Rhawn Gabriel Joseph, Ph.D.
BrainMind.com
Many people experience emotion as a potentially overwhelming force that warrants and yet resists control - as something irrational that can happen to you ("you make me so angry" - "I'm madly in love"). Perhaps in part, this schism between the rational and the emotional is attributable to the raw energy of emotion having its source in the nuclei of the ancient limbic lobe - what some have referred to as the reptilian brain, a series of nuclei that first made their phylogenetic appearance long before man walked upon this earth. Although over the course of evolution a new brain (neocortex) has developed, we remain creatures of emotion. We have not completely emerged from the phylogenetic swamps of our original psychic existence. The old limbic brain has not been replaced.
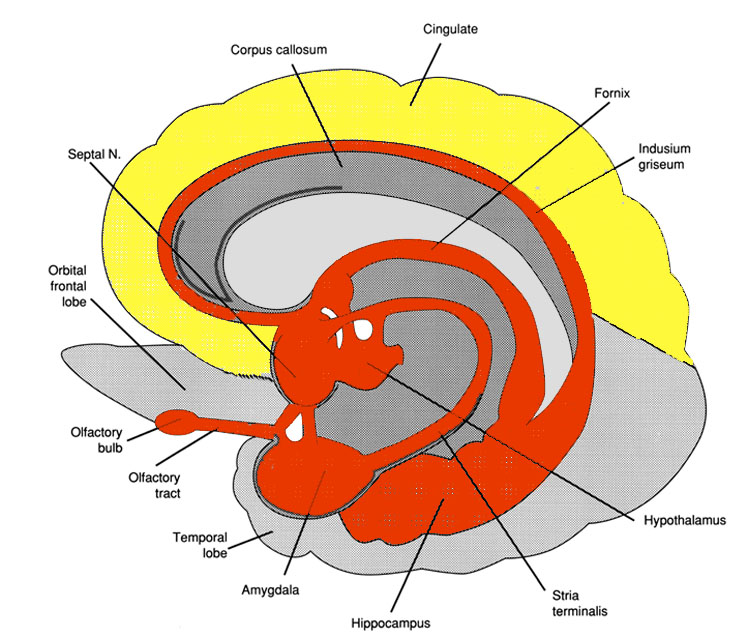
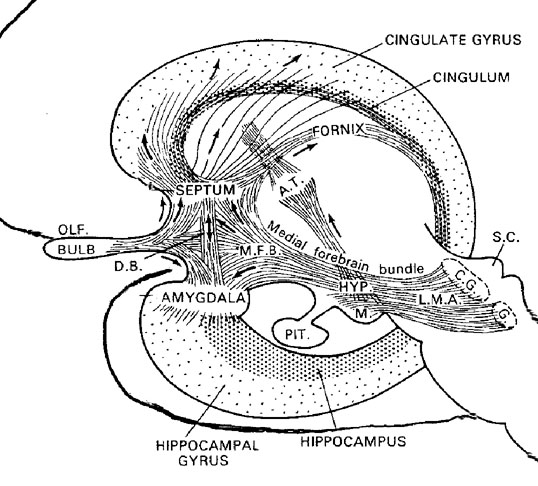
Buried within the depths of the cerebrum are several large aggregates of limbic neurons that are preeminent in the mediation and expression of emotional, motivational, sexual, and social behavior and that control and monitor internal homeostasis and basic needs, such as hunger and thirst. These regions include the hypothalamus, amygdala, hippocampus, septal nuclei, anterior and posterior cingulate, various thalamic nuclei, portions of the reticular activating system, the orbital frontal lobes, certain nuclei of the cerebellum, and other structures that together form the limbic system.
Of specific concern in this chapter are the hypothalamus, amygdala, hippocampus, and septal nuclei, the social - emotional and psychic functions they mediate, and the neural circuitry that supports their activity. Limbic laterality, temporal lobe epilepsy, hallucinations and memory, and select aspects of psychological and unconscious development, including the pleasure principle and primary process, are also briefly discussed.
Emotionality serves a protective function, either to promote survival of the individual (e.g., fight or flight) of that of the species (e.g., sexual activity). The first and most primitive manifestations of emotion are elicited in response to olfactory (e.g., pheromones and other externally secreted chemical messengers) and tactile sensory stimulation. These primitive emotions are expressed as withdrawal (fear) or approach reactions to pain or threat and are elicited in response to motivational needs, such as the seeking of a sexual partner or for the procurement of food (prey) and nourishment (Graeber, 1980; Michael & Keverne, 1974; Savage, 1980; Wilson, 1962).
Olfaction was originally crucial to evolutionary and phylogenetic development, as it informed the organism about the environment at a distance, without the necessity of physical contact. By means of olfactory cues and the detection of pheromones, the organism is able to detect and track food, determine the intent and social - emotional status of conspecies, as well as signal its own intent, motivation, social position, and/or sexual availability (Michael & Keverne, 1974; Wilson, 1962).
For example, consider pheromones, substances secreted by the skin through specialized glands or found in urine and feces. Chemical communication through pheromones is used by moths, social insects, dogs, cats, and primates, as well as amphibian, sharks, and reptiles. Although detection of pheromones among insects is often accomplished through specialized chemoreceptors located on various parts of the body (Wilson, 1962), mammals and pirates rely on olfactory receptors within the nostrils that transmit this information to the olfactory bulb (or lobe in some species) and the telencephalon (Graeber, 1980; Savage, 1980).
Among humans olfaction appears to have lost its leading role in signaling motivationally significant information. However, olfactory cues are still employed for indicating sexuality (e.g., perfume), and odor exerts a powerful influence on what is considered socially acceptable, hence the abundance of artificial chemicals designed to eliminate various body fragrances. Moreover, one need only suffer a severe cold to appreciate the dominant function of smell in the ability to detect and appreciate fully the flavor of food and thereby experience pleasure in eating (Fig 19). Odors also affect learning and memory and have the capability of triggering vivid recollections of some far away and past event.
Ontogenetically, although influenced by olfactory cues, humans first experience or express emotionally in relationship to the body and in response to tactile sensations or rapid changes in position (Emde & Koenig, 1969; Spitz & Wolf, 1946). Pain is also first experienced in relationship to the body, and is somesthetically rooted, although some have argued that pain is not an emotion.
Among human infants, the earliest smiles are induced through tactile stimulation (e.g., light stroking or even blowing on the skin), whereas loss of support is the most powerful stimulus for triggering an emotional reaction in the newborn (Emde & Koenig, 1969; Sptiz & Wolf, 1946). The earliest and most consistent manifestation of emotion in the infant consists of screaming and crying, whereas positive affect is limited to an attitude of acceptance and quiescence (Sptiz & Wolf, 1946), emotions first mediated by the hypothalamus that may or may not be true emotions at all.
In their journey from the external to the internal environment, olfactory and tactile input are transmitted to various limbic nuclei, such as the lateral hypothalamus and entorhinal area of the hippocampus (olfactory only) and the amygdala (olfaction and somesthesis). Indeed, it is through nuclei such as the amygdala (as opposed to the hypothalamus) that the first true (or rather, felt) aspects of emotion appear to be generated. It is also because of the tremendous input of olfactory information to various limbic nuclei that this part of the brain was at one time referred to as the rhinencephalon, literally "nose brain."
HYPOTHALAMUS
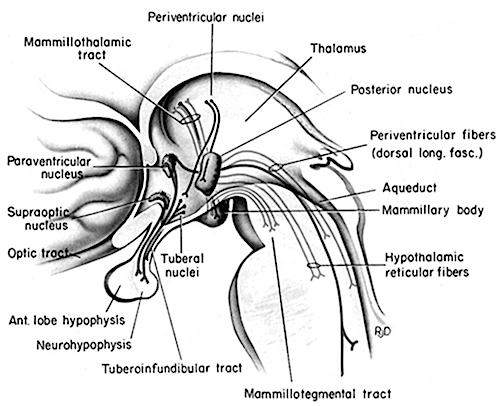
The hypothalamus is a very ancient structure. Unlike most other brain regions, it has maintained a striking similarity in structure throughout phylogeny and apparently over the course of evolution (Crosby, DeJonge, & Schneider, 1966). Located in the most medial aspect of the brain, along the walls and floor of the third ventricle, this nucleus is fully functional at birth and is the central core from which all emotions derive their motive force.
The hypothalamus is highly involved in all aspects of endocrine, hormonal, visceral, and autonomic functions; it mediates or exerts controlling influences on eating, drinking, the experience of pleasure, rage, and aversion. The hypothalamus is also sexually dimorphic; i.e., both structurally and functionally, the hypothalamus of men and women is sexually dissimilar.
Sexual Dimorphism in the Hypothalamus
As is well known, sexual differentiation is strongly influenced by the presence or absence of gonadal steroid hormones during certain critical periods of prenatal development in many species, including humans. However, not only are the external genitalia and other physical features sexually differentiated, but certain regions of the brain have also been found to be sexually dimorphic and differentially sensitive to steroids, particularly the preoptic area and ventromedial nucleus of the hypothalamus, as well as the amygdala (Bleier, Byne, & Siggelkow, 1982; Dorner, 1976; Gorski, Gordon, Shyrne, & Southam, 1978; Rainbow, Parsons, & McEwen, 1982; Raisman & Field, 1971, 1973). Specifically, the presence or absence of the male hormone testosterone, during this critical neonatal period, directly affects and determines the pattern of interconnections between the amygdala and hypothalamus and between axons and dendrites in these nuclei, and thereby the organization of specific neural circuits. In the absence of testosterone, the female pattern of neuronal development occurs.
That the preoptic and other hypothalamic regions are sexually dimorphic is not surprising, in that it has long been known that this area is critical in controlling the basal output of gonadotropins in females before ovulation and is heavily involved in mediating cyclic changes in hormone levels, such as follicle-stimulating estrogen, hormone (FSH), luteinizing hormone (LH), and progesterone. Chemical and electrical stimulation of the preoptic and ventromedial thalamic nuclei also triggers sexual behavior and even sexual posturing in females and males (Lisk, 1967, 1971).
In primates, electrical simulation of the preoptic area increases sexual behavior in males and significantly increases the frequency of erections, copulations, and ejaculations, we well as pelvic thrusting followed by an explosive discharge of semen even in the absence of a mate (Maclean, 1973). Conversely, lesions to the preoptic and posterior hypothalamus eliminates male sexual behavior and results in gonadal atrophy.
Lateral and Ventromedial Hypothalamic Nuclei
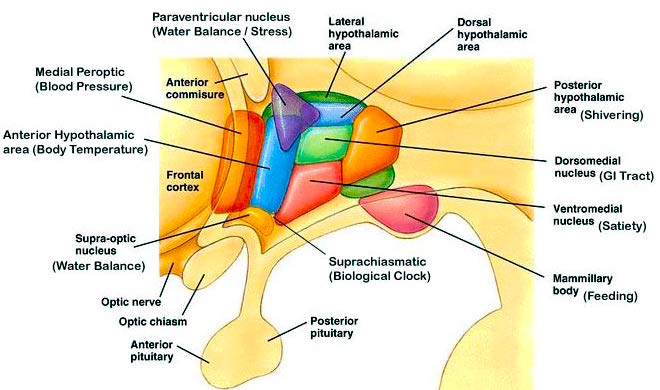
Although consisting of several nuclear subgroups, the lateral and medial (ventromedial) hypothalamic nuclei play particularly important roles in the control of the autonomic nervous system, the experience of pleasure and aversion, eating and drinking, and raw (undirected) emotionality. These nuclei also appear to share a somewhat antagonistic relationship (fig. 20).
For example, the medial hypothalamus controls parasympathetic activities (e.g., reduction in heart rate, increased peripheral circulation) and exerts a dampening effect on certain forms of emotional/motivational arousal. The lateral hypothalamus mediates sympathetic activity (increasing heart rate, elevating blood pressure) and is involved in controlling the metabolic and somatic correlates of heightened emotionality. In this regard, the lateral and medial region act to exert counterbalancing influences on each other.
Hunger and Thirst
The lateral and medial region are highly involved in monitoring internal homeostasis and motivating the organism to respond to internal needs such as hunger and thirst. For example, both nuclei appear to contain receptors that are sensitive to the body's fat content (lipostatic receptors) and to circulating metabolites (e.g., glucose), which together indicate the need for food and nourishment. The lateral hypothalamus also appears to contain osmoreceptors (Joynt, 1966) that determine whether water intake should be altered.
Electrophysiologically , it has been determined that the hypothalamus not only becomes highly active immediately before and while the organism is eating or drinking, but the lateral region alters its activity when the subject is hungry and is simply looking at food (Hamburg, 1971; Rolls, Burton, and Mora, 1976). In fact, if the lateral hypothalamus is electrically stimulated, a compulsion to eat and drink results (Delgado & Anand, 1953). Conversely, if the lateral area is destroyed bilaterally, aphagia and adipsia are so severe that animals will die unless force fed (Teitelbaum & Epstein, 1962).
If the medial hypothalamus is surgically destroyed, inhibitory influences on the lateral region appear to be abolished such that hypothalamic hyperphagia and severe obesity result (Teitelbaum, 1961). Thus, the medial area seems to act as a satiety center but as a center that can be overridden.
