Rhawn Gabriel Joseph, Ph.D.
Brain Research Laboratory
BrainMind.com
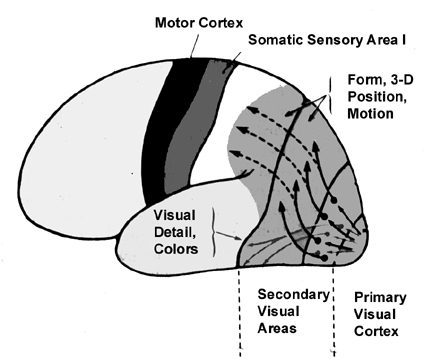

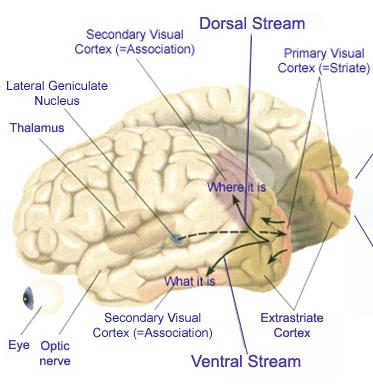
Likewise, visual functioning is not restricted to the occipital lobes for over half the primate neocortex is also concerned with visual functions (Reid, 2012; Zeki, 2007). In fact, although much of thalamic-visual- retinal input is directed to the primary visual receptive areas in the occipital lobe (Broadmann's area 17), visual input is also transmitted directly to these other visual areas such as the temporal and parietal lobes, including signals related to rapid motion and object recognition (Haxby et al., 2015; Sereno et al. 2015; Zeki, 2007). For example, and as detailed in chapters 20 and 21, the superior parietal lobe also contains visual cortex and receives peripheral and lower visual input via the optic radiations, whereas the middle and inferior temporal lobes receive foveal and upper visual field input via these fibers (Doty, 1983; Kaas & Krubitzer 2015; Lin et al. 1982; Sereno et al. 2015).
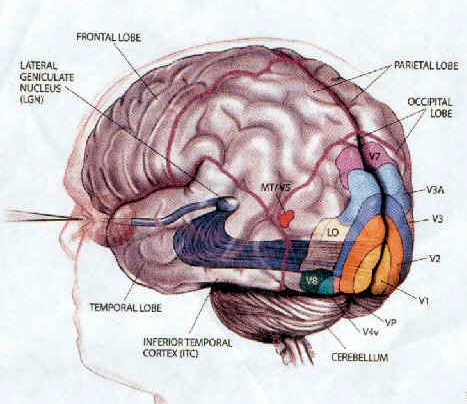
There are thus parallel and independent as well as semi-independent visual projection/reception systems within the occipital lobe as well as surrounding visual areas, with the different areas being functionally specialized to analyze different visual details such as color, motion, and object recognition (Zeki, 2007). For example, as based on functional imaging, a cortical area located at the lateral occipital temporal border (referred to as V5), can be independently activated by rapid visual motion, whereas parallel activation of area 17 (also referred to as V1) is not always observed (Zeki, 2007). As will be discussed below, this independent and semi-independent organization of the visual cortex (and other parts of the brain) and the fact that there are mutliple visual areas each of which are functionally specialized, has important implications regarding "blind sight" as well as other disconnection syndromes (see chapter 2). That is, these extensive and multiple visual areas also promote multiple fields of visual conscious awareness.
There are in fact, over 30 different visual areas that have been distinguished as based on functional specificity, chemistry, physiology, and cytoarchitexture (Felleman & van Essen, 2015; Zeki, 2007). That so much of the human/primate neocortex is concerned with visual functioning is in part a reflection of our arboreal and semi-carnivorous ancestry. That is, early in our evolutionary history, the eyes migrated to the front of the head thereby providing for stereoscopic vision, and adaption which promoted life among the trees and the hunting and killing of small animals. Moreover, cortical layers I and VII (6b) are phylogentically ancient in organization and structure and resemble and may well be an extension of midbrain tissue (Marin-Padilla 1988a) which in turn is visually sensitive. As detailed in chapter 5, it appears that these two cellular layers were initially (and presently) concerned with photoanalysis.
THE PRIMARY & ASSOCIATION VISUAL CORTEX
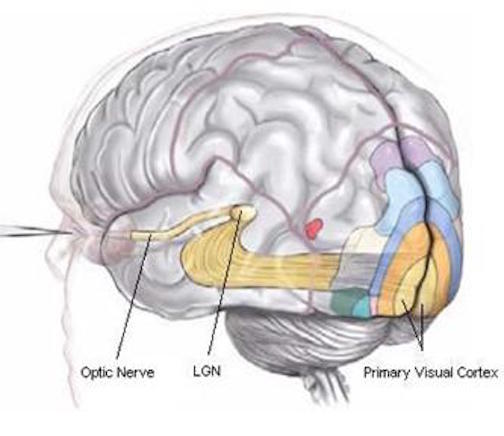
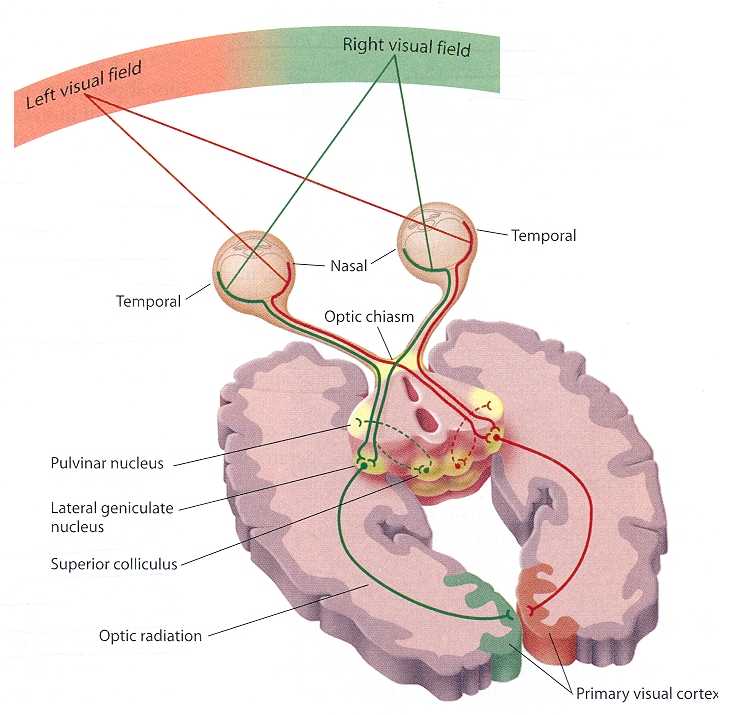
In primates and those carnivoes with their forward facing eyes, axons from the retina form the optic nerve, are organized so that visual information from the same points in visual space can be combined and rerouted at the otpic chiasm, and then directed so that all information arising from the left vs right half of the visual fields are directed to the right vs left half of the occipital lobes and visual cortex. That is, visual information arising in the nasal portion of the right eye, is combined with that from the lateral-temporal portion of the left eye (and vice versa), such that the primary visual area in the right hemisphere receives information from the left half of visual space. However, this information is first received in the lateral geniculate nucleus of the thalamus, each layer of which receives input from only one eye (Casagrande & Joseph, 1978, 1980), the bulk of which is then relayed to the primary visual cortex.
