Rhawn Gabriel Joseph
BrainMind.com
ABSTRACT
Discussed evidence and assumptions that concern hemispheric laterality and asymmetrical functional representation. It is hypothesized that the asymmetrical linguistic-motor vs. sensory-spatial-affective representation of function may be a result of differential rates of cortical, subcortical and spinal motor-sensory maturation. Evidence with regard to embryological and early postnatal neurological development is reviewed. It is argued that motor areas mature before sensory and that the left hemisphere develops prior to the right, such that the left hemisphere gains a competitive advantage in the acquisition of motor representation, whereas the later maturing right has an advantage in the establishment of sensory-affective synaptic representation, including that of limbic mediation. The influences of these differing maturational events on cognitive and psychic functioning are examined, particularly with regard to limbic influences on the development of language, thought, and mental imagery, and the effects of early emotional experience on later behavior. Thinking is viewed in part as a left hemisphere internalization of egocentric language, the internalization of which corresponds to the increasing maturation of intra-cortical and subcortical structures and fiber pathways, and the myelination of the corpus callosum connections that subserve information transfer between the hemispheres. It is argued that thought is a means of organizing, interpreting, and explaining impulses that arise in the non-linguistic portions of the nervous system so that the language dependent regions may achieve understanding. In addition, the neurodynamics and mechanisms involved in the mislabeling, misinterpretation, and inhibition of impulses, desires, and emotional expression are discussed in relation to disturbances in psychic functioning.
The Origins of Thought
Thinking is clearly a form of communication and, as such, is a form of language, an organized hierarchy of associations, symbols, and labels that appear before an observer. In the process of thinking, one acts to organize information that is 'not thought out' and that is not clearly understood, so that it may become 'thought out' and thus comprehended (Ach, 1951; James, 1961; Schilder, 1951). Thought is a means of deduction, clarification, plan and goal formation, and reality manipulation. However, it is also a progression, an associative advance that leads from inner or outer perception to linguistic-motor expression (Freud, 1900) and an elaboration that some have argued appears with an initial or leading idea that is followed by a series of related ideations, or as originating developmentally from the non-accessible regions of the mind (Freud, 1900; James, 1961; Jung, 1954; Piaget, 1962). That is, it appears to be based on information that exists prior to thought as tacit and non-organized informational variables (Ach, 1951; Franks, 1974; Joseph, 1980; Polanyi, 1966) and is dependent on the reality-based organization of previous thought-like ideations, e.g., primary, symbolic and intuitive 'thought,' (Freud, 1900; Jung, 1956; Piaget, 1962).
Thought is thus a means of explanation through which ideas, impulses, desires, plans, or thing-in-the-world may be understood and possibly acted upon. Paradoxically, it is a process by which one explains things to oneself and thus necessitates that one apprehend and organize information that is possessed prior to its explanation. However, the fact that one acts as both the explainee and the explainer raises a curious question, 'who is explaining what to whom?'
The concern of this paper is the development of thought and the substrate from which it originates, the nervous system. Thought and language are viewed as integrally related to the motor predominance of the left hemisphere and the differential rates of hemispheric maturation. It is argued that the early maturation of the motor fibers of the peripheral nervous system and the motor cortex of the left hemisphere, coupled with the later maturation of the right hemisphere and sensory systems, results in differential acquisition and function, such that the left predominates in motoric processes and relies on temporal and sequential modes of operations, whereas the right is concerned primarily with receptive-sensory non-linguistic functions and the parallel-wholistic realization of information. Although considerable overlap of function is noted, thinking is viewed as a left hemisphere linked internalization of language, the internalization of which corresponds to the increasing maturation of intra-cortical/subcortical structures and pathways, and the myelination of the callosal fiber connections that subserve information transfer between the two hemispheres. Hence, thought serves in part as a means of organizing, interpreting, and explaining impulses that arise in the non-linguistic portions of the nervous system so that the language dependent regions may achieve understanding. In addition, disturbances in the formation of thought are discussed.
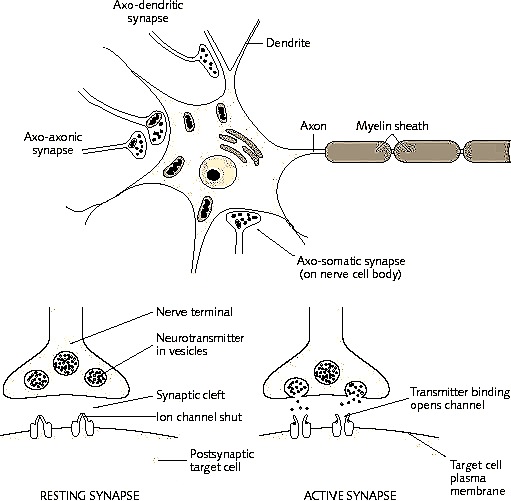
Although some lateralization of function has been noted in non-human vertebrates (cf. Dennesberg, 1979; Nottebohm, 1979; Walker, 1980), which in turn may reflect differential rates of hemispheric or central nervous maturation, laterality, or asymmetrical functional specialization of language and other higher cortical activities appear to be the hallmark of the human brain. Each hemisphere has, in the course of evolution, developed its own unique strategy and capability for processing information, and a neuroanatomical substrate to subserve each independent function. Curiously, the human brain is organized so that two potentially independent mental systems coexist, literally side by side (Bogen, 1969; Galin, 1976; Gazzaniga & LeDoux, 1978; Ornstein, 1972; Sperry, 1974), such that each hemisphere or mental system may act independently on specific information, initially without interference from the other (Dimond & Beaumont, 1974). A result of this curious arrangement is presumably the dual processing of sensory information (Dimond & Beaumont, 1974; Levy, 1974; Levy & Trevarthen, 1976). Interference, as well as the possibility of dual responding, probably is prevented by the corpus callosum, which both aids and inhibits interhemispheric transfer.
Not only are these systems dual, but they are asymmetrical as each hemisphere utilizes predominantly verbal-analytical or visual-spatial, sensory-affective associational strategies in the analysis and expression of information (Bogen, 1969; Levy, 1974; Luria, 1973; Sperry, 1974). In this regard, when one hemisphere learns or 'experiences,' the information analyzed then cab be transferred via the corpus callosum for further analysis or so that the other hemisphere also may 'learn,' being a beneficiary of the other's activity (Bogen, 1969; Dimond & Beaumont, 1974; Gazzaniga & LeDoux, 1978; Levy, 1974; Sperry, 1974). Although admittedly there is considerable overlap of function, in that both hemispheres may act to analyze all input, and given that some types of information are dealt with more efficiently by one than the other, in the long run this relationship is very adaptive as the range and speed of information analysis is enlarged and the efficiency of response is increased (Dimond & Beaumont, 1974; Levy, 1974; Levy & Trevarthen, 1976).
For example, when attempting to identify a block with few details, although the left may have a slight advantage, the two hemispheres may perform comparably. With the addition of details, the performance of the left beings to deteriorate, whereas the right continues to function efficiently regardless of the increasing complexity (Dimong & Beaumont, 1974; Levy & Sperry, 1968). In general, these qualities are characteristics of the differential strategies best employed by the two hemispheres during information analysis, the left relies on the analysis of temporal sequences, details (or parts), whereas the right is responsive to melodic and environmental sounds, visual-spatial analyses, and appears to form a gestalt.
However, in that the left codes, processes, and stores information using linguistic, categorical, functional, and motoric referents, whereas the right relies on spatial, emotional, and non-linguistic modes of understanding (Blumsetein & Cooper, 1974; Carmon & Nachson, 1971; Knox & Kimura, 1970; Levy, 1974; Sperry, 1974), complete and efficient inter-hemispheric communication may not always be possible (Joseph, Gallagher, Kahn & Holloway, 1984). At least in regard to consciousness or linguistic processing, there is the potential for much miscommunication, misinterpretation, and distortion regardless of the direction of transfer. We will return to this topic in the later half of this paper (cf. Neurodynamics).
The right and left hemisphere intact and dissected showing the corpus callosum
The right and left hemisphere
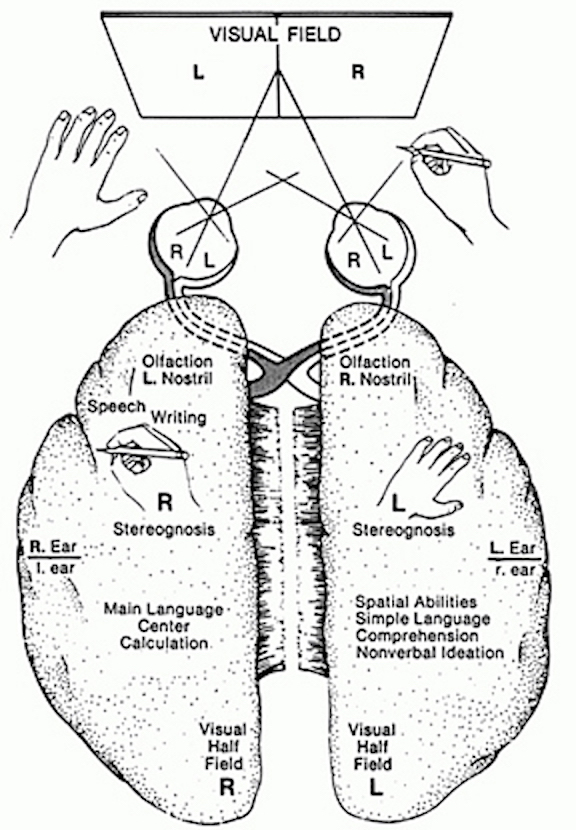
The right hemisphere. In general, the right hemisphere appears to be polysensory, expressively non-linguistic, affectively endowed, and responsible for the sensory integration of complex internal and external environmental variables (Critchley, 1953; Kimura & Durnford, 1974; Luria, 1973; Schwartz et al., 1975; Tucker, 1981). It is concerned with spatial orientation of perceived stimuli, analysis of the body's position in space, geometrical realization of spatial relationships, and the conceptualization of the whole of a stimulus configuration including form, figure-ground, and depth (Kimura & Durnford, 1974; Levy, 1974). The right hemisphere is also 'dominant' for the realization of tactile and propioceptive information (Critchley, 1953; Luria, 1973; Semmes, 1968), is the principal receptive organ for sensory impressions that arise from one's own body, and is preeminent for the realization of the body image (Critchley, 1953; Nathanson, Bergman, & Gordon, 1952; Roth, 1944, 1939; Sandifer, 1946; Weinstein & Kahn, 1950). Hence, we sometimes find with damage, particularly in the posterior cortical regions, that there is a subsequent failure to recognize or attend to the left half of the body, or space, such that the left half of the environment may be abnormally perceived or actively neglected and the sensory attributes and spatial relationships within the environment severely confused (Bisiach & Luzzatti, 1978; Critcheley, 1953; Gainooti, Messerli, & Tissot, 1972; Heilman, 1979; Nathanson et al., 1952). In addition, patients literally may deny the existence of the left side of the body, such that when engaged in daily activities they may brush only the right side of their head, and wash or dress only the right side of the body. In that hemi-neglect occurs much less frequently with left hemisphere damage, suggests that the right hemisphere normally attends to information that arises from both sides of the body and space, whereas the left hemisphere at best, and the perhaps only following right hemisphere parietal dysfunction, attends to the right side. In this regard, it certainly could be argued that the right hemisphere, in fact, has superior awareness (cf. Joseph, 1980) of the environment-body continuum than its left brain counterpart.
The right hemisphere also is concerned with the mediation of emotional arousal and expression, and with discerning the affective significance of facial contours (DeRenzi & Spinnler, 1966; Gilbert & Bakan, 1973; Schwartz et al., 1975; Tucker, 1981). When damaged, patients may no longer be able to control their emotions and may appear labile, manic or depressed, blunted, indifferent, or otherwise inappropriate (Bear & Fedio, 1977; Flor-Henry, 1969; Gainotti, 1972; Goldstein, 1948; Luria, 1973; Sherwin, 1977). However, it is important to note that although the left hemisphere does onto mediate emotion per se, it may act to inhibit emotional expression as generated in the limbic areas or the right half of the brain.
