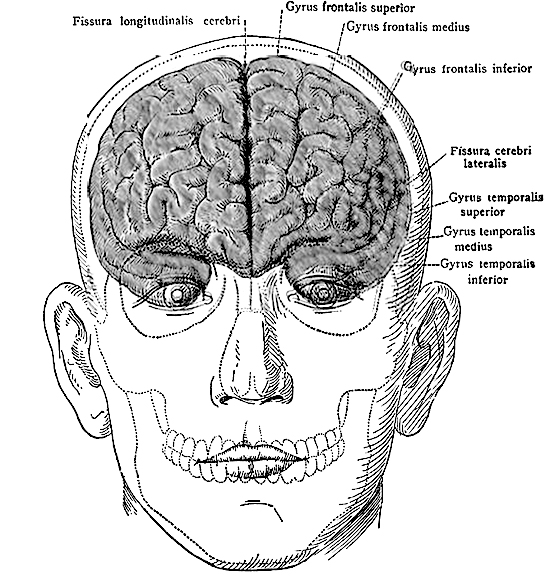
Frontal lobe injury is thus associated with the development of emotionally disinhibited infantile and childish behavior. Moreover, frontal injury is associated with a tendency to urinate and defecate on one's self, such as while lying in bed or waving goodbye--behaviors also associated with infants and young children. In this regard it is noteworthy that the frontal lobes do not begin to display a significant degree of functional maturation, until around the end of the first postnatal year-- as based on patterns of myelination, metabolic and electrophysiological activity (Bell & Fox, 1994; Blinkov & Glezer, 1968; Chugani, 1994; Conel, 1937; Fechsig, 1901; Hudspeth & Pribram, 2012; Huttenlocher, 1990; Kellaway, 2006; Ohtahara, 1981; Schore, 1994)--and that this structure in fact takes well over 10 years to fully mature and to fully establish inhibitory control over the limbic system.
Hence, frontal lobe immaturity may well explain why the behavior of infants and children is so infantile and childish (Luria, 1932; Schore, 2014; Steklis & Kling, 1985). When coupled with increasingly limbic system maturation, frontal lobe immaturity may also contribute to the development of the emotional oppositional stage, the so called "terrible twos."
Conversely, it is the maturation of the frontal lobes, the orbital region in particular, which also contributes to the development of socially acceptable behavior including the establishment of inhibitory control over the bladder and bowels. However, in this regard, it has also been proposed that the orbital region may also contribute to the so called "anal stage" (Schore, 2014) and even the "phallic stage" of "psychosexual development during which the child displays an inordinate interest in their genitals. In fact, injury or abnormal activity localized to the frontal lobes (as well as the amygdala) has been associated with genital exhibitionism and sexual seizures (see chapter 19). Of course, it should also be emphasized that any association between the frontal lobe and the so called "anal" and "phallic" stages of development, is purely speculation.
On the other hand, it can be concluded the maturation of the frontal lobes and neocortex are responsible for the gradual establishment of inhibitory control and guidance over limbic system affective functioning. In this regard, although the limbic system provides the social-emotional foundation, it is the frontal lobes (coupled with early environmental experience and parental training) which channel, mold, and restrict these impulses so as to fashion them into socially acceptable patterns of behavior.
Again, initially this begins to occur around one year of age, at which point the the immature neocortex and frontal lobes slowly begin to exert hierarchical and inhibitory control over the limbic forebrain and brainstem around 12 months of age. For example, the frontal lobes display increased metabolic activity around 12 months of age (Chugani, 1994), and it is also around the end of the first year that the EEG begins to resemble a more adult pattern (Bell & Fox, 1994; Kellaway, 2006; Ohtahara, 1981). Nevertheless, at one year the frontal lobes are exceedingly immature, and in fact double in size by age two (Blinkov & Glezer 1968; Flechsig, 1901)--a developmental process which is extraordinarily protracted.
It is noteworthy, however, that the right frontal lobe (and thus the right orbital area) appears to initially mature in advance of the left (Scheibel, 1991, 1993), which in turn provides it with a competitive advantage in the establishment of hierarchical control over the limbic system as well as arousal (Joseph, 1982, 2018a, 1999a). Indeed, the right frontal lobe exerts bilateral inhibitory influences over arousal (see chapter 19). Likewise, the nonmotor, sensory-receptive areas of the right hemisphere initially develop more rapidly than their left sided nonmotor counterparts (Chi et al., 1977; Scheibel, 1991, 1993; Thatcher 2012), which in turn enables the right hemisphere to gain a competitive dominance over the left in regard to the hierarchical representation of social-emotional limbic system functions (Chiron, Jambaque, Nabbout, Lounes, Syrota, & Dulac, 2017; Joseph, 1982), including emotional speech (Heilman et al., 1984; Joseph, 1982, 2018a; Ross, 1993; Tucker, et al., 1977). Thus, whereas the right frontal and orbital areas increasingly establish inhibitory control over the limbic system, the right hemisphere hierarchically represents and becomes dominate for the perception and expression of all aspects of emotion.
CONCLUSION
Over the course of neonatal and infant development, social-emotional behavior, including affective speech, becomes increasingly complex as the brainstem followed by the hypothalamus, amygdala, septal nucleus, and anterior cingulate gyrus mature and develop. Indeed, the progressive maturation and myelinization of these structures parallel and govern the different stages of social, emotional, affective-motor, and emotional speech development that ensue over the first year of life, beginning with reflexive behavior, crying and rudimentary smiling (brainstem/medial hypothalamus), followed by pleasure and true smiles (lateral hypothalamus/amygdala), indiscriminate contact seeking, wariness and fear (amygdala), and the establishment of specific attachments and separation anxiety (septal nuclei/anterior cingulate). Likewise, the emergence of sex differences appears to parallel the maturation of the above limbic structures; and in this regard, it should be emphasized that the limbic system continues to mature and develop beyond the first decade--again paralleling increasingly sexually differentiated behavior of males and females including the emergence of sexual behavior.
It is only around the end of the first year that the neocortex and orbital frontal lobes begin to mature and begin to establish hierarchical and inhibitory executive control over the limbic forebrain and brainstem. And like the limbic system, the sexually differentiated frontal lobes (and other neocortices) continue to mature over the ensuing years. In fact, it is only over the ensuing ten to twenty years that the orbital (and right) frontal lobe fully establish inhibitory executive control so as to affectively and consistently guide, promote, and maintain socially acceptable emotional behavior.
REFERENCES