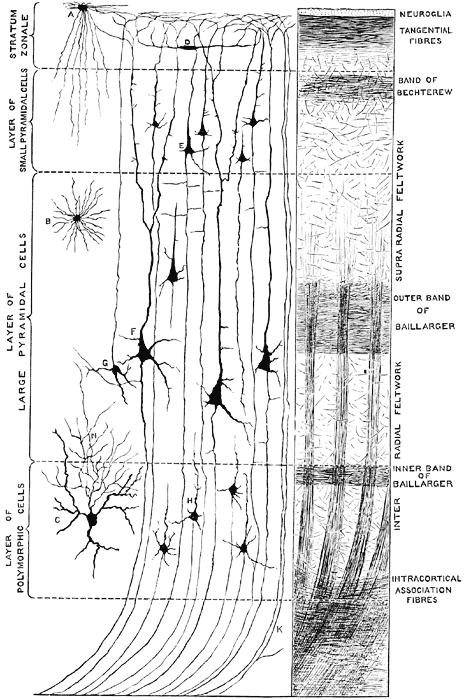
The primary visual receiving area (i.e. striate cortex, area 17) is located predominantly within the medial walls and floor of the calcarine sulcus and extends around the lateral convexity. Area 17 is also characterized by rather thin cortical layers, particularly layers II and II, and by its striped appearance which is due to the structure and composition of layer IV. That is, layer IV is divided into three sublayers, with the middle layer containing a rather thick band of cortex (the band of Baillarger/Gennari) which is visible to the naked eye.
The association cortices (areas 18 & 19) are also located medially and along the lateral convexity. However, there are also extra-striatal visual association and assimilaton areas located in the superior parietal (area 7) and inferior and middle temporal lobes (Kaas & Krubitzer 2015; Nakamura et al. 2004; Sereno et al. 2015; Tovee et al. 2004).

PRE-CORTICAL VISUAL ANALYSIS
There is much processing of visual input prior to its reception in the occipital lobe and some visual signals appear to initially bypass the primary and association visual areas (Zeki, 2007). Initially, visual information analysis takes place in the receptor cells (rods and cones) within the retina and then undergoes successive hierarchical stagess of analysis as it is passed through the sequential cell layers of which the retina is composed; i.e. horizontal cells, bipolar cells, amacrine cells, ganglion cells. Information is then relayed via the optic nerve to the lateral geniculate nucleus of the thalamus (Casagrande & Joseph, 1978, 1980; Kaas & Krubitzer 2015) as well as to the pulvinar and superior colliculus, where yet further forms of analysis are performed (see Reid, 2012, for a detailed generalized review).
As per the lateral geniculate nucleus (LGN), this structure is organized into 6 layers, each of which receives input from only one eye. However, if for any reason visual input was lost from one eye during early development, those cells innervated by the normal eye apparently grow axonal collaterals which innervate and perhaps functionally suppress the neurons in the adjoining layers which are being denied visual input (Casagrande & Joseph, 1978, 1980). These deprived cells, in turn, grow smaller and atrophy. However, if the normal eye is removed, or if the deprived eye is opened and given forced experience, some of these cells will recover, and so will some aspects of visual functioning (Joseph & Casagrande, 1978, 1980); which indicates that some degree of perceptual processing is occurring within the LGN.
Specifically, the LGN appears to engage in the preprocessing of color (processed by "P" cells), and contrast (processed by "K" cells"). Moreover, these cells are segregated and project to specific sublayers of the visual cortex; i.e. layer IVa,b,c.
From the lateral geniculate and pulvinar, visual stimuli are then transmitted via the optic radiations to the primary visual receiving area, the striate cortex as well as to surrounding areas; e.g. via the pulvinar and superior colliculus (Doty, 1983; Kaas & Krubitzer 2015; Snyder & Diamond, 1968; Tigges et al. 1983). These pathways leading to the visual cortex maintain a strict topographical relationship. Within the visual cortex immediately adjacent groups of neurons respond to visual information from neighboring regions within the retina (Kaas & Krubitzer 2015).
As noted, most of these signals, at least those from the LGN, are received in layer IVa,b,c --layers which contain "simple cells" (described below) and to a lesser extent layer VI and VII or area 17. From the visual cortex this information is then transferred back to the lateral geniculate nucleus of the thalamus (from which it was first relayed), to the superior colliculus (Kawamuara et al. 1974) and to the association areas 18 and 19 as well as the middle temporal lobe (Buchel et al., 1998; Doty, 1983; Kaas & Krubitzer 2015; Lin et al. 1982; Price, 2007; Sereno et al. 2015) where higher order processing occurs (Tovee et al. 2004). For example, when viewing or reading words, the left medial extrastriate visual cortex is activated (Peterson et al., 1990), whereas the right inferior occipital lobe becomes active when looking at pictures (Price, 2007). These visual association areas also transmit signals back to the primary area, such that highly processed information is passed back and forth, perhaps on a need to know basis. However, as noted, these visual areas are also capable of acting autonomously without pre- or post processing in the primary visual cortex such as via projections form the pulvinar to V5 (Zeki, 2007).
A similar arrangement seems to characterized information reception and transfer in the auditory, somesthetic, and motor cortices as well. That is, information transferred from the thalamus to the neocortex is then transferred back to the thalamus as well as to the adjacent association areas which then transfer information back and forth on a need to know basis. In this manner a feedback loop is constructed so that information transmission from the thalamus to the cortex and within the neocortex, can be enhanced, diminished, or altered depending on neocortical requirements.