Although propositional language is mediated by the left hemisphere, the right is also capable of perceiving and comprehending some spoken language and concrete words (Gaazaniga, 1970; Zaidel, 1977), but its ability seems to be limited to nouns and simple sentences. However, it is largely non-linguistic, except for the ability to curse, sing, and pray. IT cannot analyze the phonemic elements of words due to its difficulty in preserving sequential information (Zaidel, 1977), or can it generate phonology from meaning (Levy, 1974; Marin, Schwartz, & Saffran, 1979). Rather, its comprehension is limited to a rather impoverished lexicon that consists of the ability to comprehend connotative (Gazzaniga, 1970; Zadel, 1976, 1977) and emotional components of speech and auditory stimuli (Carmon & Nachson; 1973; Haggard & Parkinson, 1971). The recognition of environmental sounds (e.g., wind and rain the rolling of thunder) and the reception and production of tonal patterns, intonational contours, and melody are mediated by the right as well. Not surprisingly, individuals with right hemisphere damage may have considerable difficulty comprehending affective speech and may loose a great deal of their ability to appreciate music (Gordon & Bogen, 1974; Heilman, Scholes &Watson, 1975; Luria, 1973; Milner, 1962; Tucker, Watson, & Heilman, Scholes & Watson, 1975; Luria, 1973; Milner, 1962; Tucker, Watson, & Heilman, 1977), whereas speech and language functions generally remain unaffected.
Interestingly, even with extensive damage to the left hemisphere, the majority of individuals continue to respond and interact emotionally with the environment and may retain the ability to sing familiar songs and learn new ones, with few or no articulatory errors (Bogen, 1969; Critchley, 1953; Geschwind, 1965; Geschwind, Quadfasel, & Segarra, 1968; Head, 1926; Smith, 1966). Moreover, although propositional speech may be absent, the ability to pray may be retained. The retention of these operations is truly amazing when one considers that massive left hemisphere damage destroys the linguistic portions of the brain, and the individual in all other respects may appear to be without comprehension or thought, and no longer interact with the environment in an intelligible manner.
Interestingly, singing, praying, and cursing appear to be dependent on the integrity of the right hemisphere and seem to be linked affectively, in that all 3 are affectively stamped, contain emotional components, and are dependent on an emotional motive force for the arousal of their expression (cf. Goldstein, 1943, 1948). Singing, praying, and cursing thus appear to be retained after left hemisphere dysfunction due to the mediation of emotional and musical production/reception by the right hemisphere (Bogen, 1969; Chase, 1967; King & Kimura, 1970; Luria 1973; Milner, 1962; Smith, 1966).
Right hemisphere overview. The right hemisphere appears to be concerned predominantly with the reception and realization of non-linguistics, non-sequential, non-temporal sensory information that arises from one's own body or the surrounding environment. It is expressively non-linguistic, and does not seem to explicitly label, classify, or perform differential analysis on the elements of stimuli, but rather perceives things as a whole without disturbing or creating relationships. Possibly as an outgrowth of its superior sensory-receptive capacities, the right hemisphere also is concerned with the realization of spatial relationships, depth perception, emotional mediation and expression, and the appreciation of emotionally related sensory experiences. Interestingly, there is some provocative evidence that lends itself to the suggestion that right hemisphere activity during sleep provides the matrix from which dreams are derived and is responsible during waking for creative output.
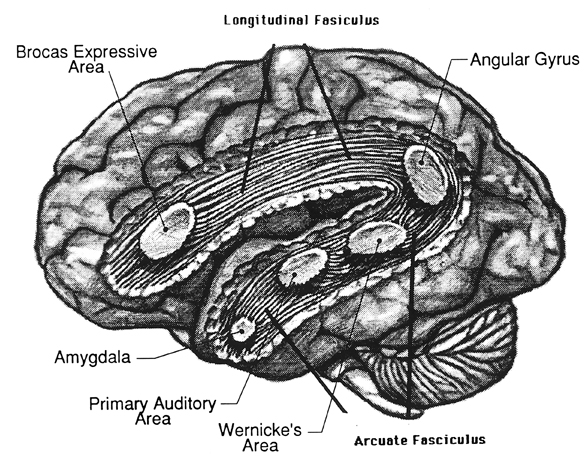
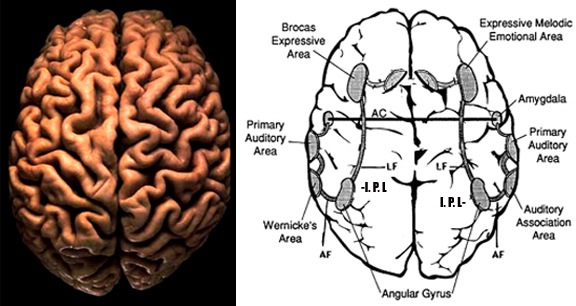
The left hemisphere. The left hemisphere is most notable for containing the neural substrate for the expression of language (Broca's are in the frontal lobe) and its analysis and comprehension (Wernicke's area in the temporal region and the angular gyrus in the parietal lobe, see Table 2). It subserves gestural communication, the organization of motoric output and purposeful actions, and includes systems for the perception and labeling of material that can be coded linguistically (Brown, 1975; Galaburda & Sanides, 1980; Luria, 1973; Sanides, 1975). It also is associated with the organization and categorization of material into discrete temporal and analytical functions, verbal concept formation, and the distinction of sound by specific articulatory features (Critchley, 1953; Kimura, 1973; Kimura & Vanderwolf, 1970; Riese, 1956).
When the left hemisphere is damaged, performance on problems that involve temporal order is selectively impaired (Carmon & Nachson, 1971; Efron, 1963; Lackner & Teuber, 1973), as is nonverbal manual or oral performance (DeRenzi, Faglionia, Saoiardo, & Vignolo, 1966; Kimura, 1976, 1977), the copying of meaningless movements (Kimura & Archibald, 1974), rapidity of limb (Wyke, 1968) and pursuit-rotor movements, and the analysis of temporal sequencing of speech (Lenneberg, 1967). Hence, with a lesion in the posterior portions of the temporal lobe the individual may have difficulty organizing these thoughts for expression. That individuals with Wernicke's aphasia have been noted to complain 'everything sounds like a foreign language' or that 'words don't separate' (Lenneberg, 1967, p. 191) indicates that the specialization for performing temporal analysis and sequencing operations is mediated by this region (Albert, 1972; Carmon & Nachson, 1971; Halperin, Nachson & Carmon, 1973), and that the ability to extract meaning from perceived sequences of sound is dependent on the ability to organize and coordinate units into a temporal and interrelated linear series; an ability at which the left hemisphere excels (Kimura, 1973, 1977; KInsbourne, 1978; Shankweiler & Studdert-Kennedy, 1975).
After left-hemisphere damage, individuals may have difficulty carrying out simple commands (e.g., 'touch your nose, then your left elbow') and may make gross errors in the attempt (Brodal, 1969; Brown, 1972). In addition, it has been noted that the right hand, more so than the left, becomes activated during verbal communication (cf. Kimura, 1977). The fact that both hands and sides of the body may become apraxic after left hemisphere damage (Brown, 1972; Geschwind, 1965; Luria, 1973) and that both hands may become activated during vocal communication, indicates that the left hemisphere may exert bilateral influence or control over muscular output, at least of the upper musculature. However, its greatest influences is over the right hand, which, in the majority of the population, is dominant for grasping, manipulating, exploring, creating, and communicating. In fact, right hand usage appears to be in some manner a motoric extension of the language function as both seem to share some common neural substrates. This motoric relationship is demonstrated further by the findings that left hemisphere damage results in difficulty copying hand movements, that deaf mutes with left sided damage have deficits in gestural communication, and that individuals with right hemisphere speech demonstrate more left hand than right sided movements while speaking (cf. Kimura, 1976, 1977). Moreover, while speaking, the ability to simultaneously sequence, position, or maintain stabilization of one's limbs or hand is reduced (Hicks, 1975; Kimura & Archibald, 1974; Kinsbourne, & Cook, 1971). These observations have led to the suggestion that language in fact may be an evolutionary outgrowth of left hemisphere motor predominance.
Left hemisphere overview. The left half of the brain is preeminent for motoric-language functions, including motor aspects of cognitive activity. Its efficiency in the organization and coordination of movements in sequence and its mediation of analytical-mathematical and temporal processes, including the linguistic labeling and categorization of experience, enable it to play the dominant role in language dependent cognitive operations, such as thought, speech, and gestural communication. Nevertheless, it is important to note that the two hemispheres are not strictly dichotomous, as there is considerable overlap in functional representation and expression.
Competition for Synaptic Space: Sensory and Motor Development
The human brain, although conceptualized by some as functionally lateralized and asymmetrical arranged, is a composite of multitudinous neuronal cell assemblies, each of which subserves specific functions and large numbers of which are aggregated in discrete regions of both hemispheres. There is abundant multi-representation of both sensory and motor function throughout the cortices of both brain halves. Nevertheless, these representations are not symmetrical because the left, in humans, has become preeminent for motor and language functions, whereas the right appears to be concerned with visual-spatial, sensory-affective-emotional aspects of experience. Given the earlier maturation and development of the motor layer of the cortex embryologically across species, and the comparatively latter development and differentiation of the sensory cortex (Arey, 1965; Hamilton & Mossman, 1972), it appears that the motor-sensory dichotomy may be in part a function of the differential rates of cortical maturation of the two hemispheres.
1. the generation of the cell.
2. the migration of the cell from its birth cite to its terminal substrate.
3. the aggregation of cells within specific brain regions.
4. cellular differentiation and the growth of axons and dendrites.
5. the formation of synaptic connections.
6. Myelination and the elimination of cells, axons, and dendrites; a continual process that spans the individual's lifetime.
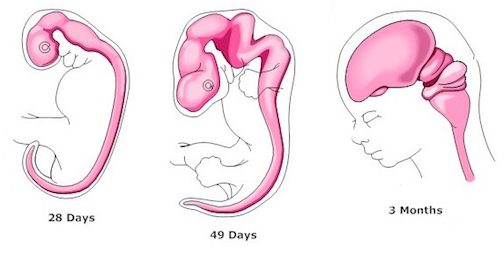
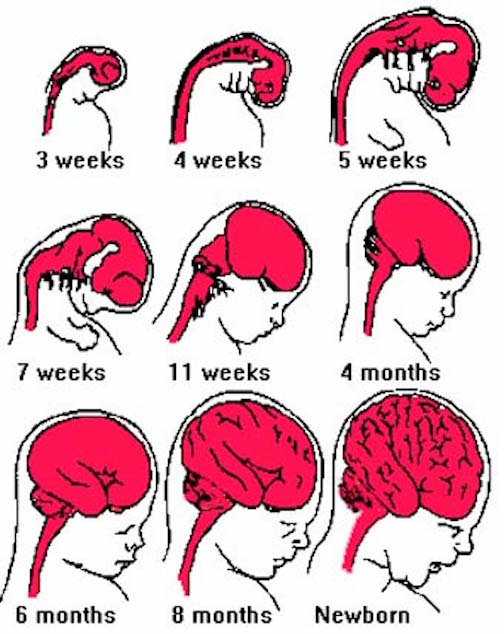
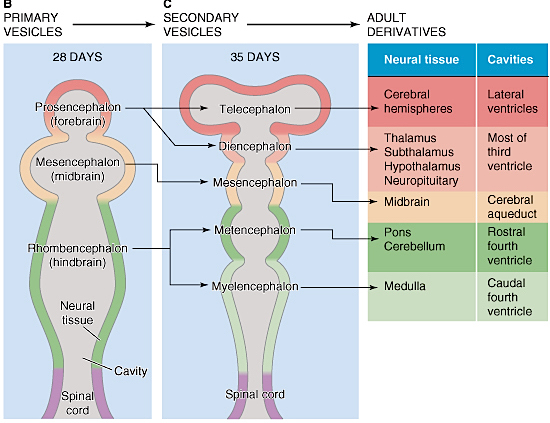
At approximately the fourth week of embryonic development, three swellings begin to develop at the head of the neural tube (the substrate from which the central nervous system develops): The forebrain, midbrain, and hindbrain.
