Rhawn Gabriel Joseph, Ph.D.
BrainMind.com
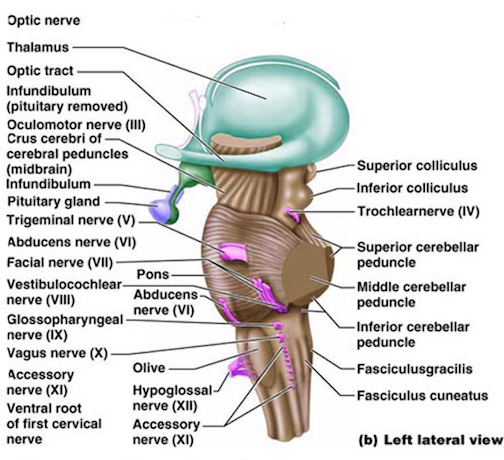
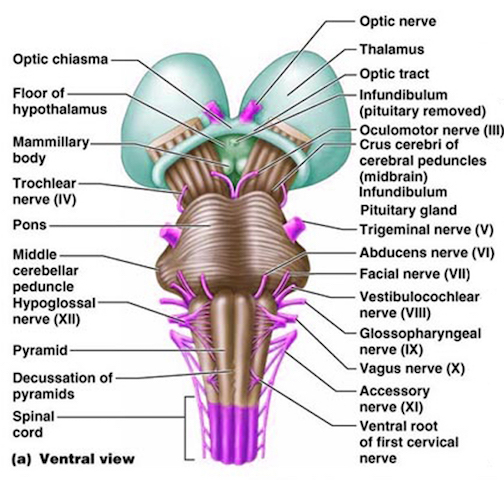
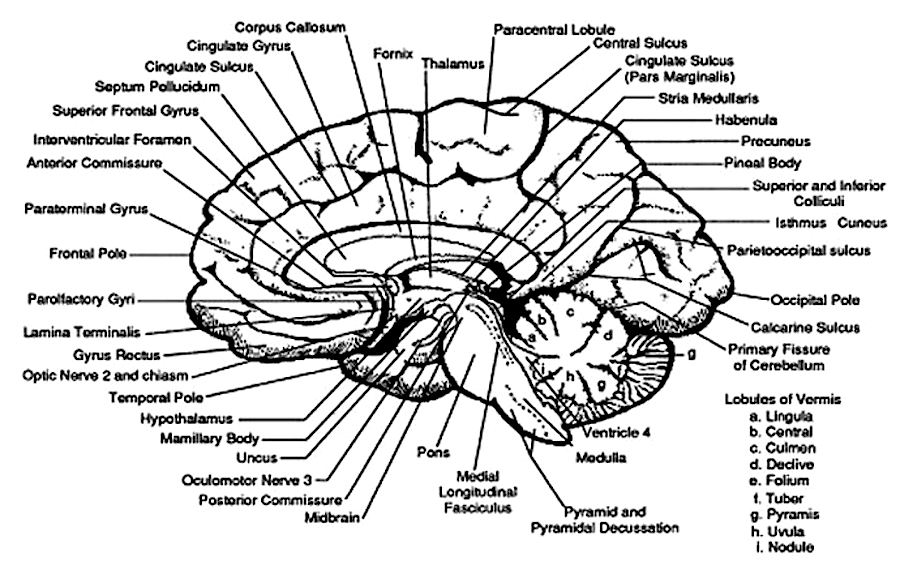
The brainstem consists of the medulla (myelencephalon), the pons (metencephalon), and the midbrain (mesencephalon). Straddling the dorsal surface of the pons is the cerebellum and spanning the length of the brainstem are the nuclei for cranial nerves 3-12 (see Carpenter 1991; Parent 1995) which will be briefly reviewed below.