Nevertheless, although different areas can act autonomously, in general, visual stimuli are received in the primary area and then relayed to the association areas18 and 19 (where further analyses take place) this information is transmitted back to area 17 and to the temporal and parietal lobe where poly modal associations are performed (Beckers & Zeki 2015; Martinez-Millan & Hollander, 1975; Tigges et al. 1983; Tovee et al. 2004; Zeki. 1978b).
NEOCORTICAL COLUMNAR ORGANIZATION
Throughout the striate cortex neurons with similar receptive properties are stacked in columns (Hubel & Wiesel, 1968, 1974). Indeed, one column of cells may respond to a certain visual orientation and the cells in the next column to an orientation of a slightly different angle. Moreover, columns exist for color (Zeki, 1974), location, movement, etc. In addition, since certain cells respond predominantly to input from one eye, there are ocular (eye) dominance columns as well (Hubel & Wiesel, 1968, 1974). A similar columnar arrangement in regard to somesthetic input is maintained in the parietal lobe.
Within these ocular dominance columns, the visual cortex is also organized so that they match and parallel input received within the retina, thus creating a retinotopic map. That is, adjacent cells in these columns receive input from adjacent cells in the retina. However, there is also almost a 50% overlap between columns, such that there are shared receptive fields, such that single cells can perceive more than one point in space--which in turn allows for a smooth transition which making eye and head movements. Indeed, neurons of a particular column, although communicating predominantly with those in the same column, also communicate with immediately adjacent columns, such that a considerable amount of parrallel communication occurs (Dow, 1974; Kaas & Krubitzer 2015). That is, information is anlyazed vertically and horizontally. so as to create a series of superimposed mosaics of the visual word.
SIMPLE, COMPLEX, LOWER- & HIGHER ORDER HYPERCOMPLEX FEATURE DETECTORS
The visual cortex is made up of a variety of cell types each of which is concerned with the analysis of different visual features (Ferster, et al., 1996; Hubel & Wiesel, 1959, 1962, 1968; Kaas & Krubitzer 2015; Sereno et al. 2015). These include simple, complex, and (higher & lower order) hypercomplex cells which are distributed disproportionately throughout areas 17,18, 19.
To briefly summarize, simple cells appear to be involved in the initial analysis of incoming visual cortical input, and are most sensitive to slowly moving stimuli. They are found predominantly within area 17 and in layer IVa,b,c,. Some are sensitive to stimuli moving in one direction, whereas others may respond to stimuli moving in any direction. In fact, almost 95% of the neurons in area 17 are responsive to stimuli moving only in one direction, but not the direction of movement. In addition, simple cells are responsive to the particular position and orientation a stimulus may take. However, for a simple cell to fire, a stimulus must assume a specific orientation and position.
Simple cells relay this processed information to the far more numerous complex cells which are found predominantly in layers II and III and V, which interact and communicate with one another including with layer IV which receives thalamic input. Each complex cell receives input from several simple cells. Complex cells are also concerned with orientation of the stimulus. However, these cells are more flexible and will respond and analyze a stimulus regardless of its particular orientation. These cells via the combined input from simple cells, are probably involved in the earliest stages of actual form perception, i.e. the determination of the outline of an object. A considerable number of complex cells receive converging input from both eyes, the remainder being monocular. Complex cells are found predominantly within area 18.
Hypercomplex cells are concerned with the analysis of discontinuity, angles and corners, as well as movement, position, and orientation. That is, these cells respond selectively to certain visual configurations and thus act so as to determine precise geometric form. It is also via the action of these cells (in conjuction with visual neurons in the temporal lobe) that the first stages of visual closure are initiated. This in part requires that the functional activity of these cells be suppressed such that when presented with an incomplete figure these cells are overridden and the brain is able to "fill in the gaps" in stimuli perceived. It is also for this reason that one does not notice his or her "blind spot"; it is filled in. Hypercomplex cells are found predominantly within area 19.
STRIATE CORTEX: AREA 17
The primary visual cortex, area 17, is located predominantly within the medial walls of the cerebral hemispheres, extending only minimally along the lateral convexity. This area is often referred to as "striate" because the incoming fibers from the optic radiations form a stripe along the cortical surface which can be seen by the naked eye. Areas 18 and 19 do not have this striped appearance.
Like the primary motor and somesthetic cortices, a greater degree of cellular representation is maintained for those areas which are the most densely innervated and of the most sensory importance, i.e., the fovea (Daniel & Whitteridge, 1961; Hubel & Wiesel, 1979; Kaas & Krubitzer 2015). Indeed, the central part of the retina has a cortical representation which is 35 times more detailed than that of the periphery. This is particularly important in that the fovea contains cells which are most sensitive to the detection and representation of form.
Although all neuron types are found within the striate cortex, simple and complex cells predominate. Hence, the primary receiving area is predominantly involved in the analysis of color, slow movement, position, and orientation (Zeki, 2007); i.e. the most elementary aspects of form and visual stimulus perception.
The primary visual cortex, however, receives fibers from non-visual brain areas as well. These include brainstem nuclei, the pontine and mesencephalic reticular formation, the lateral amygdala, and lateral hypothalamus (Doty, 1983; Tigges et al. 1983). Processing in the primary region can thus be enhanced or diminished via reticular influences and emotional-motivational concerns. In this manner, if a stimulus is emotionally significant greater visual attention will be directed at the object.
Conversely, bilateral destruction of area 17 can result in loss of visual recognition capabilities -even with sparing of the association areas (Humphrey & Weiskrantz 1967; Weiskrantz & Cowey 1963). However, awareness of moving objects and visual-spatial orientation is preserved. Visual preservation with primary occipital destruction has been referred to as "blind sight" (see below).
HALLUCINATIONS.
Electrical stimulation, tumors, seizures, or trauma involving the striate cortex may produces simple visual hallucinations, such as sparks, tongues of flames, colors and flashes of lights (Penfield, 1954; Tarachow, 1941). Objects may seem to become exceedingly large (macropsia) or small (micropsia), blurred in terms of outline, stretched out in a single dimension, or colors may become modified or even erased (Hecaen & Albert, 1978). Sometimes simple geometric forms may be reported. Usually the hallucination is restricted to one half of the visual field. That is, if the seizure is in the right occipital lobe, the hallucination will appear in the left visual field.
Although elementary hallucinations are usually associated with abnormalities involving the occipital lobe they may occur with temporal lobe lesions or electrical stimulation (Penfield & Rasmussen, 1950; Tarachow, 1941).