Specifically, the brainstem and cerebellum begin to develop in advance of the forebrain, which in turn matures in a rostral arc, i.e. diencephalon, limbic system, striatum, neocortex (unpublished observations). And these maturational events continue well into late childhood, adolescence and adulthood.
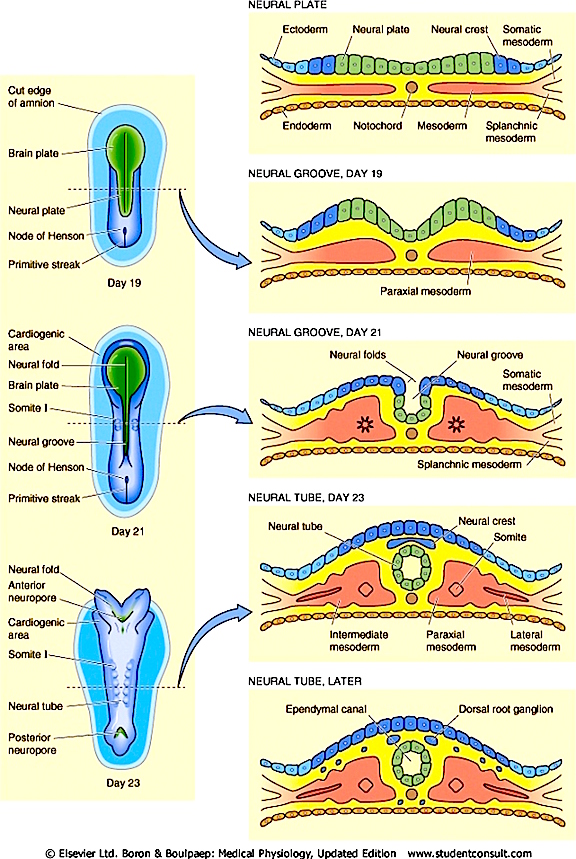
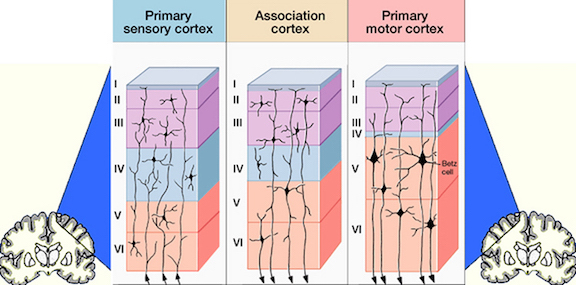
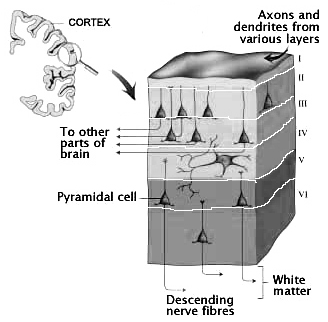
However, prior to the development of the neocortex, as the brain continues to form and unfold, a germinal layer forms that surrounds the lateral ventricle. From this germinal layer, neuroblasts (primitive neuronal cell bodies) begin to migrate outward to form an outer cortical or marginal zone, the primordial cortex. This zone subsequently becomes filled with cell bodies at about the third month of prenatal development as it receives massive migrations of cells from the inner regions of the brain (Arey, 1965; Hamilton & Mossman, 1972; Truex & Carpenter, 1969). It is within the primordial cortex that the six to seven concentric layers of the cerebral neocortex are formed (Arey, 1965). Nevertheless, not all six concentric layers are formed at once. This is important, in that the different layers subserve different functions.
The first layers to be established are the deepest layers, 6 and 5, which consist primarily of pyramidal cells from which the cortico-spinal (pyramidal) tract develops, and which subserve motor functions. Similarly, during this period, the anterior or motor regions of the cerebral cortex (frontal lobes) and the deep layers of the temporal lobes (including hippocampal and other limbic areas) begin to form (Milner, 1967; Truax & Carpenter, 1969). Later in development there is a second wave of neuroblast migration into the primordial cortex, and layers 4 then 3 and 2 are formed. Although they contain some pyramidal cells, these layers have predominantly receptive functions, receiving specific afferent and sensory fibers directly from the thalamus (particularly layer 4) or association fibers from other cortical regions (Brodal, 1969). Layer 1, the most superficial, is the last to develop and receives the final wave of neuroblast migration.
Thus the neocortex, which consists of numerous sublayers, develops in an "inside-out" fashion, with the inner, deeper layers forming in advance of the outerlayer, thus forming a scaffolding or ladder.
Moreover, differentiation of these last three receptive layers is not complete until middle childhood (Arey, 1965), which suggests that the motor (pyramidal) layers develop (or rather appear) well in advance of the sensory regions of the cerebral cortex.
Nevertheless, although at birth motor maturation is much more advanced than sensory, particularly in the regions that control hand and arm movement, the primary motor strip (Conel, 1939; McGraw, 1969), both sensory and motor neurons are quite immature, and appear functionally isolated and unable to exert regulatory control over peripheral processes until later in development (Arey, 1965; Brodal, 1969; McGraw, 1969; Milner, 1967).
Although the six to seven layered neocortex is formed well before birth, with the exception of a few pyramidal cells in the primary motor cortex of the frontal lobe, there is no evidence of myelinization of metabolic activity within this region of the brain at birth (Fleshig, 1901; Langworthy, 1937).
Moreover, even at one year of age, the frontal, temporal, occipital, and parietal lobes are only about half the size of the lobes of the adult brain (Blinkov & Glezer, 1968). In fact, the neocortex of the frontal, parietal, occipital, and temporal lobes can take well over 7, 10, or more years to fully mature.
For example, we find that the cortico-spinal tract (the long axons from the pyramidal-motor layers of the cortex) does not completely establish its functional connections or become completely myelinated until the seventh month after birth (Yakovlev & Lecours, 1967). Hence, most early behavior is mediated by subcortical, limbic, and spinal mechanisms (Arey, 1965; Brodal, 1969; Conel, 1939; Hamilton & Mossman, 1972; Langworthy, 1933; Milner, 1969). It is largely due to this incomplete information and regulatory exchange that the behavior of anencephalic monsters and normal infants is largely indistinguishable for the first few days of life (Mcgraw, 1969).
Cell death and synaptic maintenance. Although growth continues throughout the brain after birth there are anywhere from 15 to 85% more neurons in the infant as compared to the adult brain due to cell death. Presumably because of the immaturity of the brain and the fact that functional and synaptic interconnections are largely incomplete or not yet established, a large number of these neurons die and/or their processes are retracted within the first few years of life (Arey, 1965; Cowan, 1979; Hamilton & Mossman, 1972). A number of interactions, however, appear to be responsible for this decline, such as the maturity of the synaptic substrate during the period of proliferative growth, hormonal environment, sensory-motor activity, early environmental experience, the number of synaptic and functional contacts available, and the number of competitors that are vying for a connection (Casagrande & Joseph, 1978; 1980; Diamond, Johnson, Mizono, Lee & Wells, 1977; Goodman & Horel, 1966; Greenough, 1976; Hubel & Wiesel, 1970; Joseph & Casagrande, 1978; Lund, 1978; Rakic, 1977; Rosenzweig, Bennet, & Diamond, 1972; see also Wolstenholme & O'Connor, 1968).
Because of neocortical (and subcortical/limbic) immaturity, and as these tissues are also experience-expectant they require considerable stimulation early in life, in order for neurons to grow, mature, divide, and establish and maintain synaptic connections. Indeed, it is well established that early environmental influences exert significant organizing effects on neural growth throughout the neocortex and limbic system, determining neocortical thickness, the density, size, shape and growth of dendrites and synapses, and the die off and drop out rate of glia, neurons, and axons. An enriched rearing environment can increase neural growth and establish billions if not trillions of synapses throughout the brain and neocortex and increase a thousand fold the number of synapses per axon (Greer, Diamond, & Tang, 1982). In the occipital cortex, for example, the dendritic fields are increased by about 20% among those reared in an enriched visual environment.
The optimal maintenance and preservation of a neuron, its axon, dendrites, and synaptic connections require not only experience-expectant stimulation, but that the neuron and terminal substrate with which it is expected to form its synaptic connection functionally mature and interact at similar times. Witness, for example, the motor and sensory systems. Cortical motor layers develop prior to sensory neurons, spinal and subcortical motor fibers become myelinated before the sensory systems (Langworthy, 1933; Milner, 1967; Yakovlev & Lecours, 1967), and the motor roots of the spinal cord are completely myelinated at birth or soon thereafter, whereas the sensory roots are not complete until the fourth to seventh month of postnatal development (Yakovlev & Lecours, 1967). Presumably, this possible coordination of substrate maturation allows the axons of the cortico-spinal tract to establish functional connections with the spinal motor neurons, whereas viable synaptic connections with processes terminating in the superficial layers of the neocortex as they mature at a later date.
We may suppose that many of those neurons that subsequently die off do so because they, their processes, or the fibers with which they were to make synaptic connections matured at the wrong time or were not functionally active or functionally maintained. On the other hand, death may be due to their terminal substrate being occupied by fibers from a competing neuron (cf. Casagrande & Joseph, 1978, 1980; Joseph & Casagrande, 1978). This is particularly important as stimulus to growth is exerted by non-occupied synaptic space which attracts axons and stimulate fiber growth (Raisman, 1969). However, as noted, the maintenance of terminal space (i.e., a neuronal cell body and its dendrites) is dependent on the establishment of functional innervations, the maintenance of which is experience-expectant.
Experience-Expectant Stimulation & Deprivation.
Abnormal and deprived early experience during specific critical developmental periods can result in the elimination of millions of neurons, axons, dendrites and synapses, and may even induce functional invasion by competing neuronal cell assemblies which establish functional dominance over those neurons that have been insufficiently or adversely stimulated (e.g., Casagrande & Joseph, 1978, 1980; Hirsch & Spinelli, 1971; Joseph, 1986b; Spinelli et al., 1972). As demonstrated in animal (Hirsch & Spinelli,1971; Spinelli et al., 1972) and in human studies (Novelly & Joseph, in press), neocortical and limbic neurons deprived of normal experience sometimes come to subserve wholly different functions. In consequence, even when normal experience is later provided, deprived neurons are unable to function normally.
For example, kittens reared in a visual environment consisting only of thick horizontal lines were later unable to see lines or objects oriented in a vertical direction. They would bump into table legs and furniture as if blind (Hirsch & Spinelli, 1971; Spinelli et al., 1972). In addition, neurons in the visual cortex became unresponsive to vertical stimuli, and instead could only react to objects oriented in a horizontal direction. Moreover, kittens that were provided considerable visual input, but were denied the opportunity to motorically interact with their environment (as they were strapped into a carriage and only allowed to move freely in the dark), were later found to be blind.