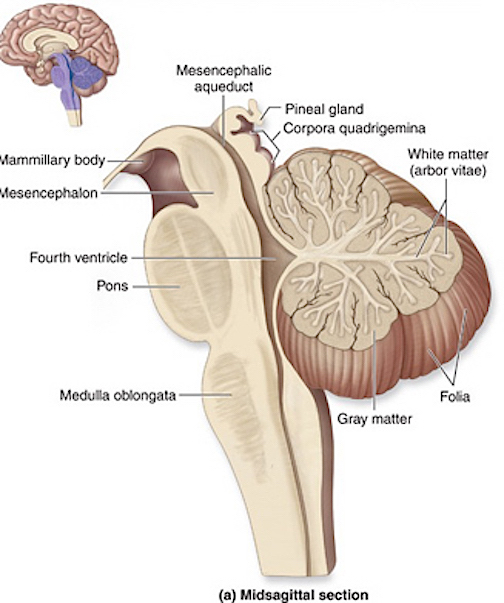
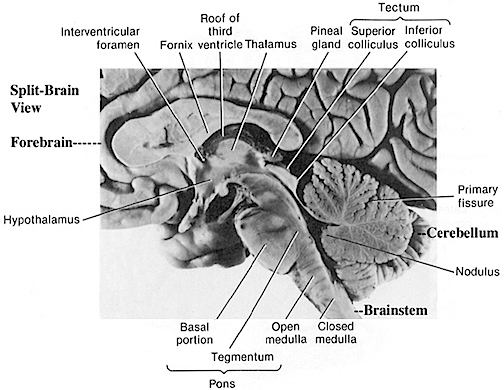
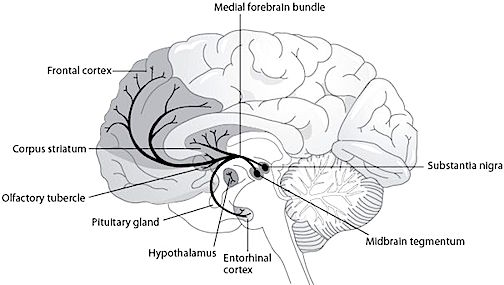
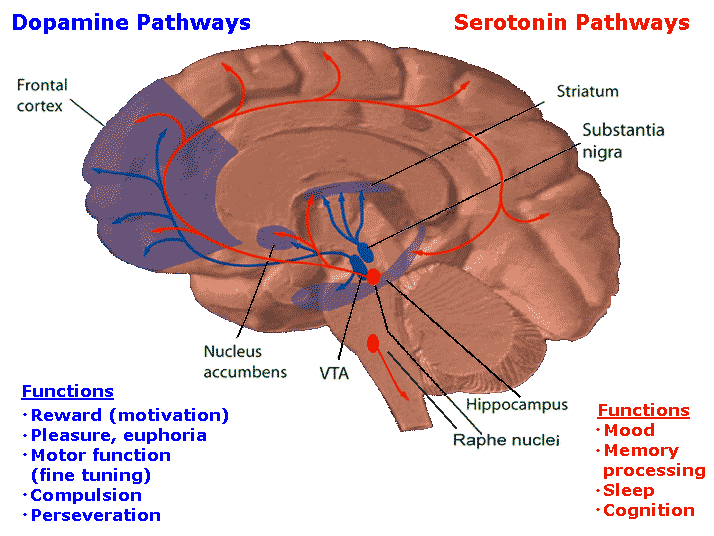
The most rostral portion of the brainstem, i.e. the midbrain merges with the diencephalon which in turn is surrounded by the cerebral hemispheres. The more posterior portion of the midbrain consists of the superior (visual) and inferior (auditory) collicululi which are directly related to the thalamus, and the red nucleus which receives motor fibers from the cerebellum and frontal and parietal lobe. The dorsal roof of the midbrain is referred to as the tegmentum (which produces dopamine) whereas more centrally located is the substantia nigra, a major source of corpus striatal dopamine.
The pons represents the most rostral portion of the "hindbrain" and it is separated from the midbrain via the superior pontine sulcus, and from the medulla by the inferior pontine sulcus. The two components of the vestibulocochlear nerve (VIII), and the facial (VII), abducens (VI) -which also lies in the floor of the fourth ventricle- and the trigeminal nerve (V) are the cranial nerve nuclei associated with the pons.