ASSOCIATION AREAS 18 & 19
Areas 18 and 19 are are involved in the translation and interpretations of visual impressions transmitted from area 17. Although simple and complex cells are found in the association cortex, this region is predominantly populated by hypercomplex (both higher and lower order) neurons --most of which are concerned with the determination of precise geometric form as well as the assimilation of signals transmitted from the primary cortex (Kaas & Krubitzer 2015).
In contrast to the neurons within area 17, many of the cells within area 18 receive binocular input and can be activated by either eye (Hubel & Wiesel, 1970). This same pattern of bincularity is evident in the parietal association area. It is via the action of these cells that one is able to gather information regarding distance, discrepancies in stimulus location and thus determine depth and achieve stereoscopic vision (Blakemore, 1970a; Hubel & Wiesel, 1970a). Indeed, some association neurons will only fire when a target is a definite distance from the eye.
Many of the neurons in this region, particularly area 19, receive higher order converging input from the parietal and temporal lobe. For example, in addition to visual input, neurons in the superior portions of area 19 respond to tactile and proprioceptive stimuli, whereas those in the inferior portions respond to auditory signals (Morrell, 1967). It is probably in this manner (in conjunction with subcortical connections with, for example, the superior colliculus) that one is able to orient toward and gaze upon an auditory stimulus as well as maintain stablization of the head (via proprioceptive-vestbibular input) while engaged in visual search.
Hence, overall, the visual association area appears to be involved in the initial analysis of form, distance, and depth perception, as well as the performance of visual closure. It is thus heavily involved in the association of various visual attributes so that a variety of qualities may be ascertained. This would include an objects shape, length, thickness, and color (Sereno et al. 2015).
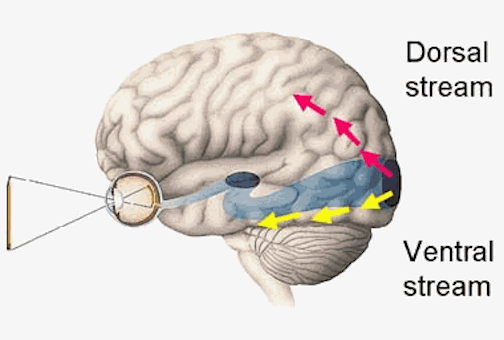
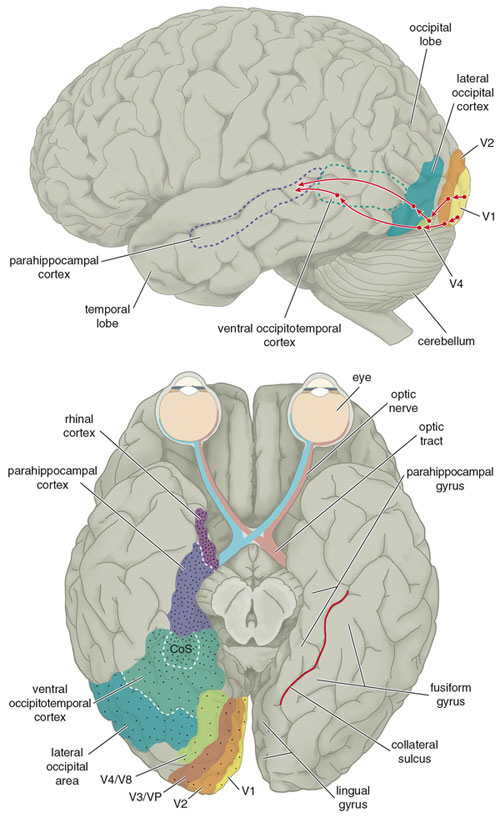
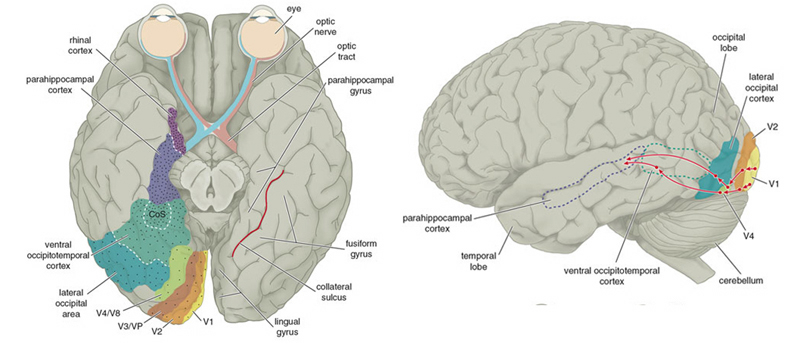
THE TEMPORAL/PARIETAL VISUAL AREAS
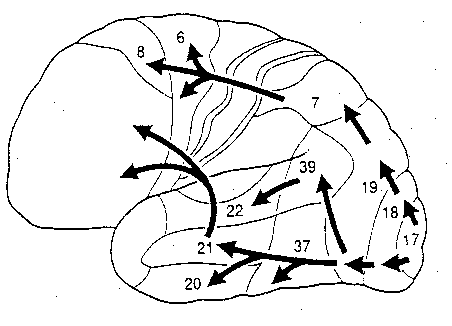
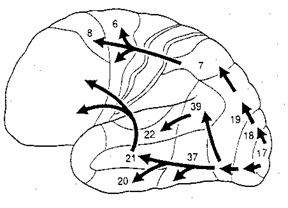
The visual association areas also maintain intimate relationships with the parietal visual regions (area 7) as well as the visual areas in the middle and inferior temporal lobes (ares 37) including V5 (the lateral occipital-temporal junction). The temporal visual areas are in turn reciprocally interconnected with area 7 and areas 17, 18, 19.
As noted, whereas the temporal lobes perform complex form recognition and central and upper visual field analysis, the parietal lobes observe the periphery and lower visual field (where the hands and feet are most likely to be viewed) and together these cortical regions compute and make possible, eye-hand, or hand-object coordination. Hence, a complex interactional visual loop is maintained.
For example, the inferior-medial temporal lobe is concerned with form perception and the analysis of emotional-motivational significance and transmits this information to area 7. Area 7 is involved in visual attention, visual fixation, and the analysis of distance, depth, and objects within grasping distance. Area V5 (at the temporal occipital border) perceive motion. Hence, through this temporal/occipital/parietal pathway, the individual can recognize and fixate on the object (temporal lobe), determine the speed at which it is traveling (V5) and reach out and grasp it (parietal lobe).