Likewise, as demonstrated in human (Freeman & Bradley, 1980) and non-human primates (Casagrande & Joseph, 1978, 1980; Joseph & Casagrande, 1978, 1980), abnormal and deprived experience during early critical developmental periods may even induce perceptual blindness. This blindness is secondary to functional invasion and occupation by competing neuronal cell assemblies which were provided normal input and then took over those neurons that have been insufficiently or adversely stimulated. For example, in non-human primates, if one eye is sutured shut soon after birth and patterned visual input is prevented from reaching target cells in the lateral geniculate nucleus of the thalamus and visual neocortex, target neurons and cellular layers and columns become smaller and functionally suppressed by adjacent cells receiving normal input (Casagrande & Joseph, 1978, 1980; Hubel &Wiesel, 1968). Cortical columns and layers innervated by the deprived eye shrink and those of the experienced eye grow larger, filling in the vacated space. Perceptual functioning becomes exceedingly abnormal and the deprived eye is unable to see objects, places, or smiling faces.
Because developing neurons are experience-expectant when denied that experience, they may cease to function normally, or come to be inhibited by competing neural assemblies (Casagrande & Joseph, 1978, 1980; Freeman & Bradley, 1980; Joseph & Casagrande, 1980). Likewise, if the developing limbic system is denied sufficient emotional and social stimulation, or if the child is subject to abuse and emotional stress, it too may become "blind" and fail to respond or respond in an abnormal fashion to social-emotional nuances even when normal input is later provided. Indeed, children (Ainsworth,et al, 1978; Bowlby, 1982; Koluchova, 1976; Spitz, 1945) and non-human primates and mammals (Harlow & Harlow, 1965a,b; Joseph, 1979; Joseph & Casagrande, 1980; Joseph & Gallagher, 1980) reared under maternally deprived, abusive, or neglectful conditions, suffer severe social, emotional, cognitive, perceptual, and intellectual deficits. These disturbances include diminished curiosity and a lack of exploratory behavior, a reduced ability to anticipate consequences or to inhibit irrelevant or inappropriate behaviors which lead to punishment, and an impaired ability to form loving attachments. Even the ability to function in a sexually normal fashion is disrupted (Langmeier & Matejcek, 1975; Moberg, 1985).
Some severely neglected and deprived children, including those who have been severely sexually abused, fail to shown any sexual drive, or they may repeatedly expose themselves and masturbate, or sexually attack other children (reviewed in Langmeier & Matejcek, 1975). Sexuality often becomes abnormal, because sexuality is associated with the functional integrity of the limbic system, the amygdala, septal nuclei, and hypothalamus in particular. If sexually abused, these structures may become abnormally sexually differentiated and/or develop other neural abnormalities which result in abnormal behavior or traumatic sexualization.
Competition and the cerebral hemispheres. Although this has not yet been well demonstrated (cf. Corballis & Morgan, 1978), for reasons yet unknown the motor cortex of the left hemisphere appears to mature embryologically at a faster rate than the right, as the axons from the pyramidal cells in the deep layers of the cortex (the cortico-spinal tract) begin to grow (Kertesz & Geschwind, 1971; Yakovlev & Rakic, 1966) and presumably establish functional synaptic connections with subcortical and spinal mechanisms in advance of the right. This suggestion is further supported behaviorally by evidence that indicates that the majority of infants demonstrate right-sided motor activity (e.g., right-sided turning of the body and head) almost four times more often than activity to the left (Saling, 1979; Siqueland & Lipsitt, 1966; Turkewitz, Gordon, & Birch, 1965). However, the left hemisphere is believed to influence if not program a large-portion of left as well as right-sided motor activity (Geschwind, 1965). Although this may be accomplished via cortico-cortico interactions given that numerous collaterals are given off by the pyramidal tract before decussating in the medulla (Brodal, 1969) and perhaps by the anterior cortico-spinal tract as it descends before crossing in the spinal cord, it is possible that both left- and right-sided subcortical and spinal motor neurons receive an abundance of left hemisphere pyramidal collaterals due to its competitive developmental advantage in establishing synaptic contact.
It appears that the earlier development of the left hemisphere is not confined to the motor layers of the cortex and its pyramidal-cortico-spinal tract, but may include the outer sensory-receptive layers as well. Ultimately, synaptic and cellular growth in these receptive layers possibly is reduced, as is the direct acquisition of afferent-sensory processes, due to the maturation and myelination of the dorsal roots, spinal afferents and post-thalamic sensory fibers not coinciding with left hemisphere development. Moreover, (albeit hypothesized) accelerated maturation (which is not reflected in size) may reduce growth and proliferation of the superficial layers in the left hemisphere as well as the eventual formation of its gyri and convolutions in a variety of neocortical areas (cf. N. Kopp, pp. 302-303, in Corballis & Morgan, 1978). Hence, although the superficial layers of the left have begun to develop in advance of the right, the later and protracted rate of development in the right hemisphere (which is in part demonstrated by its capacity to recover and acquire functions of the left subsequent to left-sided cortical damage; see Plasticity & Recovery, this paper) coincides with the later development of sensory and afferent systems, which come to be represented more greatly in these regions. In consequence, not only are a number of neocortical areas larger in the right, but during neonatal development gyral formation in some of these regions quickly surpass in size their counterparts in the left and are thus 'recognizable' somewhat sooner (Chi, Dooling, & Gilles, 1977). However, one also could argue that certain areas in the right actually begin to develop in advance of similar areas in the left, but that the maturational cycle is much more prolonged.
Nevertheless, it appears that the motor neurons and fibers within the left hemisphere (including afferents feeding back from subcortical nuclei) may act to reduce or rather come to partially occupy substrate that would potentially be available for sensory representation. That is, the motor fibers of the left hemisphere may have a competitive advantage over sensory-afferent representation in the left half of the brain due to early maturation of both the left hemisphere and the motor systems. The later developing sensory processes thus are relegated to greater representation in the right, in which they subsequently terminate in greater numbers. A situation thus evolves in which as the sensory fibers and systems develop and the sensory-afferent-dorsal roots mature, there is more available space in the later developing right hemisphere (which has less space committed to motor functions). Presumably it is this unequal representation of receptive capacity and acquisition that predisposes the right hemisphere to demonstrating somewhat earlier and greater receptive activity in the infant and adult (Andreassi, 1980; Crowell, Jones, Kapuniai, & Nakagawa, 1973).
In general, it appears that the motor-sensory dichotomy of hemispheric function may be in part a function of differences in rate of neuronal maturation as well as the availability of space and synaptic connections in the course of development. Variations in the course of these maturational events, in turn, would result in variations in functional representation and, consequently, cognitive functioning. For example, vocal development and sound production correspond to maturational stages in motor development (Lenneberg, 1967) and are related closely to the maturation of the motor roots of the cranial nerves (Lecours, 1975). Accordingly, early maturing individuals perform better on verbal tests, whereas later maturing Ss perform better on tests of spatial ability (Zurif & Carson, 1970), variations which apparently reflect functional representation of sensory and motor processes. Similarly, maturational lag is associated with language and abnormalities (Bakker, 1972; Leong, 1976; Satz & Van Nostrand, 1973), which may be due to the failure to establish sufficient anatomical representation of the necessary motor units during the critical period of motor development. However, it is important to note that sensory feedback is crucial for the development of motor precision in language functions, as well as later behavioral functioning in general (Joseph & Casagrande, 1978, 1980; Joseph & Gallagher, 1980).
It must be cautioned that evidence of anatomical asymmetries is based on gross measurements of brain volume. However, it is interesting to note that the planum temporal of the left hemisphere (which contains Wernicke's area) is larger than that of the right in the majority of infants and adults (Geschwind & Levitsky, 1968; Wittelson & Palli, 1973), whereas the right hemisphere weights more than the left (Lemay, 1976)�a function, possibly, of greater abundance of white matter and reciprocal interconnections with sensory and limbic modalities. These anatomical asymmetries are not, however, complete, as there is considerable overlap in acquisition.
NeuroPlasticity and Recovery
The right hemisphere plasticity and immaturity hypothesis is supported by a number of independent investigations, which have shown that if the left hemisphere of a young child is severely damaged, speech functions reappear, presumably mediated by the right hemisphere (Basser, 1962; Byers & Mclean, 1962; Dennis & Whitaker, 1976; Hecaen, 1976; Krashen, 1973; Lenneberg, 1967). Although the ability to structure and produce syntactic components is somewhat defective after recovery (Dennis & Kohn, 1975; Dennis & Whitaker, 1976), it appears that removal of left hemisphere synaptic influences allows the right to acquire belatedly the motor representation necessary for language. For example, in the visual system, if a single eye is given a competitive advantage in development and experience through the suturing shut of the opposing eye, its synaptic representation in the thalamus (lateral geniculate body) and visual cortex is increased, whereas the cells normally innervated by the disadvantaged eye become smaller and/or innervated by the normal eye. When reverse sutures are performed and the disadvantaged eye is opened (for example, 6-9 months after birth), the animals seems blind, and the cells innervated by that eye appear non-functional except in regions where competitive influences are null (the monocular segment). However, if the normal eye is removed surgically, the disadvantaged eye will regain function and vision returns (Casagrande & Joseph, 1978, 1980; Joseph & Casagrande, 1978, 1980).
After cerebral or peripheral damage, it has been demonstrated repeatedly that the lesion is accompanied subsequently by sprouting and regeneration of damaged and undamaged neurons such that vacated sites are reinnervated (Cunningham, 1972; Devor, 1976; Murray & Golderberger, 1974; Raisman, 1969). In mammals, however, central neurons show much less plasticity than peripheral structures (Bernstein, 1967). Nevertheless, two basic types of reconstructive changes may occur: (1) The severed axons will exhibit regenerative sprouting if its cell body remains intact; and (2) intact axons or neurons from surrounding border regions will grow processes and reinnervate a region that has been separated from its afferent input (Goodman & Horel, 1966; Raisman, 1969). These two processes then may compete for the newly available terminal space. However, the stimulus to growth suddenly will cease when the available region is reinnervated (Raisman, 1969). This demonstrates that unoccupied synaptic space acts as a powerful growth stimulus that exerts a strong attractive influence on the severed as well as intact axonal neighbors.
The fact that recovery of language function greatly decreases after age 6 (Hacaen, 1976; Krashen, 1973) indicates a decline in the availability of non-damaged synaptic space. Prior to this, it appears that the deinnervated space in the peripheral structures and deinnervated axons from the sensory regions and motor musculature that subserve expressive and receptive language act as either a possible attractive influence on fibers that originate in the right hemisphere, or regenerate and then successfully compete for synaptic space with the available substrate in the right hemisphere. Because deinnervated motor areas may compete for space in the immature right half of the brain, anatomical representation of motor functions possibly expands due to the increased pressure for motor mediation. On the other hand, with massive damage to the right hemisphere in early infancy, we would expect to find some functional (but incomplete) recovery of sensory function (e.g., spatial ability, etc.) as a greater number of sensory fibers compete for left hemisphere space. Because of this, the representation of both sensory and motor function would be decreased. This is why, for instance, early right hemisphere injury affects speech more than equivalent damage in adults (Basser, 1962; Hecaen, 1976), and why complete removal of a hemisphere in infancy affects cognitive functioning in general (cf. Kohn & Dennis, 1974).
The sensory-limbic Axis
Sensory functions, pleasure, pain, hunger, etc., are associated naturally in that all are essentially emotional and limbic. Even sensations such as light, pressure, etc. come to have limbic and thus emotional characteristics if sufficiently intense. Emotion is, in fact, somatosensory linked and interdependent. The inability to feel is the inability to experience emotion.