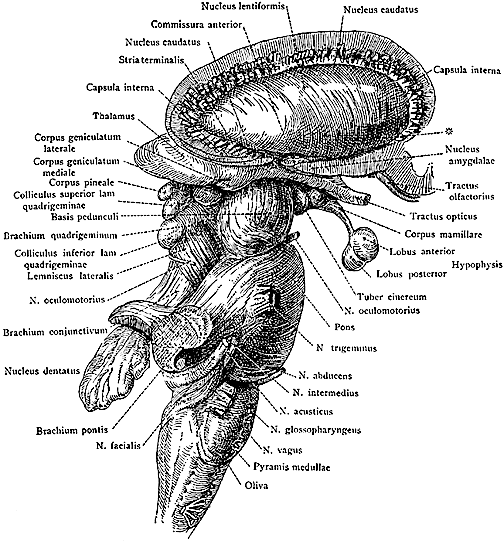
The medulla is the most caudal portion of the brainstem. The transition from spinal cord to medulla, however, is not obvious, though located at the medulla-spinal junction are the paired medullary pyramids through which course the cortico-spinal/pyramidal tract as they cross over before descending into the spinal cord. Basically the medulla extends from the level of the foramen magnum to the inferior pontine sulcus. The hypoglossal (XII), accessory (XI), vagus (X), and glossopharygeal (IX), cranial nerves are associated with the medulla, whereas the vestibulocochlear (VIII) nerve emerges at the junction of the medulla, pons and cerebellum.
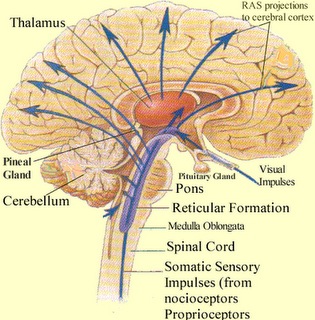
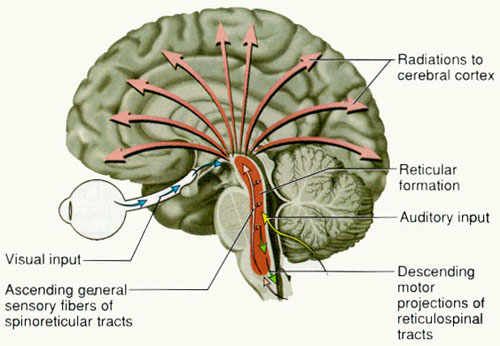
Spanning the medial and lateral lengths of the brainstem is a complex reticulum of richly interconnected cells with long ascending and descending axons, collectively referred to as the reticular activating system, which is concerned with generalized and selective arousal and activation of the neuroaxis. The pontine tegmentum and the more dorsal portion of the midbrain tegmentum is also considered part of the reticular formation through which course ascending sensory fibers and descending motor fibers.
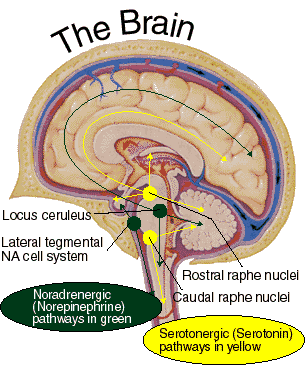
Neurons of the reticular formation receive specific sensory input from the skin, muscles, joints, and vestibular system which they then integrate. That is, the reticular formation as a whole, is not only concerned with arousal, but the integration and coordination of behavior in response to arousal, such as through the integration of trunkal, limb, arm, head and eye movements. Hence, the reticular formation exerts both generalized and specific activating influences and is thus concerned with sensory-motor integration (mediated by the excitatory and inhibitory neurotransmitters, glutamate and GABA) and modulatory functions (mediated by norepinephrine and serotonin).
In part, the activating influences of the reticular formation are subserved by clusters of chemically active neurons which secrete and distribute norepinephrine (NE), serotonin, (5HT), and acetylcholine (ACh). Specifically, clusters of NE neurons (designated A1 through A7 by Dahlstrom & Fuxe, 19), are scattered throughout the lateral, ventral, and anterior reticular formation and tegmentum.