As noted, the temporal parietal visual areas are also interconnected with areas 17, 18, and 19, and receive their own autonomous visual input, via, for example, the pulvinar and midbrain. Moreover, the temporal parietal visual areas receive some visual signals in advance of the primary and association visual areas. Area V5, for example, perceive rapid movement, and if injured or deactivated, the ability to perceive rapid movement is diminished or abolished, whereas slow motion perception is maintained due to preservation of the occipital visual areas (Zeki, 2007). Moreover, fast moving stimuli are perceived and activate area V5 in advance of area 17, which in turn is excited by slow moving stimuli in advance of area V5. Hence, V5 receives information from the retina regarding fast moving objects, information that bypasses the primary visual area (Zeki, 2007); a very adaptive relationship, at least from an evolutionary and life-preservative perspective, for under some circumstances it would be most advantageous to perceive something that was moving rapidly, such as a predator, than something less dangerous that was moving slowly.
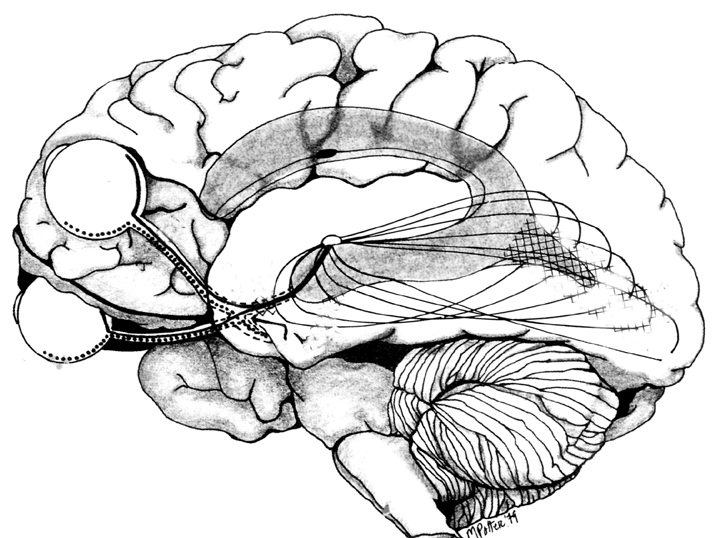
MULTIPLE VISUAL REALITIES
As detailed in chapters 2, 10, the brain is not only interactional, but is characterized by normal discontinuities where some areas do not always communicate together efficiently. That is, the functioning of the brain is not always in parallel, such that some areas function in semi-isolation or semi-independently giving rise to multiple streams of conscious awareness which may not always correspond to reality. The same can be said even regarding those areas of the brain which are ostensibly concerned with the same visual stimulus, such as auditory or vision as is the case with V5 and areas 17, such that when these stimuli are processed, an internal reality which is slightly different from external reality may be produced.
According to Zeki (2007, pp. 170-171) the evidence has "led us to the concept of dynamic parallelism, by which we mean that while both areas can be healthy and functional in a normal brain, which area gets activated first depends on the nature of the stimulus." However, as also pointed out by Zeki (2007), even within the visual system the various "perceptual systems are therefore different as are the processing systems" and "that there is no synchronizer in the brain capable of setting the results of the operations of the two processing-perceptual systems to time zero... that we thought was the hallmark of our visual experience.... the brain, therefore missychronizes and misbinds or rather more accurately, it binds the results of its processing-perceptual systems, not what happens in real time in the real world."
HOMONYMOUS HEMIANOPSIA & QUADRANTANOPSIA
Massive unilateral destruction of the visual cortices results in blindness in the contralateral temporal visual field and ipsilateral nasal visual field. Hence, a left visual cortex lesion produced a right homonymous hemianopsia. However, these visual disturbances may also result from destruction of the optic radiations or optic tract. It is noteworthy that patients are often unaware of having lost half their sight, particularly with right occipital lesions. Nevertheless, patients may complain of bumping into people and objects they cannot see.
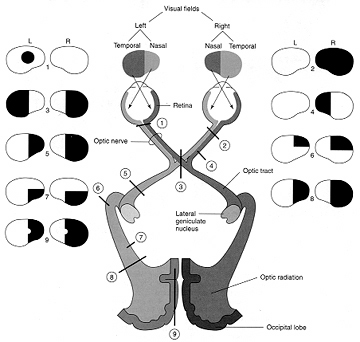
As noted, above, the parietal lobes are concerned with the lower visual fields, whereas the temporal lobes receive massive upper visual field projections. Thus, lesions to the inferior or superior occipital lobe can result in an upper or lower visual field homonymous quadrantanopsia.
HALLUCINATIONS
Electrical stimulation of or lesions involving areas 18 and 19 can produce complex visual hallucinations (Foerster, 1929, cited by Brodal, 1981 & Hecaen & Albert, 1978; Tarachow, 1941), such as images of men, animals, various objects and geometric figures, liliputian-type individuals, including micropsias and macropsias (see Luria, 1980; & Hecaen & Albert, 1978, for review). Sometimes objects may seem to become telescoped and far away, wheras in other situations, when approached, objects may seem to loom and become exceedingly large.
Complex hallucinations are usually quite vivid and fully formed and the patient may think what he sees is a real (Hecaen & Albert, 1978). Foerester (1928, cited by Heacen & Albert, 1978) reported a ptient who hallucinated a butterfly then attempted to catch it when area 19 was elecrically stimulated. Another hallucinated a dog and then called to it, denying the possibility that it was not real.
Complex hallucinations, although usually associated with tumors or abnormal activation of the visual association area, have also been reported with parietal-occipital involvement (Russell & Whitty, 1955), occipital-temporal, or inferior-temporal damage (Mullan & Penfield, 1959; Tarachow, 1941; Teuber et al., 1960), or with lesions of the occipital pole and convexity (Hecaen & Albert, 1978)
Laterality.
According to Hecaen and Albert (1978) based on their review of the international literature, although simple hallucinations are likely following damage to either hemisphere, complex hallucinations are usually associated with right rather than left cerebral lesions (Teuber et al., 1960; Mullan & Penfield, 1959; Hecaen & Albert, 1978).
CORTICAL BLINDNESS
Lesions of the occipital lobe, especially if the entire visual cortex is ablated, result in cortical blindness, such that pattern and form vision is lost. Rather, what is left is the ability to discriminate only between different fluexes in luminous energy, i.e. lightness and darkness (Brindley et al. 1969; Brodal, 1981, Hecaen & Albert, 1978; Weiskrantz, 1963). If damage is restricted to the occipital lobe of only one of the hemispheres, patients will lose patterned vision for the opposite half of the visual field (i.e. a hemianopsia). This is not the same as unilateral neglect or inattention. However, if the lesion is sufficiently large and involves the right parietal area as well, the patient may suffer from both hemianopsia and neglect.