Overall, it appears that the lateral hypothalamus is involved in the initiation of eating and acts to maintain a lower weight limit such that when the limit is reached the organism is stimulated to eat. Conversely, the medial regions seems to be involved in setting a higher weight limit such that when these levels are approached, it triggers the cessation of eating. In part, these nuclei exert these differential influences on eating and drinking by means of motivational/emotional influences they exert on other brain nuclei (e.g., by reward or punishment).
Pleasure and Reward
In 1952, Heath (cited by Maclean, 1969) reported what was then considered remarkable. Electrical stimulation near the septal nuclei elicited feelings of pleasure in human subjects: "I have a glowing feeling. I feel good!" Subsequently, Olds and Milner (1954) reported that rats would tirelessly perform operants to receive electrical stimulation in this same region and concluded that stimulation "has an effect that is apparently equivalent to that of a conventional primary reward." Even hungry animals would demonstrate a preference for self-stimulation over food.
Feelings of pleasure (as demonstrated by self-stimulation) have been obtained following excitation to a number of diverse limbic areas, including the olfactory bulbs, amygdala, hippocampus, cingulated, substantia nigra ( a major source of dopamine), locus coeruleus (a major source of norepinephrine), raphe nucleus (serotonin), caudate, putamen, thalamus, reticular formation, medial forebrain bundle, and orbital frontal lobes (Brady, 1960; Lilly, 1960; Olds & Forbes, 1981; Stein & Ray, 1959; Waraczynski & Stellar, 1987).
In mapping the brain for positive loci for self-stimulation, Olds (1956) found that the medial forebrain bundle (MFB) was a major pathway that supported this activity. Although the MFB interconnects the hippocampus, hypothalamus, septum, amygdala, and orbital frontal lobes (areas that give rise to self-stimulation), Olds discovered that in its course up to the lateral hypothalamus, reward sites become more densely packed. Moreover, the greatest area of concentration and the highest rates of self-stimulatory activity were found to occur not in the MFB but in the lateral hypothalamus (Olds, 1956; Olds & Forbes, 1981). Indeed, animals "would continue to stimulate as rapidly as possible until physical fatigue forced them to slow or to sleep" (Olds, 1956).
Electrophysiological studies of a single lateral hypothalamic neurons have also indicated that these cells become highly active in response to rewarding food items (Nakamura & Ono, 1986). In fact, many of these cells will become aroused by neutral stimuli repeatedly associated with rewards such as a cue tone - even in the absence of the actual reward (Nakamura & Ono, 1986; Nishino, Sasaki, Fukuda, & Muramoto, 1980). However, this ability to form associations appears to be secondary to amygdaloid and other source of input (Fukuda, Ono, & Nakamura, 1987).
Nevertheless, if the lateral region is destroyed, the experience of pleasure and emotional responsiveness is almost completely attenuated. For example, in primates, faces become blank and expressionless, whereas of the lesion is unilateral, marked neglect and indifference regarding all sensory events occurring on the contralateral side occur (Marshall & Teitelbaum, 1974). Animals will in fact cease to eat and will die.
Aversion
In contrast to the lateral hypothalamus and its involvement in pleasurable self-stimulation, activation of the medial hypothalamus is apparently so aversive that subjects will work to reduce it (Olds & Forbes, 1981). Thus, electrical stimulation of the medial region leads to behavior that terminates the stimulation - apparently so as to obtain relief (e.g., active avoidance). In this regard, when considering behavior such as eating, it might be postulated that when the upper weight limits (or nutritional requirements) are met, the medial region becomes activated, which in turn leads to behavior (e.g., cessation of eating) that terminates its activation.
It is possible, however, that medial hypothalamic activity may also lead to a state of quiescence such that the organism is motivated simply to cease to respond. In some instances, this quiescent state may be physiologically neutral, whereas in other situations the result may be highly aversive. Nevertheless, quiescence is associated with parasympathetic activity, which is also mediated by the medial area.
Hypothalamic Damage and Emotional Incontinence: Laughter and Rage
When electrically stimulated, the hypothalamus responds by triggering two seemingly oppositional feeling states: pleasure and unpleasure/aversion. The generation of these emotional reactions in turn influences the organism to respond so as to increase or decrease what is being experienced. Through its rich interconnections with other limbic regions, including the neocortex and frontal lobes, the hypothalamus is able to mobilize and motivate the organism either to cease or to continue to behave. Nevertheless, at the level of the hypothalamus, the emotional states elicited are very primitive, diffuse, undirected and unrefined. The organism feels pleasure in general, or aversion/unpleasure in general. Higher-order emotional reactions (e.g., desire, love, hate) require the involvement of other limbic regions as well as neocortical participation.
Emotional functioning at the level of the hypothalamus is not only quite limited and primitive, it is also largely reflexive. For example, when induced by stimulation, the moment the electrical stimulus is turned off, the emotion elicited is immediately abolished. By contrast, true emotions (which require other limbic interactions) are not simply turned on or off but can last from minutes to hours to days and weeks before completely dissipating.
Nevertheless, in humans, disturbances of hypothalamic functioning (e.g., caused by an irritating lesion such as tumor) can give rise to seemingly complex, higher-order behavioral - emotional reactions, such as pathological uncontrollable laughter and crying. However, in some cases, when patients are questioned, they may deny having any feelings that correspond to emotion displayed (Davison & Kelman, 1939; Ironside, 1956; Martin, 1950). In part, these reactions are sometimes due to disinhibitory release of brainstem structures involved in respiration, whereas in other instances the resulting behavior is caused by hypothalamic triggering of other limbic nuclei.
Uncontrolled Laughter
Pathological laughter has frequently been reported to occur with hypophyseal and midline tumors involving the hypothalamus, aneurysm in this vicinity, hemorrhage, astrocytoma, or papilloma of the third ventricle (resulting in hypothalamic compression), as well as surgical manipulation of this nucleus (Davison & Kelman, 1939; Dott, 1938; Foerster & Gagel, 1933; Martin, 1950; Money & Hosta, 1967; Ironside, 1956; List, Downman, & Bagheiv, 1958).
For example, Martin (1950) describes a man who, while "attending his mother's funeral was seized at the graveside with an attack of uncontrollable laughter which embarrassed and distressed him considerably" (p. 455). Although this particular attack dissipated, it was soon accompanied by several further fits of laughter and he died soon thereafter. At postmortem examination, a large ruptured aneurysm was found compressing the mamillary bodies and hypothalamus.
In a similar case (Anderson, 1936, cited by Martin, 1950), a patient literally died laughing following eruption of the posterior communicating artery that resulted in compression (by hemorrhage) of the hypothalamus. "She was shaken by laughter and could not stop: short expirations followed each other in spasms, without the patient being able to make an adequate inspiration of air, she became cyanosed and nothing could stop the spasm of laughter which eventually became noiseless and little more than a grimace. After 24 hours of profound coma, she died."
Because laughter in these instances has not been accompanied by corresponding feeling states, this pseudoemotional condition has been referred to as sham mirth (Martin, 1950). However, in some cases, abnormal stimulation in this region (such as due to compression effects from neoplasm) has triggered corresponding emotions and behaviors - presumably due to activation of other limbic nuclei.
For example, laughter has been noted to occur with hilarious or obscene speech - usually as a prelude to stupor or death - in cases in which tumor has infiltrated the hypothalamus (Ironside, 1956). In several instances, it has been reported by one group of neurosurgeons (Foerster & Gagel, 1933) that while swabbing the blood from the floor of the third ventricle, patients "became lively, talkative, joking, and whistling each time the infundibular region of the hypothalamus was manipulated." In one case, the patient became excited and began to sing.
Hypothalamic Rage
Stimulation of the lateral hypothalamus can induce extremes in emotionality, including intense attacks of rage accompanied by biting and attack upon any moving object (Flynn, Edwards, & Bandler, 1971; Gunne & Lewander, 1966; Wasman & Flynn, 1962). If this nucleus is destroyed, aggressive and attack behavior is abolished (Karli & Vergness, 1969).
Thus, the lateral hypothalamus is responsible for rage and for aggressive behavior. The lateral hypothalamus maintains an oppositional relationship with the medial hypothalamus. Thus, stimulation of the medial region counters the lateral area such that rage reactions are reduced or eliminated (Ingram, 1952; Wheatley, 1944), whereas if the medial is destroyed. There results lateral hypothalamic release and the triggering of extreme savagery.
In man, inflammation, neoplasm, and compression of the hypothalamus have also been noted to give rise to rage attacks (Pilleri & Poeck, 1965), and surgical manipulations or tumors within the hypothalamus have been observed to elicit manic and ragelike outbursts (Alpers, 1940). These appear to be release phenomenon, however. That is, rage, attack, aggressive, and related behaviors associated with the hypothalamus appears to be under the inhibitory influence of higher order limbic nuclei such as the amygdala and septum (Siegel & Skog, 1970). When the controlling pathways between these areas are damaged (i.e., disconnection) these behaviors are sometimes elicited.
For example, Pilleri and Poeck (1965) described a man with severe damage throughout the cerebrum, including the amygdala, hippocampus, and cingulate, but with complete sparing of the hypothalamus, who continually reacted with howling, growling, and baring of teeth in response to noise or a slight touch or if approached. Hence, the hypothalamus, being released, responds reflexively in an aggressive nonspecific manner to any stimulus. Lesions of the frontal - hypothalamic pathways have been noted to result in severe rage reactions as well (Fulton & Ingraham, 1929; Kennard, 1945).
Nevertheless, like sham mirth, rage reactions elicited in response to direct electrical activation of the hypothalamus immediately and completely dissipate when the stimulation is removed. These outbursts have been referred to as sham rage.
Lateralization
Although scant, there is some evidence to suggest that the right hypothalamus may be more heavily involved in the control of neuroendocrine functioning, particularly in females. Greater right hypothalamic concentration of substancessuch as LHRH (luteinizing hormone) has also been reported (see Gerendai, 1984, for review).
Psychic Manifestations of Hypothalamic Acitivity
Phylogenetically and from an evolutionary perspective, the appearance and development of the hypothalamus predate the emergence and differentiation of all other limbic nuclei, e.g., amygdala, septal nucleus, and hippocampus (Andy & Stephen, 1961; Brown, 1983; Herrick, 1925; Humphrey, 1972). It constitutes the most primitive, archaic, reflexive, and purely biological aspect of the psyche.
Biologically, the hypothalamus serves the body tissues by attempting to maintain internal homeostasis and by providing for the immediate discharge of tenions in an almost reflexive manner. Studies of lateral and medial hypothalamic functioning indicate that it appears to act reflexively, in an almost on/off manner so as to seek or maintain the experience of pleasure and escape or avoid unpleasant noxious conditions.
Emotions elicited by the hypothalamus are largely undirected, short-lived, and unconnected with events occurring within the external environment, being triggered reflexively and without concern or understanding regarding consequences. Direct contact with the real world is quite limited and almost entirely indirect as the hypothalamus is largely concerned with the internal environment of the organism. It has no sense of morals, danger, values, logic, and so forth, and can neither feel nor express love or hate. Although quite powerful, hypothalamic emotions are largely undifferentiated, consisting of such feelings as pleasure, unpleasure, aversion, rage, hunger, and thirst.
As the hypothalamus is concerned with the internal environment, much of its activity occurs outside conscious-awareness. Moreover, being involved in maintaining internal homeostasis, through, for example, its ability to reward or punish the organism with feelings of pleasure or aversion, it tends to serve what Freud (1911) described as the pleasure principle.
The lateral and medial nuclei exert counterbalancing influences that serve to modulate activity occurring in the other. As described by Freud (1911), the pleasure principle not only serves to maximize pleasant experiences, but acts to keep the psyche as a whole free from high levels of excitation (be they pleasurable or unpleasant).
Like the hypothalamus, the pleasure principle is present from birth and for some time thereafter the search for pleasure is manifested in an unrestricted manner and with great intensity, as there are no oppositional forces (except those between the lateral and medial regions) to counter it's strivings. Indeed, higher order limbic nuclei have yet to mature.
Functionally isolated, the hypothalamus at birth has no way of reducing tension or of mobilizing the organism for any form of effective action. It is helpless. When tensions associated with immediate needs (e.g., hunger or thirst) become unpleasant the only response available to the hypothalamus is to cry and make ragelike vocalizations. When satiated, the hypothalamus can only respond with a feeling state suggesting pleasure or at least quiescence. Indeed, as is well known, for the first few months of life, the infant's awareness largely consists of a very restricted matrix involving tactile, visceral (hunger), and kinesthetic sensations, whereas emotionally the infant is capable of screaming, crying, or demonstrating very rudimentary features of pleasure, i.e., an attitude of acceptance of quiescence (McGraw, 1969; Milner, 1967; Piaget, 1952; Spitz & Wolf, 1946). It is only with the further differentiation and maturation of higher-order limbic nuclei (e.g., amygdala, septal nucleus, hippocampus) that the infant begins to achieve some awareness of external reality and begins to form memories as well as differentiate and associate externally occurring events and individuals.