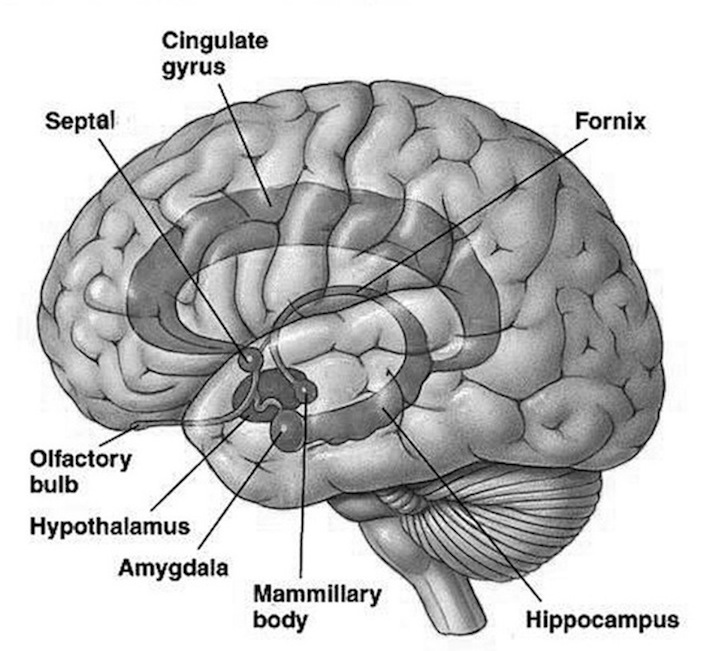
Although the right hemisphere appears to subserve emotional expression, emotionality is most clearly a function of subcortical limbic structures (Gloor, 1060; Green, 1958; Kaada, 1967; Robinson, 1967; Scoville, 1954). These structures, are in fact the major sites for the elicitation of emotional arousal (Egger & Flyn, 1962; Gloor, 1960; Green, 1958; Kaada, 1967; Ursin & Kaada, 1960) and are important mediators of sensory functions because they receive (e.g., the amygdale) projections from all sensory receptors (Gloor, 1960; Machine & Segundo 1956) and provide the neural substrate (the hippocampus) for the registration and storage of imaginal and sensory experience, and presumably memory in general (Green, 1964; Milner, 1974). Moreover, limbic structures, such as the amygdala, in addition to being the possible seat for the control of emotional tone, the enhancement of pleasure, consummatory behavior, fear, sadness, affection, and happiness (cf. Isaacson, 1974, for review) also are involved in the control of aggression (Mark & Ervin, 1970), the inhibition of emotional activity (Penfield, 1954), fight or flight responses (Ursin & Kaada, 1960), and emotional vocalization (Robinson, 1967). In this last regard, it is interesting to note that intense somatosensory stimulation and activation give rise to involuntary and emotionally connotative vocalizations, for example "ouch!"
The Amygdala, Child Development, Deprived Environments, Emotion
The development of wariness, fear, separation anxiety, and the establishment of long term emotional attachments, is mediated by, and parallels the differential rates of growth and maturation of the hypothalamus, amygdala, septal nuclei, cingulate gyrus, as well as the hippocampus.
Consider for example, fear, an emotion associated with the amygdala. The human medial amygdala is exceedingly immature at birth and doesn't become well myelinated until around the 8th postnatal month (Gilles, Leviton, & Dooling, 1983; Langworthy, 1937; Yakovlev & Lecours, 1967).
As is well known, the development of axonal myelin insulation improves neural transmission and is an indicator of functional maturation (see Grafstein, 1963). Myelinated axons do not normally undergo programmed cell death (unpublished observations).
Hence, paralleling the maturation of the medial amygdala, at 8-months, the infant first begins to experience and express feelings of fear, and then becomes progressively more fearful, such as in response to an approaching stranger (Bronson, 1972; Emde, Gaensbauer, & Harmon, 1976; Sroufe & Waters, 1976), adult male strangers in particular with female infants becoming more fearful than males infants.
This initial fearlessness (and amygdala immaturity) is exceedingly adaptive, for the generation of fear would interfere with the initial establishment of intimate emotional attachments, and the infant's need for considerable social-emotional and physical stimulation, which is initially and eagerly welcomed even from strangers. The later development of fear ensures that an infant that can crawl, will not indiscriminately crawl into the arms of a stranger, or dangerous animals, only to be whisked away or eaten.
The hypothalamus, amygdala, as well as the septal nuclei, hippocampus, and anterior cingulate, all begin to mature at different, as well as at overlapping rates, and each subserves a variety of unique as well as overlapping functions. For example, in addition to fear, the amygdala subserves the ability to form emotional attachments, whereas the hippocampus subserves memory and the cingulate is associated with separation anxiety. As these structures develop, they thus require input which may enhance memory or social emotional functioning.
Again, if denied sufficient social, maternal, and emotional experience-expectant stimulation early in development, or if reared in an abnormal, neglectful, or abusive environment, these nuclei, and other forebrain structures (including the neocortex), may atrophy, establish aberrant interconnections, and cease to function normally (Henriksen, Bloom, & McCoy, 1978), as demonstrated in humans (Heath, 1954) and nonhuman subjects (Walsh, Budtz-Olsen, Penny, & Cummins, 1969). In consequence, various or all aspects of social emotional and various aspects of cognitive and perceptual functioning may become abnormal. The brain and the limbic system require considerable stimulation in order to function normally.
For example, when experiencing hunger or thirst, the organism is motivated to seek food or water. Likewise, the experience-expectant limbic system actively seeks social stimulation, and requires emotionally "sensitive" maternal contact in order to develop normally. Hence, for the first 8 months of postnatal development infants will indiscriminately seek contact and will smile at the approach of anyone, even complete strangers (Charlesworth & Kruetzer, 1973; Spitz & Wolf 1946). It appears that the immature "experience-expectant" limbic system in fact demands emotional and social contact, and will seek it out regardless of consequences.
Hence, young animals raised in social isolation will form attachments to bare wire frames, inanimate objects, and television sets, and will seek out animals that might maul them and creatures that might eat them (Cairn, 1966; Fisher, 1955; Harlow & Harlow 1965a,b; Hoffman & DePaulo, 1977). Infant humans, non-human primates, and a variety of young creatures will desperately seek contact with mothers and caretakers who reject, physically abuse, and who nearly kill them. For example, Melzack and Scott (1957) found that dogs who were denied social emotional stimulation for the first 7 to 10 months of life, desperately sought social contact and would hover excitedly next to the experimenter even when he repeatedly stuck pieces of glass or burning matches into their snouts. Similarly, Harlow and Harlow (1965a,b) report that young monkeys would persistently attempt to make maternal contact even when severely bitten and battered. Infant humans are no different.
This "paradoxical" abusive-contact seeking behavior is not entirely surprising as the limbic system appears to be genetically programmed to respond to aversive or threatening conditions by seeking safety and contact comfort from the mother or surrogate caretaker. Hence, the infant is unable to reconcile the contradiction when subject to abuse and cannot inhibit the contact and safety seeking response, as the immature limbic system is not genetically designed to avoid abnormal mothering. On the contrary, the same limbic tissues which perceive and respond to aversive stimulation, e.g. the amygdala, cingulate, and septal nuclei (Olds & Forbes, 1981), are the same nuclei which promote and desperately require social contact and emotional stimulation.
So pervasive is this need for physical interaction and social stimulation, that when grossly reduced or denied, the result may be death. For example, in several well known studies of emotionally neglected but well nourished and otherwise healthy children warehoused in foundling homes during the early 1900's, morbidity rates for those less than one year of age was generally over 70%. Over 10,000 children were admitted to the Dublin Foundling home, and only 45 survived (reviewed in Langmeier & Matejcek, 1975).
Children reared in institutions where mothering and emotional comfort are minimized typically display low intelligence, profoundly reduced verbal skills, extreme passivity, apathy, severe attentional deficits, pathological shyness, and exceedingly bizarre social behavior (Dennis, 1975; Goldfarb 1943, 1945, 1946; Langmeier & Matejcek, 1975; Spitz, 1945, 1946). R.A. Spitz (1945, 1946) found that deterioration is in fact quite rapid, and that within less than a year unmothered children became unresponsive to social stimulation, would lay passively on their beds, and made vigorous attempts to avoid strangers or novel objects or toys. When approached they would scream and withdraw. These deprived youngsters spent hours engaged in obsessive, repetitive, stereotyped, and abusive self-stimulating movements; i.e. head banging, rocking, or repeatedly pinching the same piece of skin until sores developed.
Nor are these catastrophic consequence limited to humans, for similar disturbances characterize the behavior of other primates reared under deprived conditions. As detailed by Harlow and Harlow (1965; p. 138), monkeys raised alone, in a sterile environment, with surrogate terry cloth mothers would stereotypically "sit in their cages and stare fixedly into space, circle their cages in a repetitive stereotyped manner and clasp their heads in their hands or arms and rock for long periods of time. They develop compulsive habits, such as pinching precisely the same patch of skin between the same fingers hundreds of times a day; occasionally such behavior may become punitive and the animal may chew and tear at its body until it bleeds."
Human and non-human primates need to be held, touched, caressed, rocked, and carried about everyday for these are the adaptive evolutionary requirements of our inherited 100 million-year-old mammalian nervous system. For much of human and primate evolution infants were carried about by their mothers who would bend, twist, walk, run, climb, and gather foodstuffs with a baby strapped or clutched tightly to her body. Hence, the adaptive significance of the infant's grasp reflex; infants are provided the capacity to clutch back. And, when mother was not in attendance, the baby would likely be held by a sister, aunt, grandmother, and so on.
However, it must be emphasized that the degree of any subsequent abnormality is dependent on the nature, degree, and extent of the deprivation or abuse suffered. In less extreme case where "mothering" is inconsistent and/or if the child is seldom held or played with, the individual may merely become shy and feel awkward and uncomfortable when around others. This is because intimate physical, social, and emotional contact with a "sensitive" and attentive caretaker is a biological and limbic system necessity which directly contributes to the strength and quality of any emotional attachment and the ability to relate successfully with others (Ainsworth et al., 1978; Bowlby, 1960, 1982). When deprived of this stimulation, or when reared in an abnormal or abusive environment, the limbic system, the neocortex, and striatum, and all aspects of social, emotional, and even sexual functioning will be negatively impacted to varying degrees depending on the degree, duration, and the age at which the infant is neglected and insufficiently stimulated.
Limbic Language
Although non-humans do not have the ability to speak, mammalian vocalizations are also primarily limbic and somatosensorily mediated, being evoked in situations that involved sexual arousal, terror, anger, flight, helplessness, and separation from the primary caretaker when young (cf. Robinson, 1967). Similarly, in human infants the first vocalizations arise in response to somatosensory upheavals. Hence, primate (including human) vocalizations arise at first in a context deeply embedded in limbic activity (Jurgens, 1969; Kaada, 1967; Robinson, 1967), activity that invariably accompanies and/or calls forth involuntary and voluntary action from oneself or others for example, a signal that indicates danger, the cry of pain that accompanies a reflexive withdrawal, or a grunt involuntarily evoked as one engages in strenuous activity.
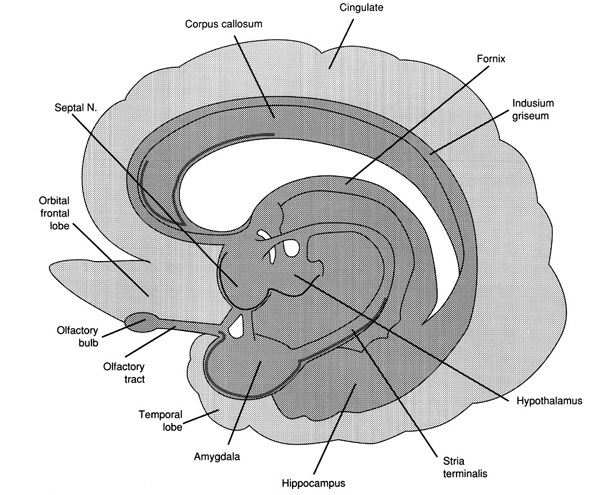
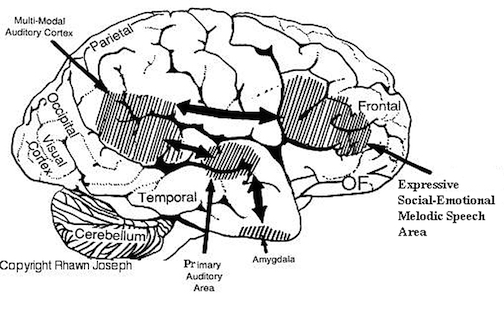
Similar vocalizations appear to arise from and are mediated by the right hemisphere and are possibly under the control or influence of the orbital regions of the frontal lobes and temporal regions, which, in turn, are outgrowths of limbic nuclei (cf. Figure 1) and which have maintained cytoarchitectural as well as functional similarities to limbic cortex (Kaada, 1967; Maclean, 1954). It is little wonder that with massive left hemisphere lesions that result in loss of expressive and receptive language, the ability to swear, cry, sing, and even pray may remain intact, as well as the capacity to respond to non-linguistic, auditory stimuli (Geschwind et al., 1968).