Nevertheless, if only a portion of the visual cortex is destroyed, vision is lost only for the corresponding quadrant of the visual field (referred to as a scotoma). However, in cases of partial cortical blindness, patients are able to make compensatory eye movements and are not terribly troubled by their disability (Luria, 1980). Indeed, frequenty patients have no awareness that they have lost a quadrant or even half of their visual field. Hence, this must be tested for.
"BLIND SIGHT"
Although considered somewhat controversial, it has been reported that although blind (due to destruction of the primary visual cortex), some individuals are able to indicate the presence or absence of a moving stimulus within the "blind" portion of their visual field (Holmes, 1918; Riddoch, 1917; Scharli, Harman & Hogben, 2012; Weiskrantz, 1986, 1996; Zeki, 2007), and even differentiate between various objects, although not knowing what they are (Humphrey & Weiskrantz 1967; Weiskrantz & Cowey 1963). Using forced choice procedures, although "blind" some patients can localize visual targets and perceive moving objects. In one case it has been reported that a patient who denied visual perception and with a diagnosis of cortical blindness, was able to correctly name objects, colors, famous faces, facial emotions, as well as read various single words with greater than 50% accuracy (Hartmann et al. 2015).
Visual preservation following area 17 lesions has been referred to as "blind sight" (Weiskrantz, et al. 1974; Weiskrantz, 1996). Although blind, these patients may avoid obstacles, and correctly retrieve desired objects, and thus appear to have some residual visual functions even though they verbally claim no conscious awareness of the visual stimulus and thus have no verbal awareness that they can see (Poppel, Held & Frost, 1973). Nevertheless, although the verbal aspects of consciousness have been disconnected from the visual cortex and claims it cannot see, the patient continues to behave as if visual input is still being received; hence the term "blind sight."
However, it has also been argued that "blind sight" is not really "blind sight" and that "useful visual function is preserved only when a critical amount of area 17 is spared (Celesia et al. 2015). For example, using PET and SPECT studies, Celesia and colleagues (2015) found that among those with visual cortical destruction and "blind sight", that islands of preserved area 17 functioning were observed. According to Celesia et al. (2015) those with complete area 17 destruction that patients do not demonstrate signs of "blind sight." This view is in error, however as "blind sight" has also been demonstrated with evidence of no functional activity in area 17 (Barbur, et al., 2003; Zeki, 2007).
Possibly, although cortically blind, patients are able to recognize moving objects, or in some cases, correctly move about in space, due to the preservation of intact subcortical nuclei involved in visual orientation, i.e. via the so called "second (retino-collicular- pulvinar-extrastriate) visual system" (Schneider, 1969; Weller 1988) which projects to temporal occipital junction, i.e. area V5. For example, Barbour and colleagues (2003), describe a patient GY, who had suffered damage to the occipital lobe at age 8, rendered hemianopi yet was able to demonstrate blindsight. Using functional imaging, it was found that although there was no activity in the primary visual area when shown a fast moving object, that V5 became highly active, and that GY was able to see a "shadow." However, when asked to clarify what he had seen, GY replied that it was more of a "feeling" (Zeki, 2007). Given that V5 became active, this suggests that blindsight, at least in this case, was due in part to preserved visual functioning in the temporal occipital junction.
Consistent with this view is the case presented by Hartmann et al. (2015). Although denying visual perception, the patient was able to name objects, colors, and so on, when the stimuli were placed in the upper visual field. The upper visual fields are associated with the inferior temporal lobe, which are the recipients of fibers from the "second visual system" as well as from the visual neocortex. Hence, in cases of disconnection and blind sight due to occipital lesions, complex visual input may still reach the temporal lobe, and may therefore be directed to the auditory areas via a secondary route so that associatd "feelings" of seeing something can be communicated, and in some cases, so that objects can be named, although the patient continues to deny visual perception.
Presumably, those who are "cortically" blind but demonstrate "blind sight" coupled, in some cases, with the ability to turn the head and orient, can accomplish these acts apparently because undamaged midbrain, thalamic, and neocortical tissues (e.g. temporal/parietal lobe) involved in visual functioning continue to function normally (Milner & Goodale, 2015; Stoerig, 1996). That is, tissues in the midbrain, thalamus, or the temporal/parietal lobe, continue to process visual input, but are nevertheless, disconnected from the "dominant" stream of verbal consciousness mediated by the left hemisphere due to the injury to the occipital lobe. Although "cortically blind" the eyes are functional, and visual impressions are transmitted via the optic nerve directly to the midbrain visual colliculus, and via the optic tracts and optic radiations to the lateral geniculate nucleus of the thalamus and other forebrain structures including the visual areas in the temporal and parietal lobe (Milner & Goodale, 2015; Stoerig, 1996) which in turn may transmit visual input to those islands of striate cortex which remain intact (Wessinger, Fendrich, & Gazzaniga, 2007), but which are unable to communicate with the language areas of the brain, which, in failing to receive visual signals, claims to have no knowledge of the visual world. Nevertheless, visual processing continues outside of linguistic conscious-awareness, and patients may report that their behavior is guided by "non-visual feelings" (Scharli, et al., 2012). Hence, although disconnected form the language axis, which reports only "non-visual feelings" these isolated visual areas may continue to mediate behavior, perhaps via connections with the midbrain--a structure which is responsive primarily to moving stimuli and which is triggers head turning in response to moving stimuli (Blessing, 2007; Klemm & Vertes, 1990).
Consider, for example, the classic cases of blindsight first described by Riddoch (1917). According to Riddoch (1917) although these patients had suffered extensive destruction of the primary visual areas, they remained "conscious of something moving when the object oscillate... and that.... the consciousness of something moving kept up a continual desire to turn the head."
The fact that these and other patients with blind sight may have a feeling of seeing movement, are/or experience a desire to turn the head, and/or correctly reach out and grasp or move around objects, directly implicates the midbrain visual colliculus as this structure is able to detect movement and different gradations of light and shadow (Davidson & Bender, 2015), and (via the lower brainstem) can direct head turning, groping and grasping, and even walking (Blessing, 2007; Klemm & Vertes, 1990). However, as noted above, due to massive destruction of primary visual cortex, and as areas 17 and V5 as well as other neocortical areas interact, there is a disruption of the feedback loop which enables the language axis to receive these visual impressions, other than, in some cases, through the experience of "feelings." Nevertheless, one does not need language or a neocortex in order to see movement or perform simple visually-guied movements, which is why creatures such as reptiles, frogs, etc., are capable of complex visually guided behaviors even though they never evolved language or neocortex.