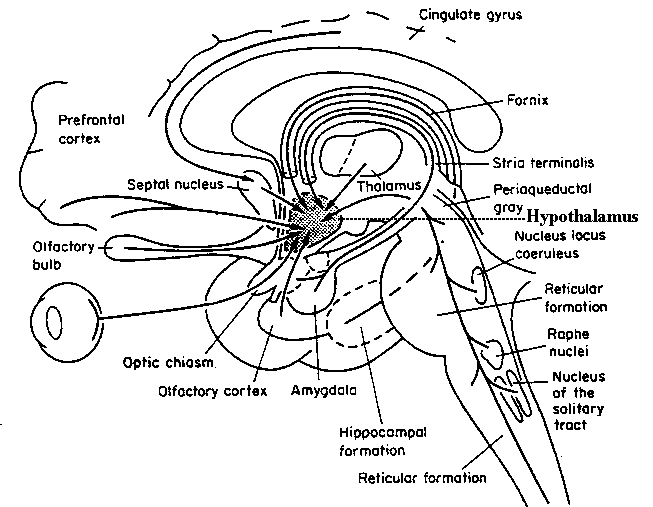
AMYGDALA
In contrast to the primitive hypothalamus, the more recently developed amygdala is preeminent in the control and mediation of all higher order emotional and motivational activities. Through its rich interconnections with various neocortical and subcortical regions, amygdaloid neurons are able to monitor and abstract from the sensory array stimuli that are of motivational significance to the organism (Steklis & Kling, 1985). This includes the ability to discern and express even subtle social - emotional nuances such as friendliness, fear, love, affection, distrust, and anger, and at a more basic level, determine whether something might be good to eat. In fact, amygdaloid neurons respond selectively to the flavor of certain preferred foods, as well as to the sight or sound of something that might be especially desirable to eat (Fukuda et al., 1987; O'Keefe & Bouma, 1969; Ono et al., 1980).
Single amygdaloid neurons receive considerable topographic input, and are predominantly polymodal, responding to a variety of stimuli from different modalities simultaneously (O'Keefe & Bouma, 1969; Perryman, Kling, & Lloyd, 1987; Sawa & Delgado, 1963; Schutze, Knuepfer, Eismann, Stumpf, & Stock, 1987; Turner, Mishkin, & Knapp, 1980; Ursin & Kaasa, 1960; Van Hoesen, 1981). The amygdala is also very sensitive to somesthetic input and physical contact such that even a slight touch in a very circumscribed area of the body can produce amygdaloid excitation. Overall, in addition to emotional and motivational functioning, because multimodal assimilation of various sensory impressions occurs in this region, it is also involved in attention, learning, and memory.
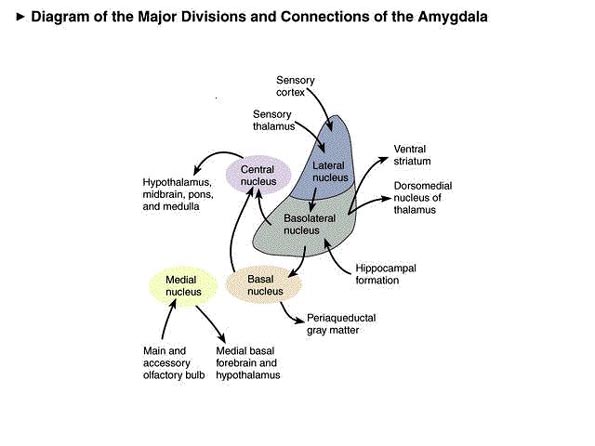
Medial and Lateral Amygdaloid Nuclei
The amygdala is buried within the depths of the anterior - inferior temporal lobe and consists of two major nuclear groups. These are a phylogenetically ancient anteromedial group (or medial amygdala) involved in olfaction and motor activity, as well as a relatively newer basolateral division (lateral amygdala) that first appears in primates (Herrick, 1925; Humphrey, 1972). Like the lateral and medial hypothalamus, these two amygdaloid nuclei subserve different functions and maintain different anatomical interconnections.
Emybryologically, the medial amygdala is the first portion of the basal ganglia striatal complex to appear during development, being formed through neuroblast migration from the epithilium of the lateral ventricle (Humphrey, 1972). As it circles in an arc from the frontal to temporal lobe, the tail of the caudate nucleus actually terminates and merges with the amygdala. This portion of the amygdala is in fact part of the basal ganglia and is heavily involved in motivating and coordinating gross, or whole-body, motor activity.
The medial amygdala receives fibers from the olfactory tract and through a rope of fibers called the stria terminalis, projects directly to and receives fibers from the medial hypothalamus (through which it exerts inhibitory influences) as well as the septal nucleus (Carlsen, De Olmos, & Heimer, 1982; Gloor, 1955; Russchen, 1982; Swanson & Cowan, 1979). In addition, the medial (and lateral) regions are rich in cells containing enkephalins, and opiate receptors can be found throughout the amygdala (Atweh & Kuhar, 1977; Uhl, Kudar, & Snyder, 1978).
Portions of the hypothalamus and amygdala are sexually dimorphic; i.e., there are male and female amygdaloid nuclei. Thus, at a very basic and primitive level, emotional and motivational perceptual/behavioral functioning becomes influenced and guided by the neuroanatomical sexual bias of the host. Electrical stimulation of the amygdala, the medial division in particular, results in sex-related behavior and activity. In females this includes ovulation, uterine contractions and lactogenetic responses, and in males penile erections (Robinson & Mishkin, 1968; Shealy & Peele, 1957).
Damage to the amygdala bilaterally often results in heightened and indiscriminant sexual activity. For example, primates and other animals (while in captivity) will engage in excessive masturbation and genital manipulation and will repeatedly attempt to copulate even with species other than their own (e.g., a cat with a dog, a dog with a turtle) regardless of their sex. With bilateral destruction, animals are not only overly active sexually but are able to identify appropriate partners (Brown & Schaefer, 1888; Kluver & Bucy, 1939). This abnormality is one aspect of a complex of symptoms sometimes referred to as the Kluver - Bucy syndrome (to be discussed in more detail below).
Lateral Amygdala
With the evolutionary ascent of primates, the relatively new lateral division of the amygdala progressively expands and differentiates. The lateral amygdala contributes fibers to the stria terminalis and gives rise to the amygdalofugal pathway, through which it projects to the lateral and medial hypothalamus (upon which it exerts inhibitory and excitatory influences, respectively), the dorsal medial thalamus (which is involved in memory, attention, and arousal), olfactory tubercle, as well as other subcortical regions (Aggleton, Burton, & Passingham, 1980; Carlsen, et al., 1982; Dreifuss et al., 1968; Gloor, 1955, 1960; Klinger & Gloor, 1960; Mehler, 1980; Russchen, 1982). It also receives fibers from the medial forebrain bundle, which in turn has its site of origin in the lateral hypothalamus (Mehler, 1980).
In general, whereas the medial amygdala is highly involved in motor, olfactory and sexual functioning, the lateral division is intimately involved in all aspects of higher order emotional activity; hence its rich interconnections with the lateral and medial hypothalamus, and the neocortex. The lateral amygdala maintains rich interconnections with the inferior, middle, and superior temporal lobes, as well as the insular temporal region, which in turn allows it to sample and influence the auditory, somesthetic, and visual information being received and processed in these areas, as well as to scrutinize this information for motivational and emotional significance (Herzog & Van Hoesen, 1976; Kling et al., 1987; Machne & Segundo, 1956; Mesulam & Mufson, 1982; O'Keefe & Bouma, 1969; Steklis & Kling, 1985; Turner et al., 1980; Van Hoesen, 1981).
Gustatory and respiratory sense are also rerepresented in this vicinity (Fukuda et al., 1987; Maclean, 1949; Ono et al., 1980), and the lateral division maintains rich interconnections with cingulated gyrus, orbital frontal lobes (Pandya, Van Hoesen, Domeskick, 1973), and the parietal cortex (O'Keefe & Bouma, 1969), through which it receives complex somesthetic information.
The lateral amygdala is highly important in analyzing information received and transferring information back to the neocortex so that further elaboration may be carried out at the cortical level. It is through the lateral division that emotional meaning and significance can be assigned to as well as extracted from that which is experienced.
The amygdala, overall, maintains a functionally interdependent relationship with the hypothalamus. It is able to modulate and even control rudimentary emotional forces governed by the hypothalamic nucleus. However, it also acts at the behest of hypothalamically induced drives. For example, if certain nutritional requirements need to be met, the hypothalamus signals the amygdala, which then surveys the external environment for something good to eat. By contrast, when, via environmental surveilance, the amygdala discovers a potentially threatening stimulus, it acts to excite and drive the hypothalamus so that the organism is mobilized to take appropriate action. Thus, when the hypothalamus is activated by the amygdala, instead of responding in an on - off manner, cellular activity continues for an appreciably longer time period (Dreifuss et al., 1968). The amygdala can tap into the reservoir of emotional energy mediated by the hypothalamus to achieve certain ends.
Attention
The amygdala acts to perform environmental surveillance and can trigger orienting responses as well as mediate the maintenance of attention if something of interest or importance were to appear (Gloor, 1955, 1960; Kaada, 1951; Ursin & Kaasa, 1960). Electrical stimulation of the lateral division can initiate quick and/or anxious glancing and searching movements of the eyes and head, such that the organism appears aroused and highly alert as if in expectation of something that is going to happen (Ursin & Kaada, 1960). The EEG becomes desynchronized (indicating arousal), heart rate becomes depressed, respiration patterns change, and the galvanic skin response significantly alters (Bagshaw & Benzies, 1968; Ursin & Kaada, 1960) - reactions that characteristically accompany the orienting response of most species. Once a stimulus of potential interest is detected, the amygdala acts to analyze its emotional - motivational importance and will act to alert other nuclei such as the hypothalamus so that appropriate action may take place.
Fear, Rage, and Aggression
Initially, electrical stimulation of the amygdala produces sustained attention and orienting reactions. If the stimulation continues fear and/or rage reactions are elicited (Ursin & Kaada, 1960). When fear follows the attention response, the pupils dilate and the subject will cringe, withdraw, and cower. This cowering reaction in turn may give way to extreme fear and/or panic such that the animal will attempt to take flight.
Among humans, the fear response is one of the most common manifestations of amygdaloid electrical stimulation. Moreover, unlike hypothalamic on/off emotional reactions, attention and fear reactions can last up to several minutes after the withdrawal of stimulation.
In addition to behavioral manifestations of heightened emotionality, amygdaloid stimulation can result in intense changes in emotional facial expression. This includes facial contortions, baring of the teeth, dilation of the pupils, widening or narrowing of the eyelids, flaring of the nostrils, tearing, as well as sniffing, licking, and chewing (Anand & Dua, 1955; Ursin & Kaada, 1960). Indeed, some of the behavioral manifestations of a seizure in this vicinity (i.e., temporal lobe epilepsy) typically include chewing, smacking of the lips, and licking.
In many instances, rather than fear, there instead results anger, irritation, and rage, which seems to build up gradually, until finally the animal or human attacks (Egger & Flynn, 1963; Gunne & Lewander, 1966; Ursin & Kaada, 1960; Zbrozyna, 1963). Unlike hypothalamic sham rage, amygdaloid activation results in attacks directed at something real or, in the absence of an actual stimulus, at something imaginary. Moreover, rage and attack will persist well beyond the termination of the electrical stimulation of the amygdala. In fact, the amygdala remains electrophysiologically active for long periods, even after a stimulus has been removed (be it external - perceptual, or internal - electrical), such that is appears to continue to process - in the abstract - information even when that information is no longer observable (O'Keefe & Bouma, 1969).
In addition to permitting sustained electrophysiological activity, the amygdala has been shown to be heavily involved in the maintenance of behavioral responsiveness even in the absence of an immediately tangible or visible objective or stimulus (O'Keefe & Bouma, 1969). This includes motivating the organism to engage in the seeking of hidden objects or continuing a certain activity in anticipation of achieving some particular long-term goal. At a more immediate level, the amygdala is probably very important in object permanence (i.e., keeping an object in mind when it is no longer visible) and concrete or abstract anticipation. Anticipation is, of course, very important in the prolongation of emotional states such as fear or anger, as well as the generation of more complex emotions such as anxiety. In this regard, the amygdala is probably important not only in regard to emotion, but in maintaining mood states.
Fear and rage reactions have also been triggered in humans following depth electrode stimulation of the amygdala (Chapman, 1960; Chapman, Schroeder, Geyer, Brazier, Fager, Poppen, Solomoan, & Yakovlev, 1954; Heath, Monroe, & Mickle, 1955; Mark, Ervin, & Sweet, 1972). Mark et al. (1972) describe one female patient who following amygdaloid stimulation became irritable and angry, and then enraged. Her lips retracted, there was extreme facial grimacing, threatening behavior, and then rage and attack - all of which persisted well beyond stimulus termination.
Similarly, Schiff et al. (1982) described a man who developed intractable aggression following a head injury and damage (determined via depth electrode) to the amygdala (i.e., abnormal electrical activity). Subsequently, he became easily enraged, sexually preoccupied (although sexually hypoactive), and developed hyperreligiosity and psuedomystical ideas. Tumors invading the amygdala have been reported to trigger rage attacks (Sweet, Ervin, & Mark, 1960; Vonderache, 1940).
The amygdala appears capable of not only triggering and steering hypothalamic activity but acting on higher level neocortical processes so that individuals form emotional ideas. Indeed, the amygdale is able to overwhelm the neocortex and the rest of the brain so that the person not only forms emotional ideas but responds to them. A famous example of this is Charles Whitman, who in 1966 climbed a tower at the University of Texas and began to indiscriminantly kill people with a rifle.