"Language was originally a system of emotive and imitative sounds--sound which express terror, fear, anger, love, etc., and sound which imitate the noises of the elements: the rushing of water, the rolling of thunder, the roaring of the wind, the cries of the animal world, and so on; and lastly, those which represent a combination of the sound perceived and the emotional reaction to it. --Jung
Apparently, right hemispheric involvement with emotional functioning is due to greater abundance of reciprocal interconnections with the limbic system. As noted, the later maturation of the right hemisphere may coincide with the maturation and myelogenetic cycle of the post-thalamic exteroceptive and proprioceptive fiber tracts (which object from thalamus to cortex), which are not complete until the end of the first year post-partum (Lecours, 1975). In that the dorsomedical nucleus of the thalamus receives major fiber connections from the amygdale, has connections with the temporal and orbital regions (Nauta, 1962), and is involved in vocalization as well as sensory functions, the possibility exists that right hemisphere emotionality is due to the availability and subsequent representation of thalamic-cortical-limbic fibers and nuclei in the later maturing right hemisphere substrate, which comes to subserve and mediate limbic functioning.
The Limbic Origins of Psyche: Emotion, Hunger, Thirst, Pleasure, Pain
The Primary processes are present in the mental apparatus from the first, while it is only during the course of life that the secondary processes unfold, and come to inhibit and overlay primary ones. In consequence of the belated appearance of the secondary processes, the core of our being remains inaccessible to understanding. --Freud
Prior to the migration of neuroblasts from the mantle layer into those zones that will become the deep motor layers of the cerebral cortex, the corpus striatum (basal ganglia and other subcortical motor areas) and diencephalons-rhinencephalon (thalamus, hypothalamus, hippocampus, amygdala, and other limbic nuclei) have begun to form and differentiate, and by birth have become functional (Arey, 1965; Hamilton & Mossman, 1972). Because the cortical motor areas do not establish functional control for some time after birth, and because of the later beginning and completion of the myelogenetic cycle of the cortico-spinal tract (eighth month of gestation to seventh month post-partum, Yakovlev & Lecours, 1967), most motoric output, including the control over reflexes, is mediated by the more advanced subcortical motor centers and spinal cord disappear within several months after birth (Brodal, 1969; Langworthy, 1933; McGraw, 1969; Milner, 1967) and reappear after extensive cortical damage. Thus the newborn's behavior largely reflects subcortical and limbic activities (Milner, 1967). Because the outer sensory and association layers of the cortex still are forming and due to the later completion of the myelogenetic cycle of the post-thalamic sensory fibers that project to these layers, sensory analysis and responding is largely limbic and thalamic.
According to Milner (1967), during the first month after birth the 'sole evidence of neocortical functioning, as well as the infant's reactivity [is] almost entirely [directed] to stimuli impinging directly on the body-surface,' and that 'sensations transmitted by the skin senses (including the mouth) provide the entirety of the neonate's experience; that is, the neonate is essentially internally oriented' (pp. 179-180). Hence, psychic functioning in the newborn is characterized by a vague, undifferentiated awareness, and a multitude of excitatory and inhibitory interactions and a series of transient feeling states and emotional upheavals that lack psychological referents or distinct features. Rather, the referents of feeling states, or limbic activations, are general and diffuse, being aimed at the reactivation of experience associated with the alleviation of displeasure or with the experience of pleasurable or painful affect. In fact, from birth to 1 month, the infant displays only two attitudes, accepting and rejecting (Milner, 1967). Because the limbic system monitors and responds to internal and external sensations (e.g., hunger, pain, etc.) with only minimal inhibitory regulation by the newborn's neocortex, most if not all 'behavior' is governed by brain stem and limbic concerns, including that of the first (and later) vocalizations: limbic speech. Hence, in response to hunger, the infant cries, and to pleasure it gurgles and babbles.
Visual Imagery. Although, admittedly, we have no direct knowledge as to psychic interactions in the neonate, it does seem reasonable to assume that as the cortex and underlying structures and fiber pathways mature, neural 'programs' are formed. That is, fiber pathways that are repetitively fired, deactivated or activated in response to sensory and affective activity, become associated with that activity, and when appropriately triggered subsequently can replay this 'learned' pattern (Penfield, 1954, Spinelli, 1970). With the repetition of affective states and accompanying motor outputs and sensory impression, certain external and internal states, actions, movements, stimuli, etc., become endowed with 'significance' and are retained as neural 'programs' and patterns of neural activity that may be revoked or 'triggered' by associated activity or stimuli. A form of 'learning,' so to speak, has occurred. When replayed, the organism presumably reexperiences to some degree the sensations, emotions, etc., originally associated and repetitively experienced. In regard to the neonate and infant, it is presumably these mechanisms that first give rise to dream-like ideation and imagery (cf. Freud, 1900).
Thus, for example, as the infant experiences hunger and stomach contractions as well as its cries of displeasure, these states become associated with the sound, smell, taste, etc., of mother and her associated movements and other stimuli that accompany being fed (cf. Piaget, 1952, pp. 37, 407-408). Repetitively experienced, the sequence from hunger to satiety evoked and becomes associated with the activation of certain neural pathways. Eventually, when the infant becomes hungry, if prolonged there is the possibility that the entire neural sequence associated may become activated and an 'image' experienced (manifested behaviorally through sucking and tongue movements in the absence of food; cf. Piaget, 1952). The organism experiences the experience of being fed. In that these sensory-motor-limbic associations make up the bulk of the neonate's experience, these rudimentary representations (images) may in themselves be indistinguishable from experience simply because in themselves they are experience. Thus the young organism, as yet unable to distinguish between representation and reality, may respond to the image as reality (cf. Freud, 1900). That is, for example, when a neural sequence is activated such as due to prolonged hunger, the infant responds to the neural 'experience' with associated neuronal activity (i.e., being fed), and for a brief period the neonate reacts as though its hunger were being satiated. Reality is replaced by an image, or rather a 'dream.' However, the organism is not long fooled, for as the pain of hunger remains and increases, limbic activity is increased, and the image falls away to be replaced by a cry of hunger and perceptual surveillance.
As the child ages and the cortex matures, the failure of the limbic induced sensory-motor neural patterns to satisfy, relieve, or achieve the ends for which they were activated impels the organism to pay renewed and ever-increasing attention to the external environment. Eventually, aided greatly by the maturation of the motor centers in the left hemisphere, the infant is able to attend selectively and to distinguish partially between reality and image and to create representations of reality in a motor context, which may be manipulated to effect the changes or satisfy the impulses desired. For example, the infant purposefully may cry or call 'mamma.'
It is the establishment and elaboration of these rudimentary patterns of neural activity that eventually provide the foundations for psychological and cognitive development.
Limbic Language and Egocentric Speech
Limbic language heralds the founding drive from which all purposeful and intellectual activities develop. Language springs from roots buried within the limbic system (cf. Jung, 1954). Limbic speech, however, is basically concerned with the expression of feelings, moods, impulses, desires, etc., which serve as commands and accompaniments to action, or may be represented subtly in tone or inflection. Nevertheless, limbic speech is not bound up with thinking, the expression of thought, or reflection, although symbolism and imagery may be inherent in its production. Limbic speech, although communicative and thus social, may occur independent of thought yet may be evoked by thought, or may provide the undifferentiated matrix from which th0ught at times originates. It is, however, primarily emotional, automatic, and yet symbolic, as a command or accompaniment to action or desire. Crying may bring forth food or other forms of somatosensory relief, babbling accompanies pleasure or social contact, or may result as a form of self-simulation, and 'mamma' brings forth mamma and all her accompanying attributes.
Initially, however, cries, babbles, or, for example, the word 'mamma' do not signify the infant's feeling states, desires, etc. Rather, these are limbic induced motoric responses to the evocation of a diffuse feeling that merely signifies itself. 'Mamma' thus can mean 'mamma come here,' 'mama give me,' 'mama I hurt,' 'I am hungry,' etc. (Piaget, 1952; Vygotsky, 1962). Although without precise definition, once vocalized it remains a means of communication. The earliest forms of communication and thus social speech, therefore are embedded in limbic activity, for limbic speech provides a context within which associations may be formed and schemas developed. Language slowly develops from the construction and association of these schemas and vocalization-experience pairings. But at first, language is only a servant to the limbic system and represents limbic needs of the organism in the form of motor output that carries beyond the crib to which the infant is confined, crossing boundaries that later will be traversed by legs and body as immediate calls to action or an immediate exercise in motor functioning.
As the left hemisphere continues to mature and develop, wresting control of the peripheral and cranial musculature from subcortical influences, a second aspect of language emerges, one that arises through interactions and nominal associations with external stimulatory activities and denotative speech. Although it springs forth from relationships originally limbic based, denotative (or social) language is concerned with nominal functions and denotative statements of fact or belief and statements of assertion. As such, denotative social language is closely bound with cognitive activities and the eventual expression of one's thoughts. Thinking, however, does not appear until much later in development. Moreover, although a semi-independent motor function, it remains influenced by social-limbic language throughout life.
Egocentric speech. According to Vygotsky (1962), egocentric speech makes its first appearance at approximately 3 years of age. According to Piaget (1952, 1962, 1974), at its peak, egocentric speech comprises almost 40-50% of the preoperational child's language; the remainder consists of social speech (denotative and emotional). Prior to this development, communication is directed strictly toward outside sources. There is no attempt to communicate with the self, for there is no internal dialogue because thought has not yet developed. At age 3, egocentric speech the peculiar linguistic structure from which thought will arise appears in the context of social-denotative vocalizations (Vygotsky, 1962). It is an essentially self-directed form of communication, which heralds the first attempts at self-explanation. It is essentially speech for oneself (Piaget, 1962; Vygotsky, 1962).
While the child is engaging in egocentric speech, he does not appear concerned with the listening needs of his audience simply because to all appearances his words are meant for his ears alone (Piaget, 1952, 1962, 1974; Vygotsky, 1962); he seems to be thinking out loud (Vygotsky, 1962). When engaged in an egocentric monologue there is no interest in influencing or explaining to others what in fact is being explained, and the child will keep up a running accompaniment to his actions, commenting on his behavior in an explanatory fashion, even while alone. Moreover, while engaged in this self-directed external monologue the child appears oblivious to the responses of others to his statements (Piaget, 1962; Vygotsky, 1962).
While the child is engaging in egocentric speech, he does not appear concerned with the listening needs of his audience simply because to all appearances, his words are meant for his ears alone (Piaget, 1952, 1962, 1974; Vygotsky, 1962); he seems to be thinking out loud (Vygotsky, 1962). When engaged in an egocentric monologue there is no interest in influencing or explaining to others what in fact is being explained, and the child will keep up a running accompaniment to his actions, commenting on his behavior in an explanatory fashion, even while alone. Moreover, while engaged in this self-directed external monologue the child appears oblivious to the responses of others to his statements (Piaget, 1962; Vygotksy, 1962).