Humans, however, have evolved neocortex, and the primary visual receiving areas hierarchically analyze these subcortical visual signals which are then transferred to the adjoining visual association areas thereby forming complex visual associations (Kaas & Krubitzer, 2015; Milner & Goodale, 2015; Sereno, Dale, & Reppas, 2015). These complex associations are then transmitted to the language axis which then names and verbally describes what the individual sees (Critchley, 1964; Geschwind, 1965; Joseph, 1982). These visual impressions come to be associated with language and thus with linguistic consciousness. However, with massive injuries to the primary visual cortex, the language axis no longer receives visual input, and the patient reports that he or she is blind and cannot see, though, in some cases, they avoid obstacles and can reach for desired objects.
Thus patients with "blind sight" demonstrate at least two disconnected streams of mental activity, one of which utilizes language to deny visual experience other than through the experience of "feelings", and a second non-verbal form of subcortical or isolated neocortical mental activity that is capable of seeing and/or controlling movements of the body in response to certain visual stimuli. Moreover, these multiple modes of conscious-awareness, including those supporting blindsight may wax and wane, "resulting in blindsight in some test sessions and in conscious awareness of the same stimuli in others... which raises the interesting question of whether the brain switches from one neural system to another during the waxing and waning of consciousness (Zeki, 2007, p. 175).
DENIAL OF BLINDNESS
More frequently, however, patients with cortical blindness (such as following massive lesions of the visual cortex) seem initially quite confused, indifferent regarding their condition, and report a variety of hallucinatory experiences which may be complex or elementary in form. Moreover, frequently these patients will initially deny they are blind (Redlich & Dorsey, 1945) --a condition referred to as Anton's syndrome. For example, a number of patients described by Redlich and Dorsey (1945), although bumping into furniture and unable to recognize objects held before them, invented elaborate excuses for their errors and failure to see; e.g. claiming that it is a little dark and they need their glasses, or conversely, that they see better at home. That is, these patients confabulate.
As discussed in chapter 10, these confabulatory abnormalities are sometimes due to a disconnection such that the Language Axis, failing to receive information from the visual cortex (i.e. that it cannot see), responds instead to associations from intact areas which concern "seeing". That is, the Language Axis does not know that it is blind because information concerning blindness is not being received from the proper neural channels.
It is also possible that these patients deny being blind, because subcortically they are still able to see. Hence, although at a neocortical level there is no sight, subcortically there remains an unconscious awareness of the visual world.
VISUAL AGNOSIA & ALEXIA
As based on functional imaging studies, the left medial extrastriate visual cortex becomes activated when reading or viewing words (Peterson et al., 1990), whereas the right inferior occipital lobe becomes active when looking at pictures (Price, 2007). In addition to letters, words, and pictures, complex form and object recognition is also subserved by the extra-striate visual areas in the occipital lobe, the ventral-inferior areas in particular, adjacent to the temporal lobe (Buchel et al., 1998; Haxby, et al., 2015). This has been demonstrated not only by functional imaging (Buchel et al., 1998; Price, 2007), but direct cortical recoding (Nobre et al., 2004), and electrical stimulation (Luders et al., 1986). In fact, both normal, cogenitally blind, and late-blind subject display activity in this area (Buchel et al., 1998).
If injured, patients may suffer from reading and naming deficits (Rapcsak, et al., 2002); a condition referred to as phonological alexia (e.g., Miozzo & Caramazza, 1998). Moreover, patients with developmental dyslexia have been found to have abnormalities in this area (Rumsey et al., 2007). However, in addition to, or depending on the nature and exact location of the lesion, patients may suffer from agnosia.
Visual agnosia is a condition where the patient loses the capacity to visually recognize objects, although visual sensory functioning is largely normal. This condition often arises with lesions involving the inferior medial occipital lobe. In general, objects are detected but they lose the ability to evoke meaning and cannot be correctly identified or named (Critchley, 1964; Davidoff & De Bleser 2004; Shelton et al. 2004; Teuber, 1968). The percept becomes stripped of its meaning. For example, if shown a comb, the patient might have no idea as to what it is or what it might be used for. If asked to guess they may call it a harmonica or a tiny box. If shown a pair of spectacles, they may call it a "bicycle", or "two spoons". A picture of a dial telephone may be described a a "Clock", etc. (Luria, 1980)
Many patients are unable to sort pictures or objects into categories or match pictures with the actual object such that there appears to be a deficit in the ability to not only recognize but to classify visual percepts (Hecaen, et al. 1974). Moreover, in severe cases they are unable to point to objects that are named.
Nevertheless, this is not a naming disorder (e.g. Davidoff & De Bleser 2004), because regardless of modality, anomics continue to have word finding and naming difficulties. In contrast, those with agnosia show enhanced recognition if an object is presented via a second intact modality (e.g. if they palpate it by hand). Thus agnosia can often be limited to a single input channel, i.e. visual vs. tactual. Moreover, If an object is used in context, recognition can be greatly enhanced (Rubens, 2003).
Some patients may complain that objects change while they are looking at them, and /or that they disappear; a condition which suggests optic ataxia. Usually, however, the deficit is conceptual rather than perceptual.
There are however, to major forms of agnosia, and different subtypes as well, which can be produced by damage to tissues mediating complex visual perception or lesions disconnecting the IPL, for example, from visual, somesthetic, or auditory input.
For example, one type of agnosia, "apperceptive visual agnosia" refers to a disturbance in perceptual and visual-motor integration, such that patients have difficulty copying or matching various objects. This latter form of agnosia has been associated with lesions to the parietal occipital cortex (Mizuno, et al., 1996), as well as to bilateral damage to the inferior-occipital cortex (Shelton, et al., 2004). For example, although they may be able to accurately describe, draw or copy various aspects of the object they are shown (Albert, et al,. 1975; Mack & Boller, 2017; Rubens & benson, 2014) they fail to correctly draw the entire object. Moreover, if asked to trace rather than copy, these patients may trace over and over the outlines of objects or drawing but cannot recognize where they started.Thus they seem unable to synthesize visual details into an integral whole (Luria, 1980). That is, patients may recognize an isolated detail (e.g. the dial of a phone) but are unable to relate it to the headset, etc. (see Shelton et al. 2004) Hence, if asked what they have been shown, they may erroneously extrapolate from the detail perceived and thus confabulate a concept. With increasing object complexity, or if surrounded by other objects, or if the object is presented in pictorial (vs. actual) form, or if unnecessary lines are drawn across the picture, the abilty to recognize the object deteriorates even futher (Luria, 1980; Rubens & Benson, 2014).