Whitman had initially consulted a psychiatrist about his periodic and uncontrollable violent impulses but was unable to obtain relief. Prior to climbing the tower, he wrote himself a letter (Sweet et al., 1969): I don't really understand myself these days. Lately I have been a victim of many unusual and irrational thoughts. These thoughts constantly recur, and it requires a tremendous mental effort to concentrate. I talked to a doctor once for about two hours and tried to convey to him my fears that I felt overcome by overwhelming violent impulses. After one session I never saw the Doctor again, and since then I have been fighting my mental turmoil alone. After my death I wish that an autopsy would be performed to see if there is any visible physical disorder. I have had tremendous headaches in the past.
Later he wrote: It was after much thought that I decided to kill my wife, Kathy, tonight after I pick her up from work....I love her dearly, and she has been a fine wife to me as any man could ever hope to have. I cannot rationally pinpoint any specific reason for doing this....
That evening, he killed his wife and mother and wrote: I imagine that it appears that I brutally killed both of my loved ones. I was only trying to do a good thorough job....
The following morning, he climbed the University tower carrying a high-powered hunting rifle and for the next 90 min he shot at everything that moved, killing 14 and wounding 38. Postmortem autopsy of his brain demonstrated a glioblastoma multiforme tumor the size of a walnut compressing the amygdaloid nucleus.
Docility and Amygdaloid Destruction
Bilateral destruction of the amygdala usually results in increased tameness, docility, and reduced aggressiveness in cats, monkeys and other animals (Schreiner and Kling, 1956; Weiskrantz, 1956; Vochteloo & Koolhas, 1987), including purportedly ferocious creatures such as the agouti and lynx (Schreiner and Kling, 1956). In man, bilateral amygdala destruction (by neurosurgery) has been reported to reduce and/or eliminate paroxysmal aggressive and violent behavior (Terzian & Ore, 1955).
In some creatures, however, bilateral ablation of the amygdala has been reported to at least initially result in increased aggressive responding (Bard & Mountcastle, 1948) and, if sufficiently aroused or irritated, even the most placid of amygdalectomized animals can be induced to fight fiercely (Fuller, Rosvold, & Pribram, 1957). However, these aggressive responses are very short-lived and appear to be reflexively mediated by the hypothalamus. Thus, these findings (and the data reviewed above) suggest that true aggressive feelings are dependent on the functional integrity of the amygdala (versus the hypothalamus).
Social - Emotional Agnosia
Among primates and mammals, bilateral destruction of the amygdala significantly disturbs the ability to determine and identify the motivational and emotional significance of externally occurring events, to discern social - emotional nuances conveyed by others, or to select what behavior is appropriate for a specific social context (Bunnel, 1966; Fuller, Rosvold & Pribram, 1957; Gloor, 1960; Kluver & Bucy, 1939). Bilateral lesions lower responsiveness to aversive and social stimuli, reduce aggressiveness, fearfulness, competitiveness, dominance, and social interest (Rosvold, Mirsky, & Pribram, 1954). Indeed, this condition is so pervasive that subjects seem to have tremendous difficulty discerning the meaning or recognizing the significance of even common objects - a condition sometimes referred to as psychic blindness or the Kluver - Bucy syndrome.
Thus, animals with bilateral amygdaloid destruction, although able to see and interact with their environment, respond in an emotionally blunted manner and seem unable to recognize what they see, feel, and experience. Things seem stripped of meaning. Like an infant (who similarly is without a fully functional amygdala), patients with this condition engage in extreme orality and will indiscriminantly pick up various objects and place them in their mouth regardless of its appropriateness. There is a repetitive quality to this behavior, for once they put it down they seem to have forgotten that they had just explored it, and will immediately pick it up and place it again in their mouth as if it were a completely unfamiliar object.
Although ostensibly exploratory, there is thus a failure to learn, to remember, to discern motivational significance, to habituate with repeated contact, or to discriminate between appropriate and inappropriate stimuli. Rather, when the amygdala has been removed bilaterally, the organism reverts to the most basic and primitive modes of object interaction such that everything that is seen and touched is placed in the mouth (Brown & Schaffer, 1888; Gloor, 1960; Kluver & Bucy, 1939). This condition pervades all aspects of higher-level social - emotional functioning, including the ability to interact appropriately with loved ones.
For example, Terzian and Ore (1955) described a young man who, following bilateral removal of the amygdala, subsequently demonstrated an inability to recognize anyone, including close friends, relatives, and his mother. He ceased to respond in an emotional manner to his environment and seemed unable to recognize feelings expressed by others. He also exhibited many features of the Kluver - Bucy syndrome (perserverative oral "exploratory" behavior and psychic blindness), as well as an insatiable appetite. In addition, he became extremely socially unresponsive such that he preferred to sit in isolation, well away from others.
Among primates who have undergone bilateral amygdaloid removal, once they are released from captivity and allowed to return to their social group, a social - emotional agnosia becomes readily apparent, as they no longer respond to or seem able to appreciate or understand emotional or social nuances. Indeed, they appear to have little or no interest in social activity and persistently attempt to avoid contact with others (Dick, Myers, & Kling, 1969; Jonason & Enloe, 1971; Jonason, Enloe, Contrucci, & Meyer, 1973). If approached they withdraw, and if followed they flee. Indeed, they behave as if they have no understanding of what is expected of them or what others intend or are attempting to convey, even when the behavior is quite friendly and concerned. Among adults with bilateral lesions, total isolation seems to be preferred.
In addition, they no longer display appropriate social or emotional behaviors, and if kept in captivity will fall in dominance in a group or competitive situation - even when formerly dominant (Bunnel, 1966; Dicks et al., 1969; Fuller et al., 1957; Jonason & Enloe, 1971; Jonason et al., 1973; Rosvold, Mirsky, & Pribram, 1954). As might be expected, maternal behavior is severely affected. According to Kling (1972), mothers will behave as if their "infant were a strange object to be mouthed, bitten, and tossed around as though it were a rubber ball."
Laterality and the Amygdala
Limbic Language
Although language is usually discussed in regard to grammar and vocabulary, there is a third major feature to expression and comprehension through which a speaker may convey, and a listener determine, intent, attitude, feeling, and meaning. Language is both descriptive and emotional. A listener comprehends not only what is said, but how it is said - what a speaker feels. Feeling and attitude is generally communicated via vocal inflection, intonation, and melody such that anger, happiness, sadness, sarcasm, and so forth, are indicated by such cues as variations in pitch, timbre, and stress contours - vocal capacities associated with the functional integrity of the right cerebral hemisphere, and the right frontal and temporal regions in particular (Joseph, 1988a; see also Chapter 1, The Right Cerebral Hemisphere). For example, studies using dichotic listening have repeatedly shown that the right hemisphere is superior in distinguishing stress and pitch contours, determining frequency, amplitude, melody, duration, processing inflectional contours, and even decoding contextual information in the absence of denotative speech.
Conversely, patients who have undergone right temporal lobe neurosurgery or who have suffered damage involving this area, often demonstrate impairments in regard to many aspects of prosodic - melodic perception, such that nonverbal environmental sounds and musical stimuli fail to be adequately recognized. People who have suffered deep right frontal or temporal lobe damage also have difficulty controlling the pitch and inflection of their voice, whereas patients undergoing sodium amytal anesthetization of the right hemisphere speak in a bland monotone.
It has been argued in detail (Joseph, 1982, 1988a) that the superior capacity of the right hemisphere in processing and expressing melodic - prosodic - emotional information is directly related to lateralized limbic functioning, and in particular, a greater abundance of neuronal interconnections with various limbic nuclei such as the amygdale. Emotional vocalizations are limbic in origin and are hierarchically represented, processed, and expressed by various neocortical regions within the frontal and temporal lobes.
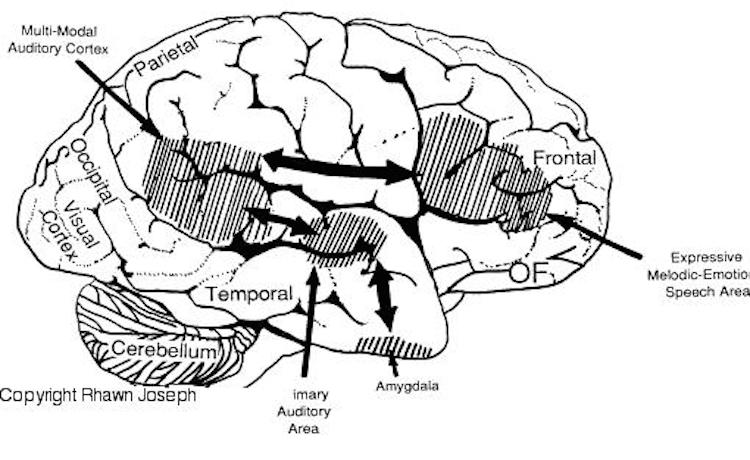
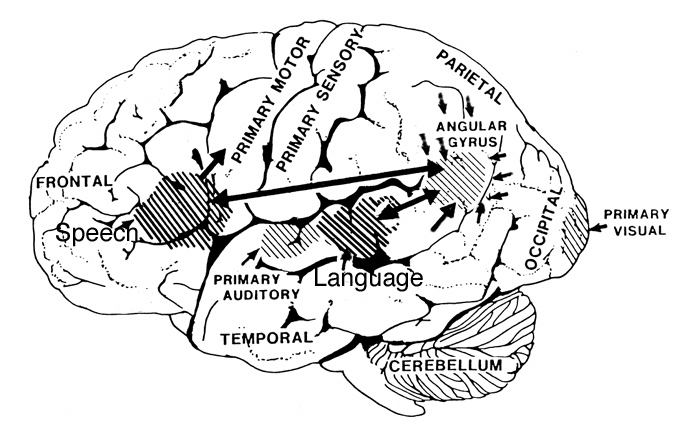
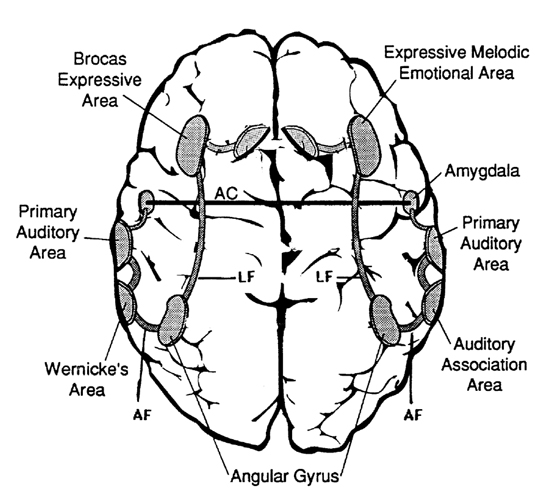
In fact, the major fiber pathway that links the language axis (Wernicke's and Broca's areas) of the left hemisphere and the melodic - prosodic - emotional Axis of the right, i.e., the arcuate fasciculus, extends from the left frontal convexity (Broca's area) through the inferior parietal lobule, and after giving off major fibers to Wernicke'e region continues down into the depths of the inferior temporal lobe where it establishes contact with the amygdala. In this way, the amygdala has a significant influence on emotional speech (Figs. 21-23).
Indeed, both phylogenetically and ontogenetically, the original impetus to vocalize springs forth from roots buried within the depths of the ancient limbic lobes (e.g., amygdala, hypothalamus, septum). For example, although nonhumans do not have the capacity to speak, they still vocalize, and these vocalizations are primarily limbic in origin, being evoked in situations involving sexual arousal, terror, anger, flight, helplessness, and separation from the primary caretaker when young. The first vocalizations of human infants are similarly emotional in origin and limbically mediated (Joseph, 1982).
Although cries and vocalizations indicative of rage or pleasure have been elicited through hypothalamic stimulation, of all limbic nuclei the amygdale is the most vocally active - particularly the lateral division (Robinson, 1967). In humans and animals, a wide range of emotional sounds have been evoked through amygdala activation, such as sounds indicative of pleasure, sadness, happiness, and anger (Robinson, 1967; Ursin & Kaada, 1960). Conversely, in man, destruction limited to the amygdala, (Freeman & Williams, 1952, 1963), the right amygdala, in particular, has abolished the ability to sing, convey melodic information, or properly enunciate by vocal inflection. Similar disturbances occur with right hemisphere damage (Joseph, 1988a). Indeed, when the right temporal region (including the amygdala) has been grossly damaged or surgically removed, the ability to perceive, process, or even vocally reproduce most aspects of musical and emotional auditory input is significantly curtailed. (See Chapter 1, The Right Cerebral Hemisphere.)
Emotion and Temporal Lobe Seizures
The amygdala is buried within the depths of the anterior - inferior temporal lobe and maintains rich interconnections with areas throughout the temporal neocortex. Because of their intimate association, damage to the temporal lobe, particularly the anterior regions, often involves and disrupts amygdaloid functioning. In fact, because the amygdala and inferior- anterior temporal lobe have, of all brain regions, the lowest seizure threshold, and are minimally resistant, and are therefore maximally vulnerable to developing abnormal seizure activity, even mild injuries may result in kindling (i.e., abnormal activation), and therefore disruption of their functional integrity. Indeed, damage to adjacent tissue has been known to spread, by kindling, to the amygdala and inferior regions. Once consequence is temporal lobe epilepsy.