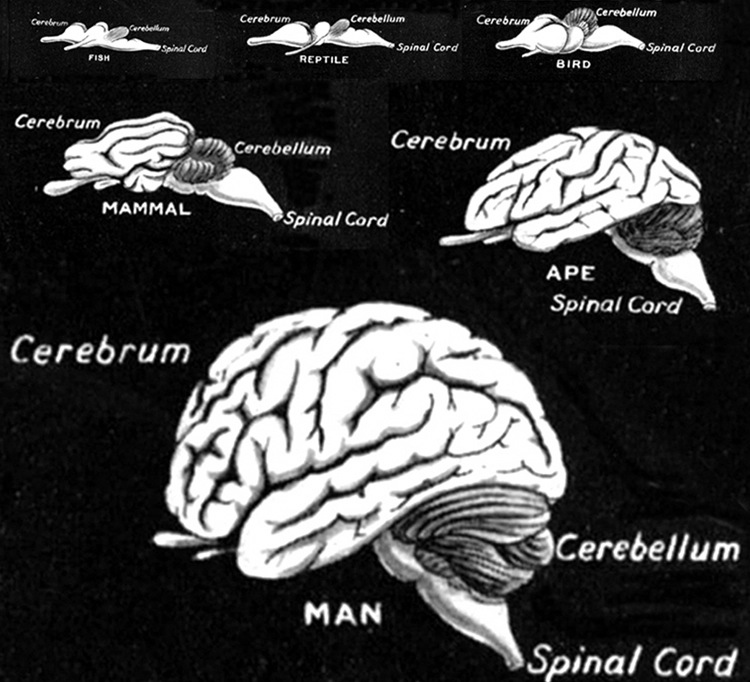
EVOLUTIONARY CONSIDERATIONS
As detailed in chapter 5, the brainstem in large part evolved from motor-sensory cells the had collected together to form like minded ganglia within the caudal regions of the head . Some of these ganglia came to constitute the portions of the spinal cord -where purely reflexive acts may be trigger (e.g. flexion or extension), whereas yet others formed the lower brainstem (and brainstem-spinal junction) and came to mediate a variety of vital vegetative functions, which for the most part occur completely outside conscious control; e.g. thermoregulation, salvation, cardiovascular functioning, swallowing, and respiration. This includes lower brainstem (medulla) motor nuclei which coordinate and program the movement of the tongue, palate, pharnyx, larynx, jaw, as well as swallowing, breathing, phonating, and "vocalization". These latter oral-facial- glutorial functions are mediated by brainstem nuclei and cranial nerves 12, 11, 10, 9, 7, and 5 (see Carpenter 1991; Parent 1995; Waxman & De Groot 1994).
Over the course of evolution, as motoric capabilities became more extensive and increasingly complex, the brainstem also evolved specific nuclei which could reflexively trigger and organize these movements. However, those nuclei concerned with the simpler aspects of movements, are also found within the lower brainstem, i.e. the medulla.
When specific brainstem nuclei (and associated neural networks) are stimulated, sucking, chewing, swallowing, swimming, stepping, walking, and running movements can be induced (Barman, 1990; Cohen, et al. 1988; Klemm, 1990; Mogenson, 1990; Steriade & McCarley, 1990; Skinner & Garcia-Rill, 1990; Vertes, 1990). However, these movements decrease in complexity as the stimulation site moves in a caudal direction from the upper pons to lower brainstem; i.e. they are hierarchically organized with those requiring the least amount of organizational or conscious control being localized near the brainstem-spinal border. Hence, at the level of the spinal cord, movement programs are exceedingly simplified and usually consist of only fragments of the entire motor display.
Conversely, as one ascends from the spinal cord to the brainstem, the degree and extent of these various motor programs and behaviors, becomes more complex. For example, at the most caudal regions of the medulla, neurons when stimulated can induce stepping motions, whereas stimulation of the more anterior midbrain pontine junction (at the level of the inferior colliculus), can initiate eye movements, head turning, controlled walking, as well as running (Cowie & Robinson 1994; Cowie et al. 1994; Skinner & Garcia-Rill, 1990). Conscious and/or forebrain/telencephalic participation is not required for these motor response.
In addition, brainstem activation can also induce involuntary, stereotyped and routine motor acts such as yawning, opening and closing the mouth, wiping the face, or lifting the legs, taking steps, walking or running (Cohen, et al. 1988; Klemm, 1990), even when forebrain influences have been eliminated via transection (Skinner & Garcia-Rill, 1990). For example, if the brainstem is completely severed from the midbrain and forebrain (by a lesion below the superior colliculi at the midbrain-pons transition) mammals may respond to facial stimulation by raising one or both paws and wiping the face (see Klemm, 1990). Or, following transection, direct stimulation of the brainstem can induce walking and running movements. Because the brainstem stores these motor programs, even infants are capable of making stepping and walking and swimming movements if properly stimulated.