Egocentric speech, although accompanying behavior, initially does not parallel action. Rather, it appears after the action has occurred (Piaget, 1952, 1962, 1974; Vygotsky, 1956). It is only as egocentric speech becomes progressively internalized that the child's comments and explanations occur earlier in the sequence of expression, until finally the child begins to explain his actions before they are performed.
Egocentric speech presents us with a curious anomaly, for we must accept that the child knows what he has done without commenting or explaining his actions; moreover, he must know hwy he has performed certain actions without need of explaining them to himself. Nevertheless, the fact that he explains and comments upon his behaviors after they occur argues otherwise. Paradoxically, the child acts as both actor and witness, explainer and explained to. Clearly, the child explains his actions to himself (Vygotksy, 1962).
According to Vygotksy (1962), after its initial appearance and elaboration, egocentric speech becomes steadily more involuted and internalized and thus progressively more covert. At its overt maximum, its traits and structures are being internalized and strengthened and so comprise a greater portion of the child's cognitive activities than may be witnessed. Egocentric speech, therefore, never diminishes, but continues to increase until it becomes completely inner speech at approximately age 7. The child then has learned to think words as well as to speak them, and to think them in a temporal and organized sequence that retains its original and primary function of self-communication.
As we know, language and its comprehension are subserved by the left hemisphere in both children and adults. Although egocentric speech contains limbic and thus right hemisphere components, it is also primarily left hemispheric because it is an elaboration of basic left hemisphere motor functions, the linguistic manipulation and organization of stimuli and sensations. That egocentric speech appears initially only after the action has occurred, indicates that the left hemisphere of the young child is responding to impulses and actions initiated outside its immediate realm of experience and understanding. It seems that the left hemisphere in the production of egocentric speech is attempting to organize the results of its experience (the observation of behavior) into a meaningful sequence, which it then linguistically communicates to itself; experiences within which it participates as a passive observer of impulses to action initiated elsewhere.
Interhemispheric Communication. The appearances of egocentric speech, its elaboration and eventual internalization occurs in response to several maturational changes in the central nervous system. Interestingly, although both hemispheres in the course of development increase their interactive influence upon the subcortex and periphery, communication between the hemispheres is exceedingly poor or nonexistent prior to age 3 (cf. Salamy, 1978) and very limited until approximately 5 (Galin et al 1977, 1979; Gallanger & Joseph, 1982; Kraft, 1977; O'Leary, 1980; Joseph et al., Note 1). Presumably, this is a function of the immaturity of the corpus callosal fiber connections between the hemispheres, which do not become completely myelinated until the end of the first decade (Yakovlev & Lecours, 1967). In fact, as recently demonstrated by Joseph and colleagues (Note 1), communication is so poor that when pictorial stimuli are presented tachistosopically to the right hemisphere, young children (age 4) will respond when questioned with large information gaps, which they erroneously fill with confabulatory responding. It thus appears that the left hemisphere of a young child has at best incomplete knowledge of the contents and activity that occur in the right.
Indeed, in that the right hemisphere retains access to the motor systems, the necessity for as well as the peculiar self-explanatory nature of egocentric commentary becomes understandable. The left hemisphere is in fact explaining behavior and impulses to action initiated by the right; information that the left hemisphere does not have direct access to due to the immaturity of the corpus callosum (Joseph et al., in press).
At one moment therefore, the infants acts impulsively, unrelated to dangers of reality and uninfluenced by them; he may attack a loved person or destroy a toy, his anger moves easily from one person or cause to another. On the other hand, an understanding and regard for the consequences of actions, a piece of reasoning, may appear intermittently as representations of higher ego activity, and interfere with the infant's free expression (Anna Freud, 1951; p. 23).
Essentially, egocentric speech is a function of the left hemisphere's attempt to organize and make sense of behavior initiated by the right half of the brain. Because interhemispheric communication is at best grossly incomplete, the left utilizes language to explain to itself the behavior in which it observes itself to be engaged. As the commisures mature and information flow within and between the hemispheres increases, the left also acts to organize linguistically its internal experience. As the organism develops, interhemispheric information exchange is increased (Joseph et al., Note 1) and the language axis (Broca's, Wernicke's, and the angular gyrus regions) increasingly acts to organize (as well as inhibit) sensory-limbic right hemispheric transmission and initiated behaviors, organizing these impulses as it organizes impulses that originate in the left cortex, so that they may be efficiently and motorically carried out. Rather than passively observing the sensory-limbic actions as they occur in the environment, as the commisures become complete, the left hemisphere now actively engages in the formulation oh behavior, achieving understanding prior to its occurrence. Essentially, commisural transmission allows the left hemisphere access to right hemisphere impulses-to-action before the action occurs rather than forcing it to make sense of the behavior after its completion.
Nevertheless, it is important to note that the resulting organization that we have defined as thought (Joseph, 1980) need not be solely linguistic, buy may consist of imaginal, spatial, pictorial or other components (cf. De Groot, 1965). In addition, as noted elsewhere (Joseph, 1980) understanding is not dependent on thought.
Although a multitude of structures and fiber pathways are involved in the final formulation of egocentric speech, thought, and language, their appearance is dependent on and a product of the language axis and a multiple series of interactions between thalamic nuclei, the angular gyrus, Broca's and Wernicke's area. Although they will not be discussed, frontal-reticular interactions necessarily are involved in the excitatory and inhibitory processes that allow for selective acquisition and rejection of various stimuli and informational sources (cf. Como, Joseph, Fiducia, & Siegel, 1979; Joseph, Forrest, Fiducia, Como, & Siegel, 1981; Luria, 1973). In addition, the actual formation of thought appears to retain in some manner a functional linkage with peripheral motor structures. For example, while engaging in logical explicit forms of linguistic thought, both manual and oral regions of the body musculature become activated as measured by EMG (McGuigan, 1978). In part, this is probably due to the activation of Broca's area, which is immediately adjacent to the supplementary motor areas that subserve the lower facial region.
Thalamus. Thalamic nuclei presumably act in an intermediary fashion in the formulation of thought, aiding in the temporal analysis and categorization of information during the initial stages of thought construction. As a possible intermediary, the thalamus, via its rich interconnections with all sensory cortices (Truax & Carpenter, 1969), may act to access various sources of information and to perform some initial integrations. If indeed this formulation is correct, thalamic structures such as the pulvinar (cf. Brown, 1975; Fazio, Sacco, & Bugiani, 1973; Penfield & Roberts, 1959; Van Buren & Borke, 1969) and ventro-lateral nucleus (cf. Fazio et al., 1973; Van Buren & Borke, 1969) probably play an important role in the regulation, differentiation, and selective attention to various sources of information. Moreover, the thalamus may act to coordinate the activities of the cortical language areas (Penfield & Roberts, 1959) at least in the initial stages of information selection.
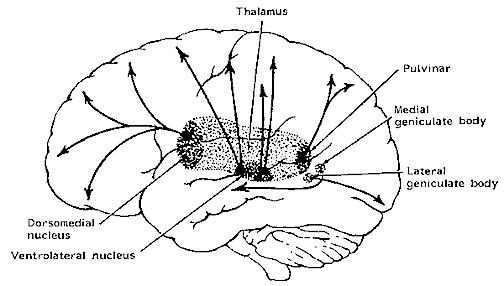
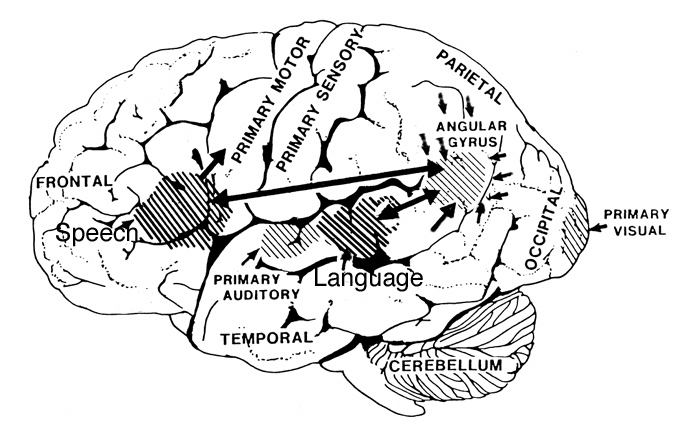
Broca's area. Broca's speech area is literally the final pathway by which thought, emotion, and other impulses come to be organized as motoric articulations for verbal communication and thought formation. Located at the foot of the third operculum in the secondary motor association area of the left and right lateral surfaces of the frontal lobes (cf. Figure 2), it is via this structure that impulses are transmitted to the adjacent motor neurons that subserve speech and the facial area (tongue, jaw, throat, lips), programming the musculature for the production of spoken language (cf. Geschwind, 1979). Although the left motor association cortex (Broca's region) receives an abundance of information via the arcuate fasciculus transmitted from the posterior speech zones (Wernicke's and the angular gyrus), due to the terminal arborization of all sensory regions in the frontal areas and the extensive connections maintained with the limbic cortex (Jones & Powell, 1970), these structures also have access to a variety of divergent forms of information and may play a role in the inhibition of stimuli or sensations deemed unnecessary or undesirable (Como et al., 1979; Luria, 1973). That is, decision-making may occur in the frontal regions (cf. Luria, 1973).
Wernicke's area . Wernicke's area lies in the secondary auditory association cortex in the posterior portions of the temporal lobe between the auditory cortex in the posterior portions of the temporal lobe between the auditory cortex and the angular gyrus, and shares fiber connections with these regions as well as with Broca's area (Geschwind, 1965, 1979). It appears that the underlying phonemic structure of all propositional thought and speech arises in this region. In conjunction with Broca's and the angular gyrus, this structure also plays an interdependent role in the formulation of programs for specific utterances because it supplies information to both poles of the language axis. Wernicke's region also plays a role in the decoding, labeling, and analysis of speech, aiding in comprehension of both written and oral language (Geschwind, 1965, 1979; Luria, 1973). Hence, when a sound is heard it is received in the auditory cortex of the temporal lobe, which transmits the impulse to Wernicke's area, where it is encoded and then transferred to other brain regions for associational analysis and comprehension. Similarly, in the organization of thought, the original signal and associations are transmitted to this area, where further encoding and labeling are performed, and the underlying structure is reinforced.
Angular gyrus. The angular gyrus, like the frontal lobes, is involved in the assimilation of diverse information variables, their integration, the calling-up of relevant associations, and function as a necessary intermediary for all conscious functioning, particularly in the development and comprehension of language and thought. Situated at the junction of the primary projection areas for vision, hearing, and somesthesis, and maintaining rich interconnections with the integration areas of the thalamus, this region is involved in the association of various stimuli, the assimilation of associations (Critchley, 1953; Geschwind, 1965, 1979; Luria, 1973; Truax & Carpenter, 1969), and the calling up of further information as an aid to left hemispheric comprehension. Through its involvement in the construction of cross-modal associations, the angular gyrus increases the capacity for the organization, categorization, and labeling of sensory-motor events. Intermodal association thus allows for motoric representation and sequential enactment as well as for more abstract representation of experience. Consequently, memory capacity is increased due to the multiple inter-classifications of associational inputs.
When activated via information transfer from other cortical/subcortical regions, the angular gyrus supplies a visual and auditory code that enables more precise linguistic labeling via Wernicke's area. When a word is read, the pattern of visual input is transmitted to the thalamus, inferior temporal region, and the angular gyrus where analyses are performed. The information then is partially integrated and transmitted to other associational structures for further processing; all of which is then transmitted to the frontal and angular region for further associational and motoric integration and temporal sequencing. Conversely, when a word is heard or a question is posed, the information is transferred to the auditory cortex and its associational areas, where the message is stabilized, prolonged, and its temporal features and phonemic components analyzed and properly labeled. The message then is sent to diverse regions, such as the angular gyrus, where cross-modal associations such as visual, somasthetic and other sensory-motor concomitants are aroused, integrated, organized, assimilated, and finally comprehended. The next stage then is initiated and the answer is sought out through the activation of closely linked associations. These are then organized, labeled, comprehended and the reply stated.