Personality, emotional, and sexual disturbances are a frequent complication of temporal lobe seizures in a significant minority of patients. Such patients may develop paranoid, hysterical, or depressive tendencies, deepening of mood, hyposexuality, and other characteristics suggestive of affective disorders (Bear, Leven, Blumer, Chetam, & Ryder, 1982; Gibbs, 1951; Herman & Chambria, 1980; Strauss, Risser, & Jones, 1982; Williams, 1956). Immediately following or during the course of a seizure 10% or more of such patients experience a change in emotionality (Herman & Chambria, 1980; Strauss et al., 1982; Williams 1956).
In part, because the highest incidence of psychiatric disorder occurs in cases in which the EEG spike focus is in the anterior temporal area (Gibbs, 1951), and because limbic nuclei such as the amygdala are frequently involved, it has been postulated that seizure activity sometimes hyperactivates these nuclei (Bear, 1979), which in turn distorts the affective meaning applied to afferent streams of visual, auditory, and somesthetic information (Gibbs, 1951).
Thus, during a seizure, these patients may be temporarily overwhelmed by feelings such as fear, or things they see or hear seem to become abnormally invested with emotional significance (Bear et al., 1979; Gibbs, 1951; Gloor et al., 1982), presumably due to abnormal amygdala activation. Interestingly, one common symptom of temporal lobe epilepsy is an aura of tastes, and more often odors, that are usually quite unpleasant (e.g., like burning wire, burning feces, burning rubber or tires).
Seizure induced emotional changes tend to predominantly involve feelings of depression, pleasure, displeasure, or fear - with fear being one of the most common emotional experiences (Gloor et al., 1982; Williams, 1956). More rarely, seizures involving sexual behavior, crying, laughing, or ragelike responses have been associated with temporal lobe epilepsy.
There is some evidence to suggest that certain emotional changes are more frequently associated with seizure originating in the right temporal lobe and/or amygdale, whereas disturbances involving thought (e.g., psychosis, schizophrenia) characterize left temporal lobe abnormalities (Flor-Henry, 1969, 1983; Offen, Davidoff, Troost, & Richey, 1976; Joseph, 1988; Sherwin, 1981; Schiff et al., 1982; Taylor, 1975; Weil, 1956).
For example, in their depth electrode study of five patients with seizure disorders, Gloor et al., (1982) found that all feelings of fear and displeasure were associated with right temporal, right amygdala, or right hippocampal activation. These findings are consistent with the observations of Slater, Beard, and Glithero (1969), Bear and Fedio (1977), Flor-Henry (1969), and others, who have noted an association between right temporal seizures and affective disorders.
Other reports, however, have been less clear cut - presumably because seizures originating in the amygdala/temporal lobe can quickly spread from hemisphere to another (through the anterior commissure) and because of the failure to employ depth electrodes to pinpoint the seizure foci. Thus, in some instances, emotions such as fear seem to arise regardless of which hemisphere is involved (Strauss et al., 1982). Nevertheless, even in these cases, an emotional dichotomy is apparent. For example, Herman and Chambria (1980) described cases with right temporal foci in whom free-floating fears developed that were not tied to something specific but that encompassed terrifying, "death-like" and "nightmarish" feelings, whereas a patient with a left temporal foci developed intense fears but were unable to describe what they were afraid of.
Stimulation of the amygdala can significantly alter facial emotional expression, including tearing. In a small minority of cases, right temporal seizures have been reported to cause paroxysmal attacks of weeping, with lacrimation, the making of mournful sounds, including sobbing and crying (Offen et al., 1976). However, crying as well as laughing seizures have also been noted to occur with left-sided involvement (Chen & Forster, 1973; Sethi & Rao, 1976). Nevertheless, without the benefit of depth electrodes, such as employed by Gloor and colleagues (1982), it is difficult to determine in which amygdala and/or temporal lobe a seizure actually originates.
The amygdala and regions of the hypothalamus are sexually dimorphic, and stimulation of either area can trigger sexual behavior. Similarly, sensations of sexual excitement, sometimes leading to orgasm, may also occur as a function of seizures originating in the temporal lobe (Currier, Little, Suess, & Andy, 1971; Freemon & Nevis, 1969; Remillard et al., 1983) and in the frontal lobe (Spencer, Spencer, Williamson, & Mattson, 1983). Of interest, 7 of 10 patients with sexual seizures described by Remillard et al., (1983) had foci originating in the right hemisphere. Similar findings have been reported by Freemon & Nevis (1969), Penfield and Rasmussen (1950, p.27), and Spencer et al. (1983).
Sexual seizures caused by temporal lobe abnormalities are often accompanied by actual sexual behavior. "The patient was sitting at the kitchen table with her daughter making out a shopping list. She stopped, appeared dazed, slumped to the floor on her back, lifted her skirt, spread her knees, and elevated her pelvis rhythmically. She made appropriate vocalizations for sexual intercourse, such as "it feels so good" and "further, further" (Currier et al., 1971, p.260).
Frontal Lobe Sexual Seizures
The frontal lobes, the orbital region in particular, maintain rich interconnections with the amygdala (Nauta, 1964), and receives olfactory projections as well (Tanabe, 1975). Epileptiform activity arising in the deep frontal (orbital) regions has also been associated with the development of sexual seizures, including exhibitionism, genital manipulation, and masturbatory activity (Spencer et al., 1983).
Similar to that described regarding right temporal emotional and sexual disturbances, Spencer et al. (1983) found that three of their four patients with sexual automatism had seizures originating in the right frontal area, whereas the remaining patient had bifrontal disturbances. Other investigators have also noted that peculiar disturbances in emotion and personality are far more likely to arise following right versus left frontal damage (Joseph, 1986, 1988; Hillbom, 1960; Lishman, 1968).
Presumably, at least in part, emotional and sexual disturbances associated with right temporal and right frontal (orbital) dysfunction are caused by activation of the limbic structures, with which they intimately interconnected. However, there is also much evidence to indicate that in some respects, inferior temporal and orbital frontal areas are in fact outgrowths of and thus part of the limbic system.
Summary
Over the course of early evolutionary development, the hypothalamus reigned supreme in the control and expression of raw and reflexive emotionality, i.e., pleasure, displeasure, aversion, and rage. Largely, however, it has acted as an eye turned inward, monitoring internal homeostasis and concerned with basic needs. With the development of the amygdala, the organism was now equipped with an eye turned outward, so that the external emotional features of reality could be tested and ascertained. When signaled by the hypothalamus the amygdala begins to search the sensory array for appropriate emotional - motivational stimuli, until what is desired is discovered and attended to.
However, with the differentiation of the amygdala, emotional functioning also became differentiated and highly refined. The amygdala hierarchically wrested control of emotion from the hypothalamus. The amygdala is primary in regard to the perception and expression of most aspects of emotionality, including fear, aggression, pleasure, happiness, and sadness, and in fact assigns emotional or motivational significance to that which is experienced. It can thus induce the organism to act on something seen, felt, heard, or anticipated. The integrity of the amygdala is essential in regard to the analysis of social-emotional nuances, the organization and mobilization of the person's internal motivational status regarding these cues, as well as the mediation of higher-order emotional expression and impulse control. When damaged or functionally compromised, social-emotional functioning becomes grossly disturbed.
Through its rich interconnections with other brain regions, the amygdaloid nucleus is able to sample and influence activity that occurs in other parts of the cerebrum and add emotional color to one's perceptions. As such, it is highly involved in the assimilation and association of divergent emotional, motivational, somesthetic, visceral, auditory, visual, motor, olfactory, and gustatory stimuli. Thus, it is very much involved with learning, memory, and attention can generate reinforcement for certain behaviors (Douglas, 1967). Moreover, through reward or punishment, it can promote the encoding, storage, and later retrieval of particular types of information. That is, learning often involves reward, and it is through the amygdala (in concert with other nuclei) that emotional consequences can be attributed to certain events, actions, or experiences, as well as extracted from the world of possibility, so that it can be attended to and remembered.
Lastly, as is evident from studies of patients displaying abnormal activity or seizures originating in or involving this nuclei, the amygdala is able to overwhelm the neocortex and gain control over behavior. As based on electrophysiological studies, the amygdala seems to be capable of literally turning off the neocortex (such as occurs during a seizure), at least for brief time periods. That is, the amygdala can induce electrophysiological slow-wave theta activity in the neocortex, which indicates low levels of arousal as well as high voltage fast activity. In the normal brain, it probably exerts similar influences such that at times people (i.e., their neocortex) lose control over themselves and respond in a highly emotionally charged manner.
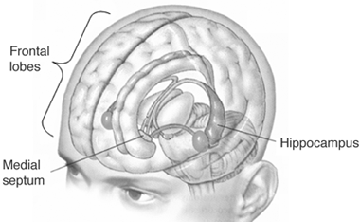
HIPPOCAMPUS
The hippocampus is an elongated structure located within the inferior medial wall of the temporal lobe (posterior to the amygdale); it surrounds, in part, the lateral ventricle. In humans, the hippocampus consists of an anterior and posterior region and is shaped somewhat like a telephone receiver.
There are three major neural pathways leading to and from the hippocampus. These include the fornix-fimbrial fiber system, a supracallosal pathway (i.e., the indusium griseum), which passes through the cingulated, and through the entorhinal area, sometimes referred to as the gateway to the hippocampus.
It is through the entorhinal area that the hippocampus receives olfactory and amygdaloid projections (Carlsen et al., 1982; Gloor, 1955; Krettek & Price, 1976a; Steward, 1977) and fibers from the orbital frontal and temporal lobes (Van Hoesen et al., 1972). It is through the fornix and fimbrial pathways that the hippocampus makes major interconnections with the thalamus, septal nuclei, medial hypothalamus, and through which it exerts either inhibitory or excitatory influences on these nuclei (Feldman, Saphier, & Conforti, 1987; Guillary, 1957; Poletti & Sujatanon, 1980).
Septal interactions: The hippocampus maintains a particularly intimate relationship with the septal nuclei (sometimes referred to as the septum - not to be confused with the septum pelucidum). The septal nucleus partly serves as in interactional relay center as it channels hippocampal influences to other structures such as the hypothalamus and reticular formation (and vice versa) and as a major link through which the hippocampus and amygdala sometimes interact (Hagino & Yamaoka, 1976).
Amygdala interactions: The hippocampus is greatly influenced by the amygdala, which in turn monitors and responds to hippocampal activity (Gloor, 1955; Green & Adey, 1956; Steriade, 1964) (Figs. 23 - 25). The amygdala also acts to relay certain forms of information from the hippocampus to the hypothalamus (Poletti & Sajatanon, 1980). Together the hippocampus and amygdala complement and interact in regard to attention, the generation of emotional and other types of imagery, as well as learning and memory.
Hippocampal Arousal, Attention, and Inhibitory Influences
Various investigators have assigned a major role to the hippocampus in information processing, including memory, new learning, cognitive mapping of the environment, voluntary movement toward a goal, as well as attention, behavioral arousal, and orienting reactions (Douglas, 1967; Grastayan et al., 1959; Green & Arduini, 1954; Isaacson, 1982; Milner, 1966, 1970, 1971; Olton, Branch, & Best, 1978; Routtenberg, 1968). For example, hippocampal cells alter their activity greatly in response to certain spatial correlates, particularly as an animal moves about in its environment (Olton et al., 1978). It develops slow-wave theta activity during arousal (Green & Arduini, 1954) or when presented with noxious or novel stimuli (Adey, Dunlop, & Hendrix, 1960). However, few studies have implicated this nucleus as important in emotional functioning per se, although responses such as "anxiety" or "bewilderment" have been observed when directly electrically stimulated (Kaada, Jansen, & Andersen, 1954).
Hippocampal - Neocortical Interactions
Desynchronization of the cortical EEG is associated with high levels of arousal and information input. As the level of input increases, the greater the level of cortical arousal (Como, Joseph, Fiducia, & Siegel, 1979; Joseph, Como, Forrest, Fiducia, & Siegel, 1981). However, when arousal levels become too great, efficiency in information processing, memory, new learning, and attention becomes compromised, as the brain becomes overwhelmed.
When the neocortex becomes desynchronized (indicating cortical arousal), the hippocampus often (but not always) develops slow-wave theta activity (Grastayan et al., 1959; Green & Arduini, 1954) such that it appears to be functioning at a much lower level of arousal. Conversely, when cortical arousal is reduced to a low level (indicated by EEG synchrony), the hippocampal EEG often becomes desynchronized.
These findings suggest when the neocortex is highly stimulated, the hippocampus functions at a much lower level in order to monitor what is being received and processed so as not to become overwhelmed. When the neocortex is not highly aroused, the hippocampus presumably compensates by increasing its own level of arousal so as to tune into information that is being processed at a low level of intensity. In situations in which both the cortex and the hippocampus become desynchronized, distractability and hyperresponsiveness result and the subject becomes overwhelmed and confused and may orient to and approach several stimuli (Grastyan et al., 1959). Attention, learning, and memory functioning are decreased. Such situations sometimes occur when one is highly anxious, upset, or when traumatized. In fact, it is likely that the hippocampus might be injured by traumatic stress, which in turn would explain why some individuals become amnesic following a prolonged trauma (Joseph, unpublished 1980).
There is also evidence to suggest that the hippocampus may act to reduce extremes in cortical arousal. For example, whereas stimulation of the reticular activating system augments cortical arousal and EEG-evoked potentials, hippocampal stimulation reduces or inhibits these potentials such that cortical responsiveness and arousal are dampened (Feldman, 1962; Redding, 1967). If cortical arousal is at a low level, hippocampal stimulation often leads to augmentation of the cortical evoked potential (Redding, 1967).