Presumably motor control is maintained via descending fibers which terminate on spinal motor neurons in the dorsal horns, and as such, components of the various motor programs that mediate these activities, are located within the spinal cord as well as the brainstem. Hence, in response to stimulation, the brainstem can act on the spinal and cranial nerves in order to trigger these complex motor acts in the absence of forebrain influences. Even the stereotyped motor components involved in sexual posturing and behavior appear to be under direct brainstem control.
SEX & THE BRAINSTEM
Portions of the caudal medulla (as well as the spinal cord) have been implicated in the motor control over sexual posturing (Benson, 1988; Rose, 1990). Specifically, there are medulla neurons which respond to hormonal influences, neurons which react to tactile-genital contact transmitted via the spinal cord, and neurons which integrate these messages as well as those transmitted by the amygdala and ventromedial hypothalamus and basal ganglia --nuclei which are also involved in sexual activities. Many of these same sexual neurons are located near cranial nerve nuclei which control phonation and respiration. Hence, when these latter neurons are activated, the subject mayseam or cry out in orgasmic pleasaure (see Rose, 1990).
Moreover, there are neurons in the medulla which when directly stimulated can induce females to assume the lordosis (or a receptive) posture. Similarly, there are brainstem nuclei, including 5HT neurons which act to suppress male sexual behavior and arousal and the capacity to sustain an erection -due to 5HT suppressive influences on related spinal motor neurons and the amygdala.
However, female sexual posturing appears to be more reflexive and "hard wired" as compared to male sexual behavior which is more complicated. That is, the female need only to stand or lay still while the male most pursue, stimulate the female, mount her, insert his penis, and discharge semen -activities which are highly dependent on sensory feedback and guidance, and more greatly under the influence of the limbic forebrain). It is because male sexual behavior is more complicated and more sensitive that it can be more easily disrupted not only by psychological factors, but lesions involving a wide variety of brainstem and forebrain nuclei.
Overall, it appears that these and related motor acts are stored in the brainstem, and do not require forebrain participation or voluntary or conscious control for their elicitation. However, most of the motor actions and "programs" stored in the brainstem can be activated by forebrain nuclei, in particular the amygdala and hippocampus, the basal ganglia, dorsal medial thalamus, and the motor areas of the frontal neocortex.
MULTI SENSORY-MOTOR BRAINSTEM NEURONS
Some brainstem neurons respond only to a single sensory system, others respond almost regardless of the nature of the stimulus. As one ascends from the spinal cord to the lower brainstem, and then from the medulla to the midbrain, neurons and nuclei become increasingly responsive to a number of different sensory modalities (Grillner & Shik, 1973; Schiebel, 1980).
For example, fibers from different sensory modalities sometimes terminate on the same neuron, indicating that many brainstem and reticular neurons have multimodal properties. Indeed, via these converging sensory fibers brainstem neurons are able to map multi-sensory space so as to create almost a three dimensional sensory representation of the environment (Schiebel, 1980), whereas others create a motor map that may be juxtaposed over the sensory map (Grillner & Shik, 1973).
Via the creation of these brainstem sensory-motor maps, the organism can respond in a reflexive manner to sensory stimuli, particularly those which are threatening, painful, or aversive, in which case reticular neurons induce generalized, or in some instances, selective arousal in response to a specific sensory signals. However, under less threatening (or reflexive) conditions, these brainstem sensory-motor neurons may preprocess stimuli in which case the corresponding region of the neocortex may subsequently become activated (see Klemm, 1990; Steriade & McCarley, 1990).
As noted, the brainstem mediates modulatory as well as sensory motor functions, and these are subserved by different transmitters. Specifically, modulatory functions are mediated by GABA and glutamate with exert inhibitory and excitatory influences, whereas sensory-motor activities are associated with NE, 5HT, and ACh. Moreover, modulatory and sensory-motor neurons differ in size and in their speed and rate of transmission.
For example, the axons of modulatory neurons tend to be small in diameter (10-25 um), have a slow rate of conduction ( 1 m s -1), slowly depolarize, and then spontaneously become self-inhibited. Presumably, this slower pace of modulatory excitatory followed by self-inhibiting activity is a consquence of the oscillation of GABA (inhibition) and glutamate (excitation) neurotransmitter release, which thus provide these neurons with "pacemaker" functions, which thus gives rise to the rhythmic nature of so many brainstem activities.