Neurodynamics and Disconnection Syndromes
As we are now well aware, the developing organism is extremely vulnerable to early environmental influences, such that both nervous system and behavior may be dramatically altered as manifested in the adult (Casagrande & Joseph, 1978, 1980; Greenough, 1976; Joseph & Casagrande, 1978, 1980; Joseph & Gallagher, 1980; Joseph, Hess & Birecree, 1978; Langmeir & Matejcek, 1975; Rosenzweig et al., 1972). In that the emerging human organism is asymmetrically arranged, with apparently little interaction and informational exchange between the cerebral hemispheres, the effects of early 'socializing' experiences could have potentially profound effects indeed. As a good deal of this early experiences is likely to have its unpleasant if not traumatic moments, it is fascinating to consider the latter ramifications of the early emotional learning occurring in the right hemisphere unbeknownst to the left; learning and associated emotional responding which may later be completely inaccessible to the language centers of the brain even when extensive interhemispheric transfer is possible (cf. Risse & Gazzaniga, 1978, for evidence that bears directly on this phenomenon). In this regard, the curious asymmetrical arrangement of function and maturation may well predispose the developing organism to later come upon situations in which it finds itself responding emotionally, nervously, anxiously, or 'neurotically' without linguistic knowledge, or without even the possibility of linguistic understanding as to the cause, purpose, eliciting stimulus, or origin of it behavior. Instead, like the egocentric child, the individual may be faced with behavior that he may explain only after it occurs: 'I don't know what came over me.'
Similar manifestations, however, may occur that are unrelated to early environmental nonlinguistic emotional learning, but result from inhibitory actions at the level of the cortex which prevent the language axis from receiving information as to the cause of the organism's behavior, or even that an impulse, desire, behavior, has emerged or occurred.
Isolation of the speech area. In children, the language centers not only are limited in their access to processes occurring in the right hemisphere, but also are not fully linked with all nervous centers in the left half of the brain (Lecours, 1975; Joseph et al., Note 1). In part, this is due to callosal immaturity and the fact that the cortex has not developed fully (Arey, 1965; Milner, 1967). The language axis of the child, therefore, may appear to be in many respects isolated from sources of information that occur in the remaining neural-psychic apparatus and thus may appear 'ignorant' when questioned about these processes (Gallagher & Joseph, 1982; Joseph et al., 1984). Functionally, in normal adults, a similar manifestation of speech area isolation at times may be evident.
As has been demonstrated in brain-injured individuals (Geschwind et al., 1968), the speech area may become completely isolated from the surrounding cortical mantle, and, if intact, form a functional unit. That is, the individual may retain the ability to repeat questions and sentences, although propositional speech is absent and linguistic comprehension of internal and external events is no longer possible. Thus, although communication between Wernicke's, Broca's and the angular gyrus is maintained, associations from other brain regions cannot reach the speech center. This is in fact called 'isolation of the speech area' (Geschwind et al., 1968). In the case described by Geschwind et al. (1968), the patient had suffered massive cortical damage, but surprisingly, although the surrounding tissue was destroyed, the speech area was completely intact. The examiners noted when the patient regained 'consciousness' that 'she sang songs and repeated questions. On several occasions when the examiner said, 'ask me no questions' she would reply 'I'll tell you no lies,' or when told, 'close your eyes' she might say 'go to sleep.' An even more striking phenomenon was observed early in the patient's illness. The patient would sing along with songs or musical commercials' (pp. 343-346). Nevertheless, she appeared to be wholly without comprehension.
Although complete isolation of the speech area may result only following severe injury to the brain, indeed, it does not require a skilled neurologist to recognize as thought their language axis does not have access to processes that occur elsewhere. Such a condition may occur when an individual would rather not be 'conscious' of knowledge he possesses and thus refuses to attend to or linguistically organize it (Joseph, 1980). Presumably, all access channels between the speech area and the neural pathways between modalities are shut down, which isolates the speech areas and the neural pathways between modalities are shut down, which isolates the speech area from its knowledge source. Not surprisingly, when questioned, especially in regard to embarrassing, threatening, or otherwise undesirable information, the language apparatus indeed has no knowledge of these processes and will respond accordingly. In such instances we in fact seem to be conversing with a self-maintained closed circuit that has established or resulted from the establishment of massive inhibitory interactions. Thus, isolated, an aroused or sought-after source of information is prevented from arriving at the language axis or becoming integrated with the major stream of activity through inhibition of the neuronal circuitry that subserves its transference to and expression by the speech centers. Such interactions, however, should not be presumed as indicative of unconscious processes.
Unless we accept the notion that the inhibition of this information transfer is automatic, such that the neuronal field creates its own inhibitory surround so as to prevent transmission, there is involved a necessary acknowledgement of the presence of this 'undesirable' material in order to inhibit its expression. As such, the isolation cannot be complete, and there must be at least some information transmission and cross talk between brain areas so that inhibition may be initiated and maintained via inhibitory feedback. Because the isolation cannot be complete (barring brain injury thus causing an anatomical separation), there must be some information leakage, which, although incomplete, may appear in fragmentary form within the language axis. How much appears would of course be a function of its own stimulus strength and the strength of inhibition.
Stimulus Anchors and the Train of Thought
In most instances in which an area of the brain is activated via internal or external probes, a train of material closely associated with the input stimuli is co-jointly, concurrently, and subsequently aroused in response. For example, if one is asked: 'What did you do in school today?' a number of associations are aroused and integrated in response to the stimulus input, all of which bear in some manner on each element of the eliciting stimulus. Finally, in the process of associational linkage, those with the strongest stimulus value, that is, those that most closely match the question in terms of appropriateness and thus with the highest probability of being the relevant, desirable, and required response, or those with the strongest stimulus strength or arousal value (Jung, 1954), rapidly take a place in a hierarchical arrangement that is being organized in a form suitable for expressing a reply (cf. Frijuda, 1972; Luria, 1973; Reitman, Grove, & Shoup, 1964). Hence, associated material of a lower probability, although aroused, nevertheless is dismissed quickly or deactivated due to its low associative value, undesirability, as well as its inappropriateness in the rapidly developing response hierarchy (Frijda, 1972; Jung, 1956).
In regard to the above question, the stimuli 'What, did, you do, in school' and associated contextual cues (Koffka, 1935) become anchors around which a train of highly associated material (Ach, 1951) is aroused, arranged, individually matched and group matched (cf. Frijda, 1972). The anchored associations that then match all sources of relevant input with sufficient value of probability act as templates of excitation that stimulate and attract other relevant associations, which then are assimilated and associated or subsequently deactivated due to their low probability in contrast to the associations already organized. Moreover, because the train of closely linked associations change in correspondence to the developing hierarchy, subsequently aroused and assimilated associations may come to have a lower probability of association within the matrix of overall activity and may be deactivated (cf. Reitman et al., 1964). The final product of this hierarchical arrangement of mutually determining associational linkages is the train of thought--a coherently organized linear sequence of interlinked associations.
The train of thought is a result and a reaction to internal and external stimulus input. It is a result of an attempt to make sense of a wide variety of information variables; the linkage of associations for the purposes of labeling, categorizing, and thus understanding (Ach, 1951; Joseph, 1980). However, what is understood is not the initiating stimulus (at least with regard to impulses that originate internally), but the resulting organization of associations. Comprehension is based on an interpretation, an interpretation which, in many instances, may be related only loosely to the original signal, impulse, need, or desire. Moreover, if the information or signal is not fully amenable to linguistic or visual labeling, or is only weakly or incompletely available for interpretation or associational linkage (due, for example, to its partial inhibition), then the speech area, having to select from a flood of probable but not completely valid associated probabilities, will organize and express those that seem to be most likely or which have the greatest strength of arousal or association (cf. Geschwind, 1965; Weinstein & Kahn, 1950; with regard to confabulation). In such instances, the signal is incompletely and thus incorrectly interpreted (depending on the degradation of the signal). As such, the individual may respond with confabulation, the formation of symptoms, or other forms of disturbance. Hence, the individual may grossly mislabel his feelings, impulses, desires, as well as the 'causes' of his behavior.
For example, let us take a stimulus that elicits a strong (lateralized) emotional reaction (e.g., intense jealousy). If the context is entirely inappropriate for its expression or realization (due to personality structure, upbringing, self-concept, situational determinants, etc.) inhibition will occur at some level of the system such that the fiber pathways that lead from the region of excitation to the language axis of the left hemisphere are functionally suppressed or deactivated. In such an instance, although fully represented in the non-linguistic portions of the neural-psychic apparatus, the linguistic-motor aspect of consciousness would in fact be ignorant of this upheaval, its significance, or the stimuli through which it was elicited. If we were to ask the left hemisphere if it is 'angry,' 'sexually aroused,' 'fearful,' 'jealous,' etc., it would truthfully respond 'no.' However, as noted by the behavior of the child who engages in egocentric speech, although non-labeled or inhibited from reaching the language axis, the source of arousal still may find expression or at least influence behavior or cognition in subtle ways. Hence, the left hemisphere taking note of these subtle effects (e.g., sweaty hands, tense muscles, fast heartbeat, etc.), may instead not the presence of some 'queer' feeling, call forth possible associations, and interpret accordingly (e.g., hungry, nervous, in need of exercise, etc.).
On the other hand, the signal or impulse may be completely intact, yet the individual may not have the correct associations available for interpretation. A similar circumstance may occur with questions that originate externally. The individual then may respond with a 'tip of the tongue' experience. Usually, however, if the correct associations are not available, several related associations may be formulated subsequently in response. Although not completely appropriate, if these associations are of sufficient associational probability and are in turn supported by other high probability associations such that all are mutually reinforcing, the train of linkage will become 'conscious' and even though erroneous, possibly vocalized and believed. The individual then would be making a guess or drawing an erroneous conclusion. In most instances, however, incorrect possibilities are deactivated, or, once expressed, discarded or corrected in response to disconfirming evidence. Of course, in some individuals an incorrect statement is maintained or upheld regardless of the availability of the correct or disconfirming evidence. These individuals are called liars.
Alpha and Omega. It would be quite erroneous to assume that the language axis of the 'normal,' 'well adjusted' brain has unrestricted access to all functional knowledge sources, for the language axis has only a limited capacity and can access and accurately report only a small bit of the information amenable to linguistic coding. Moreover, not all impulses, feelings, desires, fears, cravings, knowledge, etc., have a label and thus are not readily translatable into the codes that give rise to thought and language (Joseph, 1980). It seems that emotional expression in particular is easily amenable to misinterpretation, misidentification, and misunderstanding. The same seems to be true of most cognitive processes, however, for as Neisser (1967), Nisbett and Wilson (1977) have pointed out, most individuals have no understanding of what is involved and on what basis evaluations, judgments, problem-solving strategies, and reasons for initiating certain behaviors were arrived at. This is largely due, however, to the dependence on language for, simply, the language axis and the sequential, organizational processes which subserve consciousness in the form of thought have no direct access to the basis of these 'processes' in part because they result from them.
One's Self, the source of thought, and the Whom to whom one's thoughts are presented and explained, should not be viewed as localized in the language centers of the brain, or, for that matter, the left hemisphere. Unfortunately, the Self is most often and most readily identified with the expressible components of individuality, whereas right hemispheric, non-linguistic, non-explicit, subcortical and emotional aspects are not. This is absurd, of course, for although we are able by power of our marvelous abstraction and labeling abilities to fragment the Self into identifiable components, the fabric from which the Self and consciousness arise includes the whole person: right, left, subcortical and peripheral, as well as the environment in which one exists. As such, the Self and consciousness are a continuous process of interrelationships that defy precise localization.
REFERENCES