The hippocampal also exerts desynchronizing or synchronizing influences on various thalamic nuclei, in turn augmenting or decreasing activity in this region (Green & Adey, 1956; Guillary, 1955; Nauta, 1956, 1958). As the thalamus is the major relay nucleus to the neocortex, the hippocampus appears to be able to block or enhance information transfer to various neocortical areas.
It is likely that the hippocampus may act to influence information reception at the neocortical level as well as possibly reduce extremes in cortical arousal (be they too low or high) via inhibition or excitation, and/or it may act so that the neocortex is neither over- not underwhelmed when engaged in the reception and processing of information. That is, very high or very low states of excitation are incompatible with alertness and selective attention and with the ability to learn and retain information (Joseph et al., 1981).
Aversion and Punishment
In many ways, the hippocampus appears to act in concert with the medial hypothalamus and septal nuclei (with which it maintains rich interconnections) so as to prevent extremes in arousal and thus maintain a state of quiet alertness (or quiescence). Moreover, as with the medial hypothalamus, it has been reported that the subjective components of aversive emotion in humans is correlated with electrophysiological alternations in the hippocampus and septal area (Heath, 1976).
The hippocampus also appears to be heavily involved in the modulation of reactions to frustrations or punishment (Gray, 1970), particularly in regard to learning. For example, the hippocampus responds with trains of slow theta waves when presented with noxious stimuli but habituates with repeated presentation. Moreover, there is some evidence to suggest that this nucleus in conjunction with the amygdala and septal nuclei is important in generating negative cognitive-mood states such as anxiety.
Attention and Inhibition
The hippocampus participates in the elicitation of orienting reactions and the maintenance of an aroused state of attention (Foreman & Stevens, 1987; Grastayan et al., 1959; Green & Arduini, 1954; Routtenberg, 1968). When exposed to novel stimuli or when engaged in active searching of the environment, hippocampal theta appears (Adey et al., 1960). However, with repeated presentations of a novel stimulus, the hippocampus habituates and theta disappears (Adey et al., 1960). Thus, as information is attended to, recognized, and presumably learned and/or stored in memory, hippocampal participation diminishes. Theta also appears during the early stages of learning as well as when engaged in selective attention and the making of discriminant responses (Grastyan et al., 1959).
When the hippocampus is damaged or destroyed, animals have great difficulty inhibiting behavioral responsiveness or shifting attention. For example, Clark and Issacson (1965) found that animals with hippocampal lesions could not learn to wait 20 sec between bar presses, if first trained to respond to a continuous schedule. There is an inability to switch from a continuous to a discontinuous pattern, such that a marked degree of perseveration and inability to change sets or inhibit a pattern of behavior once initiated occurs (Douglas, 1967; Ellen, Wilson, & Powell, 1964). Habituation is largely abolished, and the ability to think or respond divergently is disrupted.
In part, this finding suggests that hippocampal damage disrupts the ability to learn and thus remember - findings that have been repeatedly demonstrated in humans. In animals, disinhibition caused by hippocampal damage can even prevent the learning of a passive-avoidance task, such as simple ceasing to move (Kimura, 1958).
When coupled with the evidence presented above, it appears that the hippocampus acts to enhance or diminish areas of neural excitation selectively, which in turn allows for differential selective attention and differential responding. When damaged, the ability to shift from one set of perceptions to another or to change behavioral patterns is disrupted, and the organism becomes overwhelmed by a particular mode of input. Learning, memory, as well as attention are greatly compromised.
Learning and Memory
The hippocampus is most usually associated with learning and memory encoding (e.g., long-term storage and retrieval of newly learned information), particularly the anterior regions (Fedio & Van Buren, 1974; Milner, 1966; 1970; Penfield & Milner, 1958; Rawlins, 1985; Scoville & Milner, 1957). Many other brain areas, such as the mamillary bodies and dorsal-medial nucleus of the thalamus, are also important in memory functioning.
Bilateral destruction of the anterior hippocampus results in striking and profound disturbances involving memory and new learning (i.e. , anterograde amnesia). For example, one such patient who underwent bilateral destruction of this nuclei (H.M.), was subsequently found to have almost completely lost the ability to recall anything experienced after surgery. If you introduced yourself to him, left the room, and then returned a few minutes later, he would have no recall of having met or spoken to you. Dr. Brenda Milner has worked with H.M. for almost 20 years, and yet she is an utter stranger to
him. H.M. is in fact so amnesic for everything that has occurred since his surgery (although memory for events prior to his surgery is comparatively exceedingly well preserved), that every time he rediscovers that his favorite uncle died (years after his surgery) he suffers the same grief as if he has just been informed for the first time.
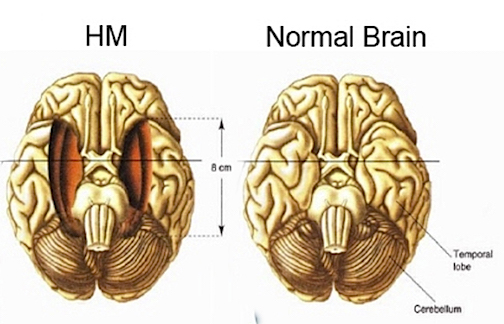
Despite his lack of memory for new (nonmotor) information, Henry (or H.M.), has adequate intelligence, is painfully aware of his deficit, and constantly apologizes for his problem. "Right now, I'm wondering" he once said, "Have I done or said anything amiss?" You see, at this moment everything looks clear to me, but what happened just before? That's what worries me. It's like waking from a dream. I just don't remember.... Every day is alone in itself, whatever enjoyment I've had, and whatever sorrow I've had.... I just don't remember.
Presumably the hippocampus acts to protect memory and the encoding of new information during the storage and consolidation phase via the gating of afferent streams of information and the filtering/exclusion (or dampening) of irrelevant and interfering stimuli. When the hippocampus is damaged, input overload results, the neuroaxis is overwhelmed by neural noise, and the consolidation phase of memory is disrupted such that relevant information is not properly stored or even attended to. Consequently, the ability to form associations (e.g., between stimulus and response) or to alter preexisting schemas (such as occurs during learning) is attenuated (Douglas, 1967).
Hippocampal and Amygdaloid Interactions: Memory
It has been argued that significant impairments involving memory (in man) cannot be produced by lesions restricted to the hippocampus (cf. Horel, 1978). Thus, in some instances with restricted lesions, good recall of new information is possible for at least several minutes (Horel, 1978; Penfield & Milner, 1958). Rather, there is evidence that strongly suggests that the hippocampus plays an interdependent role with the amygdala in regard to memory (Kesner & Andrus, 1982; Mishkin, 1978; Sarter & Markowitsch, 1985). Interestingly, nuclei such as the dorsal-medial region of the thalamus, which have also been shown to be important in memory (Squire & Moore, 1979), maintain rich interconnections with the amygdala (Krettek & Price, 1977b; Nauta, 1971).
Nevertheless, although psychic blindness is produced by damage to the amygdala, striking anterograde deficits in recall do not seem to occur (Horel, 1978; Scoville & Milner, 1957). Rather, the role of the amygdala in memory and learning seems to involve activities related to reward, orientation, and attention, as well as emotional arousal (Sarter & Markowitsch, 1985). If some event is associated with positive or negative emotional states, it is more likely to be learned and remembered.
The amygdala seems to reinforce and maintain hippocampal activity through the identification of motivationally significant information and the generation of pleasurable rewards (through action on the lateral hypothalamus). That is, reward increases the probability of attention being paid to a particular stimulus or consequence as a function of its association with reinforcement (Douglas, 1967; Kesner & Andrus, 1982).
However, the amygdala and hippocampus may act differentially in regard to the effects of positive versus negative reinforcement on learning and memory. For example, whereas the hippocampus produces theta in response to noxious stimuli, the amygdale increases its activity following the reception of a reward (Norton, 1970). Thus, if errors are made during acquisition (negative emotional state), possibly the hippocampus modulates the appropriate reaction, whereas when presented with a reward, the amygdala reinforces the organism's response.
Thus, the hippocampus acts to reduce or enhance extremes in arousal associated with information reception and storage in memory, whereas the amygdala acts to identify the social-emotional-motivational characteristics of the stimuli as well as to generate (in conjunction with the hippocampus) appropriate emotional rewards to reinforce learning and memory. We find that when both the amygdala and hippocampus are damaged, striking and profound disturbances in memory functioning result (Kesner & Andrus, 1982; Mishkin, 1978).
Laterality
It is now very well known that lesions involving the mesial-inferior temporal lobes (i.e., destruction or damage to the amygdala/hippocampus) of the left cerebral hemisphere typically produce significant disturbances involving verbal memory - particularly as contrasted with patients with right-sided destruction. Left-sided damage disrupts the ability to recall simple sentences and complex verbal narrative passages or to learn verbal paired associates or a series of digits (B. Milner, 1966, 1970, 1971).
By contrast, right temporal destruction typically produces deficits involving visual memory, such as the learning and recall of geometric patterns, visual mazes, or human faces (Corkin, 1965; Milner, 1966; Kimura, 1963). Right-sided damage also disrupts the ability to recognize (through recall) olfactory stimuli (Rausch, Serafetinides, & Crandall, 1977), emotional sounds and passages (Wechsler, 1973), sounds from the environment, as well as tactile mazes (Joseph, 1988a).
It appears, therefore, that the left amygdala/hippocampus is highly involved in processing and/or attending to verbal information, whereas the right amygdala/hippocampus is more involved in the learning, memory and recollection of nonverbal, visual-spatial, environmental, emotional, motivational, tactile, olfactory, and facial information.
THE PRIMARY PROCESS
Amygdaloid-Hippocampal Interactions during Infancy
Hallucinations
The amygdala-hippocampus complex, particularly that of the right hemisphere, is very important in the production and recollection of nonlinguistic images associated with past experience. In fact, direct electrical stimulation of this region within the temporal lobes results not only in the recollection of images but in the creation of fully formed visual and auditory hallucinations (Halgren et al., 1978; Horowitz et al., 1968; Malh et al., 1964; Penfield & Perot, 1963), as well as feelings of familiarity (e.g., de ja vu).
Indeed, it has long been known that tumors invading specific regions of the brain can trigger the formation of hallucinations that range from the simple (flashing lights) to the complex. The most complex forms of hallucination, however, are associated with tumors within the most anterior portion of the temporal lobe (Critchley, 1939; Gibbs, 1951; Horowitz et al., 1968; Tarachow, 1941), i.e., the region containing the amygdala and anterior hippocampus.
Similarly, electrical stimulation of the anterior lateral temporal cortical surface results in visual hallucinations of people, objects, faces, and various sounds (Horowitz et al., 1968) - particularly the right temporal lobe (Halgren et al., 1978). Depth electrode stimulation and thus direct activation of the amygdala and/or hippocampus is especially effective.
For example, stimulation of the right amygdala produces visual hallucinations, body sensations, de ja vu, illusions, as well as gustatory and alimentary experiences (Weingarten et al., 1977). Conversely, Freeman and Williams (1963) reported that the surgical removal of the right amygdala in one patient abolished hallucinations. Stimulation of the right hippocampus has also been associated with the production of deja-vu-, memory-, and dreamlike hallucinations (Halgren et al., 1978), and in fact, hallucinations seem to occur most frequently following hippocampal activation (Horowitz et al., 1968).
As is well known, LSD can elicit profound hallucinations involving all spheres of experience. Following the administration of LSD, high-amplitude slow waves (theta) and bursts of paroxysmal spike discharges occur in the hippocampus and amygdala (Chapman & Walter, 1965; Chapman, Walter, Ross et al., 1963), but with little cortical abnormal activity. In both humans and chimpanzees in whom the temporal lobes, amygdala, and hippocampus have been removed, LSD ceases to produce hallucinatory phenomena (Baldwin et al., 1959; Serafetinides, 1965). Moreover, LSD-induced hallucinations are significantly reduced when the right versus left temporal lobe has been surgically ablated (Serafetinides, 1965). Overall, it appears that the amygdala, hippocampus, and the neocortex of the temporal lobe are highly interactionally involved in the production of hallucinatory experiences. Presumably, it is the neocortex of the temporal lobe that acts to interpret this material (Penfield & Perot, 1963) as perceptual phenomena.
Dreaming
When hallucinations follow depth electrode or cortical stimulation, much of the material experienced is very dreamlike (Halgren et al., 1978; Malh et al., 1964) and consists of recent perceptions, ideas, feelings, and other emotions that are similarly illusionary and dreamlike. Indeed, the right hippocampus, and the right hemisphere in general (Broughton, 1982; Goldstein et al., 1972; Hodoba, 1986; Humphrey & Zangwill, 1961; Kerr & Foulkes, 1978; Meyer, Ishikawa, Hata, & Karacan, 1987), also appear to be involved in the production of dream imagery as well as REM (at least in part) during sleep.
For example, there have been reports of patients with right cerebral damage, hypoplasia, and abnormalities in the corpus callosum who have ceased to dream altogether, suffer a loss of hypnotic imagery, or tend to dream only in words (Botez, Olivier, Vezina, Botex, & Kaufman, 1985; Humphrey & Zangwill, 1951; Kerr & Foulkes, 1981; Murri, Arena, Siciliano, Mazzotta, & Muratorio, 1984). However, there have also been some reports that when the left hemisphere has been damaged, particularly the posterior portions (i.e., aphasic patients), the ability to verbally report and recall dreams also is greatly attenuated (e.g., Murri et al., 1984). Of course, aphasics have difficulty describing much of anything, let alone their dreams.