By contrast, the axons of sensory motor neurons are larger in size (50 to 75 um in diameter), fire at continuously high rates, with conduction velocities of over 100 m s -1. These neurons, therefore, are perfectly adapted for activating and arousing widespread areas of the cerebrum, and can quickly recruit (as well as inhibit) vast neural networks in response to life threatening or other emotionally or motivationally significant external events, including the presence of a potential sex partner.
As will be detailed below, modulatory and sensory-motor neurons and the neurotransmitters which serve them, also interact so as to regulate not just arousal and rhythmic motor activities, but sleep, including the generation of REM (rapid eye movement) and NREM sleep. That is, sensory motor neurons (e.g. NE and 5HT) play a significant role in the generation of dream (paradoxical) sleep, which is characterized by a high state of cerebral arousal, whereas the modulatory (pace maker) neurons (GABA and glutamate), play a significant role in the oscillation between REM and NREM.
SEROTONIN
Serontonergic (5HT) neurons exert widespread and often tonic and inhibitory influences on a variety of brain areas (Applegate, 1980; Jacobs & Azmita 1992; Soubrie, 1986; Spoont, 1992). This includes the modulation of fear and pain and emotional states such as depression, the inhibition of incoming sensory input, and the modulation of motor expression. That is 5HT restricts perceptual and information processing and in fact increases the threshold for neural responses to occur at both the neocortical and limbic level.
For example, in response to arousing stimuli, 5-HT is released (Auerbach et al. 1985; Roberts, 1984; Spoont, 1992) which acts to aid attentional and perceptual functioning so that stimuli which are the most salient are attended to. That is, 5HT appears to be involved in learning not to respond to stimuli that are irrelevant and not rewarding (See Beninger, 1989). These signals are filtered out and suppressed.
It has also been demonstrated that 5HT acts to suppress activity in the lateral (visual) geniculate nucleus of the thalamus and synaptic functioning in the visual cortex as well as the amygdala and throughout the neocortex (Curtis & Davis 1962; Marazzi & Hart 1955). By contrast, substances which block 5HT transmission -such as LSD- results in increased activity in the sensory pathways to the neocortex (Purpura 1956), which induces complex hallucinatory experiences.
As will be detailed below, 5HT is also implicated in the generation of sleep and dreaming, as well as the production of motor paralysis during sleep. For example, 5HT via descending influences, facilitates motorneuronal activity, while simultaneously decreasing or inhibiting sensory input (Morrison & Pompeiana 1965). In this manner, selective attention (via sensory filtering) can be maintained while the organisms is engaged in goal directed motor behavior. However, while sleeping and dreaming, internal sensory filtering is reduced (due to decreased 5HT) whereas motor functioning is inhibited -which prevents the subject from walking about and acting out their dreams (see below).
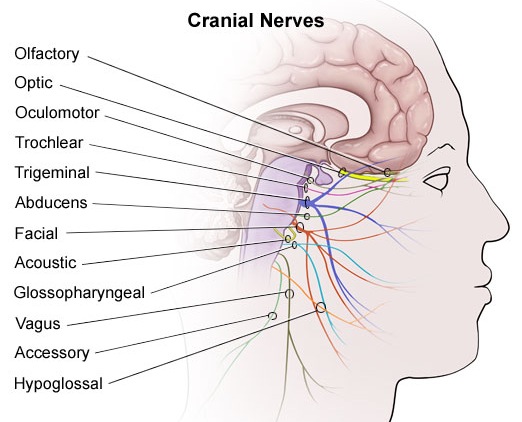
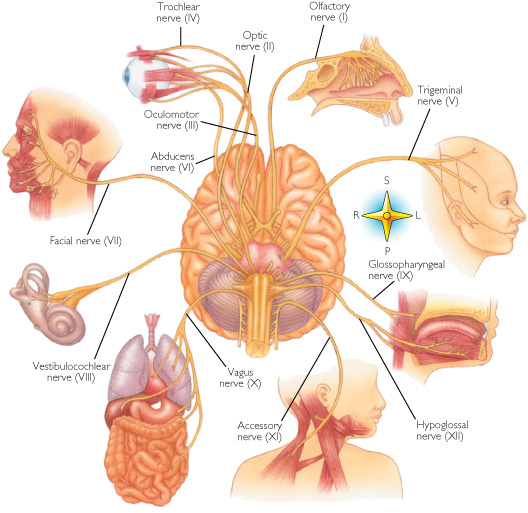
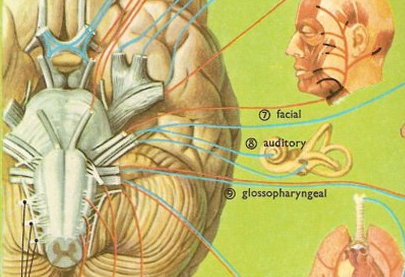
CRANIAL NERVES OF THE MEDULLA: SHOULDER, HEAD, JAW, TONGUE MOVEMENT, BREATHING, PHONATING, HEART RATE
:
CRANIAL NERVE 12: HYPOGLOSSAL The hypoglossal nucleus located in the caudal medulla gives rise to the 12th nerve which controls tongue movements via innervation of the somatic skeletal muscles. A lesion, tumor, or infarct invading this area can give rise to weakness or atrophy of the tongue. For example, if the tongue is weak and deviates to the right, the ipsilateral lower motor neuron (LMN) is involved, indicating that the right genionglussus muscle is weak. Weakness can be tested by having the patient place his tongue in one side of cheek and ask him to press against your finger (on the outside of cheek). The clinical and EMG signs of an LMN lesion of the 12th nerve are hemiatrophy, unilateral fasiculation or fibrillation, and usually severe paralysis with obvious deviation to the paralytic side when the tongue is protruded.
CRANIAL NERVE 11: SPINAL ACCESSORY
Cranial nerve 11 consists of two distinct segments; a cranial portion which, along with the vagus nerve, forms the inferior laryngeal nerve which innervates the muscles of the larynx; and a spinal portion which innervates the sternocleidomastoid and upper trapezious muscles and therefore aids in turning the head and elevating the shoulders. Injuries to this nerve may result in a sagging of the shoulder on the affected side, or weakness in turning the head -particularly when resistance is applied.
CRANIAL NERVE 10: VAGUS
The vagus nerve is actually a complex mix of nerves which innervate a variety of structures including the trachea, larynx, pharnyx, esophagus, and abdominal and thoracic viscera, the epiglottis, and the external auditory meatus. Hence, this nerve (in conjunction with the 9th nerve -both of which are derivitives of the skeletal muscles that orginally formed the brachial (gill) arches) is important in regard to oral activities, including swallowing, breathing, and speaking, pharyngeal constriction and thus with movement of the palate, pharnyx and larynx. The vagus nerve is therefore responsible for swinging the soft palate upward and backward to contact the posterior wall of the pharynx, sealing off the oropharynx from the nasopharynx when one swallows, whistles, or speaks.
An injury to this region can result in severe and enduring palatal weakness as well as a condition referred to as pseudobulbar palsy. Unless the soft palate elevates properly liquid will escape into the nose when one drinks, and when one speaks resulting in nasal speech. Indeed, damage to these nuclei can severely effect speech.
CRANIAL NERVE NINE: GLOSSOPHARYNGEAL
The 9th nerve is closely related to the vagus and both have similar functional components, including general somatic and visceral afferents and efferents. The glossopharyngeal nerve thus receives tactile, thermal, and pain sensations from the tongue, and contributes fibers to the solitary nucleus thereby forming the gustatory nucleus. In addition, the glossopharyngeal nerve receives fibers from the carotid sinus which transmit impulses regarding increases in carotid arterial pressures. The ninth nerve then transmits this data to the solitary nucleus which in turn contributes fibers to the vagus nerve. In this manner the 10th and 9th nerve can induce reductions in arterial blood pressure and heart rate.
Lesions to the 9th nerve generally results in a loss of taste and sensation in the posterior third of the tongue, and a loss of the gag reflex and the carotid sinus reflex. In some cases intense pain may be triggered by swallowing or coughing.
LATERAL MEDULLARY SYNDROME
The lateral medullary syndrome is typified by dysphonia (hoarse voice), persistent hiccup, disordered equilibrium, vertigo, nausea, and loss of thermal sense and pain involving the contralateral half of the body and the ipsilateral half of the face. Usually this condition is due to vascular abnormalities involving the vertebral arteries, or occlusion of the posterior inferior cerebellar artery.