Electrophysiologically, the right hemisphere also becomes highly active during REM, whereas, conversely, the left brain becomes more active during NREM (Goldstein et al., 1972; Hodoba, 1986). Similarly, measurements of cerebral blood flow have shown an increase in the right temporal regions during REM sleep in subjects who upon wakening report visual, hypnagogic, hallucinatory, and auditory dreaming (J.S. Meyer et al., 1987). Interestingly, abnormal and enhanced activity in the right temporal and temporal - occipital area acts to increase dreaming and REM sleep for an atypically long time period (Hodoba, 1986). Thus, it appears that there is a specific complementary relationship between REM sleep and right temporal electrophysiological activity.
In addition, during REM, the hippocampus begins to produce slow wave, theta activity (Jouvet, 1967; Olmstead, Best, & Mays, 1973; Robinson, Kramis, & Vanderwolf, 1977). Presumably, during REM, the hippocampus acts as a reservoir from which various images, words, and ideas are drawn and incorporated into the matrix of dreamlike activity being woven by the right hemisphere. It is probably just as likely that the hippocampus serves as a source from which material is drawn during the course of a daydream.
Interestingly, daydreams appear to follow the same 90- to 120-min cycle that characterize the fluctuation between REM and NREM periods, as well as fluctuations in mental capabilities associated with the right and left hemisphere (Broughton, 1982; Kripke & Sonneschein, 1973). That is, the cerebral hemispheres tend to oscillate in activity every 90- 120 min - a cycle that appears to correspond to the REM - NREM cycle and the appearance of day and night dreams.
Dreams and Infancy
In the newborn, and up until approximately 6 - 9 months, there are two distinct stages of sleep which correspond to REM and N-REM periods demonstrated by adults (Berg & berg, 1978; Dreyfus-Brisac & Monod, 1975; Parmlee, Wenner, Akimaya, Schults, & Stern, 1967). Among infants, however, REM occur during wakefulness as well as during sleep. In fact, REM can be observed when the eyes are open, when the infant is crying, fussing, eating, or sucking (Emde & Metcalf, 1970). Moreover, REM is also observed to occur within a few moments after an infant begins to engage in nutritional sucking and appears identical to that which occurs during sleep (Emde & Metcalf, 1970).
The production of REM during waking in some respects seems paradoxical and a number of different mechanism are no doubt responsible. Nevertheless, it might be safe to assume that like an adult, when the infant is in REM, he or she is dreaming, or at least, in a dreamlike state. This state might correspond to what Freud described as the primary process. That is, when produced when the infant is crying or fussing, it is dreaming of whatever relief it seeks. Correspondingly, REM that occurs while eating or sucking may have to do with the limbic structures involved not only in the production of dreamlike activity, but the identification, learning, and retention of motivationally significant information (i.e., the amygdala and hippocampus).
The Primary Process
The hypothalamus, our exceedingly ancient and primitive Id, has an "eye" that only sees inward. It can tell if the body needs nourishment but cannot determine what might be good to eat. It can feel thirst, but has no way of slaking this desire. The hypothalamus can only say: "I want," "I need," and can only signal pleasure and displeasure. However, as the seat of pleasure, the hypothalamus can be exceedingly gracious in rewarding the organism when its needs are met. Conversely, when its needs go unmet, it can respond not only with displeasure and feelings of aversion, but with undirected fury and rage. It can cause the organism to cry out. However, the cry does not produce the immediately desired relief or reduction in tension. Pressure is exerted on the limbic system and the organism to engage in environmental surveillance so as to meet the needs monitored by the hypothalamus.
Over the course of the first months of life, as the amygdala and then the hippocampus develop, the organism begins to develop an eye that not only sees outward but that can register and recall events, objects, people, and so forth associated with tension reduction, pleasure, and the satiety of the infants internal needs (e.g., the taste, smell, feeling of mother's breast and milk, the experience of sucking and relief). This is called learning.
With the maturation of these two limbic nuclei, the infant is increasingly able to differentiate what occurs in the external environment based on hypothalamically monitored needs and the emotional/motivational significance of that which is experienced. The infant can now orient, selectively attend, determine what brings satisfaction, and store this information in memory.
Primary Imagery
Although admittedly we have no direct knowledge as to the psychic interactions in the neonate, it does seem reasonable to assume that as the neocortex and underlying structures and fiber pathways mature, neural "programs" are formed that correspond to the repeated registration of experiences which are deemed significant (e.g., pleasurable). That is, neural pathways that are repetitively fired, deactivated, or activated in response to specific sensory and affective activities and experiences, become associated with that activity, such that an associated neural circuit is formed (Joseph, 1982; Penfield, 1954; Spinelli, 1970); i.e., a memory is created. Eventually, if this circuit is reactivated, the "learned" pattern is reexperienced; i.e., the organism remembers. Thus, when the amygdala/hippocampus is stimulated
by a hungry hypothalamus, the events and images associated with past experiences of pleasure not only can be searched out externally but can be recalled in imaginal form. For example, as an infant experiences hunger and stomach contractions as well as its own cries of displeasure, these states become associated with the sound, smell, taste, and other cues of mother and her associated movement, as well as other stimuli that accompany being fed (cf. Piaget, 1952, pp. 37, 407-408). Repetitively experienced, the sequence from hunger to satiety evokes and becomes associated with the activation of certain neural pathways.
Eventually, when the infant becomes hungry and that hunger is prolonged, the entire neural sequence associated with hunger and feeding (i.e., hunger, mother, food, satiety) may become involuntarily triggered and activated (through association) such that an "image" of being fed is experienced. The activation of these rudimentary and infantile memory images is probably what constitutes, at least in part, the primary process.
Behaviorally, this is manifested by REM and by sucking and tongue movements as if eating, when in fact no food is present (cf. Piaget, 1952). When hungry, the infant will begin to cry and REM might be observed; the infant will then stop crying and smack its lips and make sucking movements (mediated by the amygdala) as if it were being fed. The infant experiences being fed in the form of a dream (Joseph, 1982) or hallucination.
The brain of the human infant is quite immature for several years, in turn restricting information reception and processing (Joseph, 1982; Joseph, Gallagher, Holloway, & Kahn, 1984); also, given the limited amount of reality contact infants are able to achieve, these rudimentary memories and images (even when they occur during waking, i.e., REM) are probably indistinguishable from actual experience simply because they are experience.
Like a dream, when replayed, the infant presumably reexperiences to some degree the sensations, emotions, and so forth originally linked to tension reduction. Thus, the young infant, still unable to distinguish between representation and reality, responds to the image as reality (Freud, 1911), even while awake - as manifested by REM. When hunger is prolonged, the associations linked to feeding are triggered, and for a brief period the infant behaves as if its hunger has been sated. Reality is replaced by an image, or rather, a "dream." This is the primary process.
Since the hypothalamus (which monitors internal homeostasis) is not conscious that the dream images experienced are not real, it initially accepts the memory/dream images transmitted from the amygdala and hippocampus and ceases to cry, i.e., it responds to the imagined sources of nourishment just as it responds to a cue tone associated with a food reward (Nakamura & Ono, 1986; Ono et al., 1980). However, the hypothalamus is not long fooled, for the primary process does not offer effective long-lasting relief from tension. As the pain of hunger remains and increases, limbic activity is increased, and the image falls away, to be replaced by a cry of hunger (Joseph, 1982). The amygdala and hippocampus are thus forced to renew their surveillance of the environment in search of sources of tension reduction. Cognitive development is thus promoted (Freud, 1911): Whatever was thought of (desired) was simply imagined in an hallucinatory form, as still happens today with our dream-thoughts every night. This attempt at satisfaction by means of hallucination was abandoned only in consequence of the absence of the expected gratification, because of the disappointment experienced. Instead, the mental apparatus had to decide to form a conception of the real circumstances in the outer world and to exert itself to alter them.... The increased significance of external reality heightened the significance also of the sense-organs directed towards the outer word, and of the consciousness attached to them; the later now learned to comprehend the qualities of sense in addition to the qualities of pleasure and "pain" which hitherto had alone been of interest to it. A special function was instituted which had periodically to search the outer word in order that its data might be already familiar if an urgent need should arise; this function was attention. Its activity meets the sense-impressions halfway, instead of awaiting their appearance. At the same time there was probably introduced a system of notation, whose task was to deposit the results of this periodical activity of consciousness - a part of that which we call memory. (pp. 410-411)
SEPTAL NUCLEI
Phylogenetically, the septal nuclei develops following the appearance of the amygdala, at about the same time and rate as the hippocampus (Andy & Stephan, 1976; Brown, 1983; Humphrey, 1972). It also increases in relative size and complexity as we ascend the ancestral tree, attaining its greatest degree of development in humans.
Specifically, the septal nuclei lie in the medial portions of the hemispheres, just anterior to the third ventricle near the hypothalamus. The septum projects heavily throughout the hypothalamus and maintains rich interconnections with all regions of the hippocampus (Mesulam, Mufson, Levey, & Wainer, 1983; Siegel & Edinger, 1976). It also contributes to and receives fibers from the amygdala via the stria terminalis (Swanson & Cowan, 1979) and receives and gives rise to one of the most massive roots of the medial forebrain bundle through which it receives olfactory and ascending brainstem (reticular formation) fibers (Nauta, 1956).
Aversion and Internal Inhibition
In general, the septal nucleus appears to act in conjunction with the medial hypothalamus and hippocampus, particularly as related to internal inhibition and the exertion of quieting and dampening influences on arousal and limbic system functioning. In this regard, the septum appears to counterbalance some aspects of amygdaloid and lateral hypothalamic activity while simultaneously facilitating the actions of the hippocampus and media hypothalamus (Kolb & Whishaw, 1977; Mogenson, 1976; Petsche, Gogolack, & Van Zwieten, 1965; Petsche, Stumpf, & Gogolack, 1962).
Specifically, depending on the level of hypothalamic arousal, the septum exerts facilitatory or inhibitory influences on this nuclei (Mogenson, 1976) and from impulses it receives and relays from the reticular activating system, mediates and greatly influences hippocampal activity as well (Kolb & Whishaw, 1977; Petsche et al., 1962, 1965). The septal region (along with that of the hippocampus and medial hypothalamus) has also been reported in humans to show electrophysiological alterations in activity that correspond to subjective feelings of aversion (Heath, 1976).
The septum and amygdala appear to enjoy largely an antagonistic relationship, particularly in regard to influencing the hypothalamus. For example, the amygdala acts either to facilitate or to inhibit septal functioning, whereas septal influences on the amygdala are largely inhibitory. However, in large part, the amygdala and septal nuclei appear to exert most of their counterbalancing influences at the level of the hypothalamus, particularly in regard to emotionality.
Quiescence
A primary activity of the septal nucleus appears to be that of reducing extremes in emotionality and arousal and maintaining the organism in state of quiescence and readiness to respond. Stimulation of the septum acts to reduce blood pressure and heart rate and to induce adrenocortical secretion (Endroczi, Schreiberg, & Lissak, 1963; Kaada, 1951; Ranson, Kabat, & Magoun, 1935). It also counters lateral hypothalamic self-stimulatory activity (Mogenson, 1976).
Rage
Electrical stimulation of the septum counters and inhibits aggressive behavior (Rubenstein & Delgado, 1963) and suppresses the expression of rage reactions following hypothalamic stimulation (Siegel & Edinger, 1976). If the septal nucleus is destroyed, these counterbalancing influences are removed such that initially dramatic increases in aggressive behavior, including rage (Ahmad & Harvey, 1968; Blanchard & Blanchard, 1968; Brady & Nauta, 1953; King, 1958), results. Bilateral lesions in fact give rise to explosive emotional reactivity to tactile, visual, or auditory stimulation, which can take the form of attack (fight) or flight. If the amygdala is subsequently lesioned, the septal rage and emotional reactivity are completely attenuated (King & Meyer, 1958). Thus, septal lesions appear to result in a loss of modulatory and inhibitory restraint.
Septal Social Functioning
Eventually, within a few weeks or months, rage and aggressiveness due to septal lesions subside and/or completely disappear. However, a generalized tendency to overrespond and a generalized failure to inhibit emotional responsiveness persist (McClary, 1961; 1966; Poplawsky, 1987). In particular, rather than rage or irritability, septal lesions produce indiscriminant socializing and an extreme need for social and physical contact (Jonason & Enloe, 1971; Jonason et al., 1973; McClary, 1961, 1966; Meyer, Ruth, & Lavond, 1978).
Socialization and Contact Comfort
In contrast with amygdaloid lesions, which produce a severe social-emotional agnosia and social avoidance, septal lesions produce a dramatic and persistent increase in social cohesiveness (Jonason & Enloe, 1971; Jonason et al., 1973; D. R. Meyer et al., 1978). Thus, the normal intact amygdala appears to promote social behavior, whereas the septal nucleus seems to counter socializing tendencies.
With complete bilateral destruction of the septum in animals, the drive for social contact appears to be irresistible such that persistent attempts to make physical contact occur - even with species quite unlike their own. This occurs because the amygdala (as well as other nuclei) are no longer opposed by the septal nucleus.
Ritchie (1975, cited by D. R. Meyer et al., 1978)reported that septally lesioned rats, unlike normals, will readily seek out mice (to which they are normally indifferent) or rabbits (which they usually avoid). If presented with a choice of an empty (i.e., safe) chamber or one containing a cat, septally lesioned rats persistently attempt to huddle and crawl upon this normally feared creature, even when the cat is acting perturbed. If a group of septally lesioned animals are placed together, extreme huddling results. So intense is this need for contact comfort following septal lesions that if other animals are not available they will seek out blocks of wood, old rags, bare wire frames, or walls.
Among humans with right-sided or bilateral disturbances in septal functioning (such as due to seizure activity generated in this region), a behavior referred to as stickiness is sometimes observed. Such individuals seek to make repeated, prolonged, and often inappropriate contact with anyone who is available or who happens to be nearby so as to tell them stories or jokes or merely pass the time. They seem to have little actual concern regarding the feelings of others or how interested they might be in interacting. Indeed, they do not readily take a hint and are difficult to get rid of. In hospital situations, they can be found intruding on other patients and their families, hanging out by the nurse's station, or incessantly visiting other rooms to chat.
Attachment and Amygdaloid - Septal Developmental Interactions
Broadly considered, the various nuclei comprising the limbic system demonstrate functional maturity at different rates. Correspondingly, certain behaviors and capacities appear at various time periods, overlay previous capacities, become differentiated, and/or in turn become suppressed or eliminated.
At birth the hypothalamus reins supreme in regard to emotion. The infant freely expresses feelings of aversion and rage or pleasure or quiescence and demonstrates extreme orality and other behaviors similar to a state in which the amygdala is functionally nonexistent.
As the amygdala and hippocampus begin to develop and mature, the organism becomes more reality oriented and more social as the ability to attend selectively to externally occurring events and to store this information in memory becomes more pronounced. The pleasure principle (although still dominant) begins to be served by the reality principle (Freud, 1911).
Since the development of the amygdala precedes that of the septal nuclei, social-emotional activities mediated by the amygdala are expressed in an uninhibited fashion. That is, when the counterbalancing influences of the septum are absent (as a result of surgical removal or functional immaturity), the behavior expressed is in many respects similar to that expressed by infants, for example, indiscriminate contact seeking (e.g., attachment behavior).
Attachment
Attachment is not the same as dependency. Necessarily, the infant is dependent on its caretaker (e.g., mother). However, until the child is 6-7 months of age, it will smile at the approach of anyone, even complete strangers, and will vigorously protest any form of separation from these unknown people (e.g., if they leave the room). This state corresponds to the amygdaloid development period, during which septal influences are largely nil.
At about 7 months of age, the infant becomes more discriminant in its interactions and it is during this time period that a very real and specific attachment is formed, e.g., to one's mother - an attachment that becomes progressively more intense and stable. This period represents initial septal developmental influences such that global contact seeking becomes increasingly narrowed and restricted.
After these specific attachments have been formed, children begin to show anxiety, fear, and even flight reactions at the approach of a stranger (Spitz & Wolf, 1946). By 9 months, 70% of children respond aversively, whereas by 10 months they might cry out if a stranger were to appear (Schaffer, 1966; Waters, Matas, & Sroufe, 1975). By 1 year of age, 90% of children respond aversively to strangers (Schaffer, 1966).
Thus, during the amygdaloid phase there is indiscriminant approach and contact seeking. During the septal stage, indiscriminate social contact seeking is inhibited, whereas the specific attachments already formed are strengthened, reinforced, and maintained. The differential rates of amygdala and septal development are thus crucial in promoting survival and social interaction with significant others.
Their differential maturation rates also represent critical time periods, which in turn require specific interactional social-physical forms of stimulation, such as the presence and care of a primary caretaker. If these interactional needs are not met during the critical development period, gross abnormalities can result.
Indeed, if contact with others is restricted during these early phases, the ability to socially interact successfully at a later stage of development is retarded. This is even true among the so-called lower animals. For example, kittens that are not exposed to humans grow up to be wild and unapproachable. The phenomena of imprinting probably also requires that similar interactions take place.
Contact Comfort
As is well known, for the first 6-8 months of life, physical and social interaction with others is critical in regard to psychological, neurological, and physical development. During this period (which corresponds to the amygdaloid phase of maturation), contact seeking is indiscriminant. However, indiscriminant contact seeking and attachment during early development maximizes opportunities for physical - social interaction. So intense is the need for physical and social contact that young animals will form attachments to bare wire frames (Harlow, 1962), to television sets, to dogs that might maul them, and to creatures that might kill them (Cairn, 1966). Young human children will form attachments to mothers who might abuse them. With removal of the septum, animals will even seek contact with creatures that might eat them.
So pervasive is this need for physical interaction (especially among humans) that, when grossly reduced or denied, the result is often death. For example, in several well-known studies of children raised in foundling homes during the early 1900s, when the need for contact was not well recognized, morbidity rates for children under 1 year of age were more than 70%. Of 10,272 children admitted to the Dublin Foundling home during a single 25-year period, only 45 survived (Langmeier & Matejcek, 1975, for review).
Of those who have survived an infancy spent in institutions in which mothering and contact comfort were minimized, signs of low intelligence, extreme passivity, apathy, as well as severe attentional deficits are often characteristic (Dennis, 1960; Langmeier & Matejcek, 1975; Spitz, 1945). Such individuals have difficulty forming attachment or maintaining social interactions later in life.
In his famous series of experiments with monkeys, Harlow (1962) also showed that even those raised with surrogate terry cloth mothers develop extremely bizarre behaviors: The laboratory monkeys sit in their cages and stare fixedly into space, circle their cages in a repetitive stereotyped manner and clasp their heads in their hands or arms and rock for long periods of time. They often develop compulsive habits, such as pinching precisely the same patch of skin on their chest between the same fingers hundreds of times a day; occasionally such behavior may become punitive and the animal may chew and tear at its body until it bleeds.
It is noteworthy that the maternal behavior of chimpanzees raised in isolation is also quite abnormal and in fact similar to that of mothers who have had their amygdalas destroyed. As described by Harlow (1965): After the birth of her baby, the first of these unmothered mothers ignored the infant and sat relatively motionless at one side of the cage, staring fixedly into space hour after hour. As the infant matured desperate attempts to effect maternal contact were consistently repulsed.... Other motherless monkeys were indifferent to their babies or brutalized them, biting off their fingers or toes, pounding them, and nearly killing them until caretakers intervened. One of the most interesting findings was that despite the consistent punishment, the babies persisted in their attempts to make maternal contact.



Deprivation and Amygdaloid - Septal Functioning
In addition to mediating all aspects of higher-order emotional functioning, the amygdala is particularly responsive to tactual stimulation such that single cells may respond to touch, regardless of where on the body the person was stimulated. When there is inadequate tactile and physical-social interaction during early development, the ability of these cells to develop and function adequately is significantly reduced. That is, the amygdala (as well as other nuclei) becomes environmentally damaged.
As has been repeatedly demonstrated in studies of the visual system as well as on cerebral development, one consequence of reduced environmental input during certain critical periods of development is cell death, atrophy, and functional retardation, as well as inhibition by competing neuronal cell assemblies (Casagrande & Joseph, 1980; Greenough, 1976; Rosenzweig, 1971). Consequently, gross behavioral and perceptual abnormalities result (Harlow, 1962; Joseph & Casagrande, 1980; Joseph & Callagher, 1982).
For example, if the lids of one eye are surgically sutured shut, patterned visual input is prevented from reaching target cells in the lateral geniculate nucleus of the thalamus. When this occurs target neurons become smaller, fewer in number, and functionally suppressed by adjacent cells receiving normal input. If the sutures are reversed such that the formerly deprived eye is opened, the subject responds as if blind. However, if the normal eye is surgically removed, some vision returns to the formerly deprived eye, and some functional neuronal recovery is observed (Casagrande & Joseph, 1980), presumably because of the removal of inhibitory influences. This deprivation effect is only noted to occur during the first few months after birth in primates.
Given the similarity in social unresponsiveness and disturbances in maternal behavior demonstrated by amygdalectomized and socially-maternally deprived primates, it is likely that like the visual system, abnormalities in cellular development and function also occur within the amygdala and septum secondary to deprivation. Unfortunately, few researchers have explored this line of inquiry.
Nevertheless, it has been demonstrated that the right amygdala is larger than the left in animals reared in enriched and normal environments, whereas these differences are nonsignificant in animals reared in a restricted setting (Diamond, 1985), suggesting that deprivation differentially reduces right amygdaloid growth. This finding is significant because among humans, the right cerebral hemisphere has been shown to be dominant in regard to most aspects of social-emotional functioning. (See Chapter 1, The Right Cerebral Hemisphere.)
Heath (1972) also noted that the monkeys reared without mothers develop abnormal spiking in the septal region. Similarly, Heath (1954) found abnormal seizure-like discharges in the septum of withdrawn schizophrenics, noting that the severity of the abnormality was correlated with the severity of the psychosis.
Among those with normal limbic systems, it is presumably the interaction of these same nuclei that gives rise to attachment and bonding behavior in adults. Moreover, it is probably through the interactions mediated by these nuclei that emotions such as jealousy are generated. That some individuals respond with considerable grief, anger, and even uncontrollable rage when a "loved one" has ended a relationship probably can also be explained from a limbic perspective.
THE UNCONSCIOUS MIND
The limbic system, as defined in this paper, provides the foundations for the development of not only emotion, but social-psychological functioning as well as the more rudimentary aspects of unconscious mental activity. The limbic system, however, represents the most primitive features of the unconscious. A second, more highly developed, unconscious (i.e., nonlinguistic) mind is maintained via the functional interactions of the right cerebral hemisphere (Joseph, 1988a, b); however this mental realm only seems to be unconscious from the perspective of the left cerebral hemisphere. Nevertheless, because the interaction of various limbic nuclei is also important in learning and memory, as well as social-emotional functioning, it is likely that a variety of neurotic disturbances, needs, desires, and insecurities have as their foundation events differentially experienced and attended to by the limbic system.
This seems particularly true regarding events experienced during infancy and early development. For example, it is well known that most events experienced before age 4 are extremely difficult to recall. In part this is because of the different modes of information processing and coding employed by adults versus children (Joseph, 1988a). That is, much of what was experienced, learned, and stored in memory during early childhood occurred before the development of language. Thus, adults, relying on language, can no longer access the code in which this early information was stored. The key no longer fits the lock.
Nevertheless, because the limbic system of both an adult and infant uses nonlinguistic social-tactual-emotional-visual forms of information processing and expression, much of what occurs at this level falls outside the immediate jurisdiction of the conscious mind, which relies predominantly on language and rational, temporal-sequential linguistic thought for understanding.
Because of this, we may feel angry or sad and not consciously (i.e., linguistically) know why. However, at a limbic level, we may feel anxious or angry because something was seen, heard, or even smelled, which called forth old limbic memories of some unpleasant event - memories that are not accessible to the linguistic mind.
Take, for example, a young child, who, like many infants, at times manipulated her genitalia. Unfortunately, this little girl's mother (being very repressed and rigid) responded with shock and disgust when she discovered her daughter's action. Resolving to put an end to this "repulsive behavior," the mother shouted "No" in an angry voice and slapped the child every time her daughter touched herself.
Soon the behavior was abolished through simple stimulus - response learning. This is because, at a limbic level, every time the urge to touch the genitalia arose, the behavior was severely punished. The child's urge to touch became associated with physical pain, and later with the anxiety of anticipated pain. Only three nuclei are necessary to form this circuit - the hypothalamus, amygdala, and hippocampus - as it is purely emotional and without thought. Indeed, much of this learning can probably occur without amygdaloid or hippocampal involvement, in which case the memory may remain unconscious. In fact, if sufficiently traumatized, the hippocampus (as well as the amygdala) might be damaged, thus resulting in unconscious learning and memories that remain unconscious and which cannot be recalled due to trauma and hippocampal induced amnesia.
Nevertheless, as an adult, this young woman now has difficulty associating with men, as sometimes their mere presence brings forth terrible feelings of anxiety. She has one failed relationship after another, and soon discovers herself to be frigid. She hates sex, she hates to be touched, and men repel her.
Consciously, she may be able to generate a panoply of explanations, all of which, at some level, are believable, albeit erroneous. Consciously, she has no true idea as to the source of her difficulties. Nevertheless, at the limbic level, the explanation is very basic. Certain men trigger the onset of amorous feelings. Unfortunately, the woman never feels sexually aroused. The first inklings of arousal immediately generate feelings of anxiety and limbic memories of physical pain. This is what she experiences and responds to.
CONCLUSION
A variety of nuclei and brain regions are important in emotional functioning. In this chapter, only a few of these regions are discussed. In some respects, many might argue that such structures as the hippocampus should not be considered part of the limbic system because of its involvement with memory. Indeed, it has become increasingly fashionable to decry even the use of the term limbic system, as there are simply so many diverse cerebral regions involved in the control and mediation of emotional functioning. Even by the most liberal of anatomical definitions there can be no structural basis for the concept when all such nuclei are considered.
This is not the case, however, in regard to the highly interactional anatomical and functional system maintained by the amygdala, hypothalamus, hippocampus, and septal nuclei. Therefore, I encourage continued use of the term, particularly in that to most people limbic system implies that part of the "old" brain concerned with emotional functioning.