By contrast, associative visual agnosia, which is a deficit in naming, such that auditory equivalents cannot be matched to a visual perception, is associated with left inferior and middle temporal (area 37) occipital abnormalities (Giannokapoulos et al., 2012), which may be accompanied with alexia (Feinberg et al., 2004), as well as to lesions to and atrophy of the parietal occipital cortex (Mizuno, et al., 1996).
Agnosic individuals also often (but not always, Davidoff & De Bleser 2004) have difficulty with reading (Albert, Reches & Silverberg, 1975; Mack & Boller, 2017; Rubens & Benson, 2014) and may suffer from prosopagnosia and/or impaired color naming (Mack & Boller, 197; Shelton et al. 2004). Interestingly, in some cases visual memory be intact (Rubens, 1979).
Like alexia, agnosia can occur following lesions to the medial and deep mesial portion of the left occipital lobe (Feinberg, et al., 2004). The left inferior temporal lobe and posterior hippocampus may also be damaged in some cases (Shelton et al. 2004).
In some cases, it is likely that agnosia is due not only to tissue destruction but to tissue disconnection. That is, if the visual form recognition neurons in the temporal lobe are no longer able to receive input from the visual association areas, then this particular region becomes "cortically blind" and form recognition is prevented. However, like some other disconnection syndromes, if a different input channel is employed, i.e. if the object is verbally described or tactually explored, recognition is enhanced.
PROSOPAGNOSIA.
Prosopagnosia is a severe disturbance in the ability to recognize the faces of friends, loved ones, or pets (DeRenzi, 1986; DeRenzi, et. al., 1968; DeRenzi & Spinnler, 1966; Evans et al. 2015; Hecaen & Angelergues, 1962; Landis, et al. 1986; Levine, 1978; Whitely & Warrington, 2017l ). Some patients may in fact be unable to recognize their own face in the mirror. Nevertheless, they usually realize that a face is a face; they just don't know who the face belongs to. In this regard, prosopagnosia is not a visual agnosia as described above, where the patient cannot recognize that a chair is a chair or a clock a clock.
A number of authors have erroneously argued that prosopagnosia is due to bilateral injuries involving the inferior and medial occipital lobe visual association areas. Nevertheless, although frequently such individuals do indeed suffer from bilateral injuries, in many cases the lesions are restricted to the right hemisphere and involve the occipital and inferior temporal regions (DeRenzi, 1986; DeRenzi et. al., 1968; DeRenzi & Spinnler, 1966; Evans et al. 2015; Hecaen & Angelergues, 1962; Landis et al., 1986; Levine, 1978; Whitely & Warrington, 2017; Young et al. 2015), although frontal lesions (Braun et al. 2004), and right parietal lesions (Young et al. 2002) may also disrupt facial recognition. By contrast, prosopagnosia does not usually result from lesions restricted to the left half of the brain, whereas loss of facial recognition with frontal and parietal lesions are more likely due to visual neglect and visual scanning abnormalities.
Although prosopagnosia could be explained from a disconnection perspective, it is likely that the actual face identification neurons have been destroyed whereas neurons involved in the recognition of facial parts have been preserved.
SIMULTANAGNOSIA
Simultanagnosia occurs with left hemisphere damage and is an inability to see more than one thing, or all aspects of an item, at a time (Bousen & Humphrey, 2012; Kinsbourne & Warrington, 1962, 1964; Laeng, et al., 2012; Rizzo & Robin 1990). For example, some patients complain of seeing things only in a piecemeal fashion such that objects look fragmented. In fact, by surrounding the object with other objects perceptual recognition deteriorates even further.
This condition is sometimes accompanied by abnormal eye movements (Luria, 1980). These individuals often have difficulty shifting gaze and/or performing visual search tasks such that their ability to scan and visually explore the environment is drastically reduced (Bousen & Humphrey, 2012; Rizzo & Robin 1990). As described by Luria (1980), this is due to a breakdown in the ability to perform serial feature-by-feature visual analysis. Visual attention is often largely limited to the central visual field whereas the periphery is ignored (Hecaen & Ajuriaguerra, 1954; Luria, 1973, 1980). However, patients complain that even objects in the central visual field tend to disappear as they stare at them (Rizzo & Robin 1990).
Simultagnosia has been described following lesions to the frontal eye fields and following bilateral superior occipital lobe lesions (Rizzo & Hurtig, 2002). In many cases the lesion may be localized to the superior occipital-parietal region (area 7). Hence, the patient is no longer able to maintain visual fixation and cannot adequately focus on an object or explore its parts. This disorder has also been referred to as Balints syndrome as well as optic ataxia, paralysis of gaze, and concentric narrowing of the visual field.
IMPAIRED COLOR RECOGNITION
In this condition, although sometimes able to correctly name objects patients cannot correctly name, match, and identify colors or point to colors named by the examiner. No, this is not due to color blindness. Frequently individuals with color imperception also display prosopanosia (Green & Lessel, 2017; Meadows, 1974b).
DeRenzi & Spinnler (1967) found that 23& of those with right cerebral damaged and 12% of those with left sided destruction had difficulty with color matching (Lhermitte et al., 1969). On the otherhand, some investigators note that impairments of color perception are frequently secondary to bilateral inferior occiptial lobe damage (Green & Lessell, 2017; Meadows, 1974a; Shelton et al. 2004). In addition, almost 50% of those with aphasia demonstrate deficient color naming and color identification (DeRenzi & Spinnler, 1967; DeRenzi et al., 1972). However, color perception per se is largely intact among aphasic individuals.
OVERVIEW
The primary visual cortex is located predominantly within the medial walls of the cerebral hemispheres and is concerned with the elementary aspects of form perception. Damage limited to this area will usually affect foveal vision and/or give rise to simple hallucinations.
From the primary area information is then relayed to the association areas, 18 and 19, where complex analysis including form recognition, position and analysis of depth take place. Damage involving these areas and the primary region can cause cortical blindness or hemianopsia if only one hemisphere is lesioned. Destruction or abnormal activity in areas 18 and 19 are associated with the formation of complex hallucinations.
Visual information is next relayed to area 7 in the parietal lobe and to the inferior temporal lobule, where higher order analysis and multimodal processing occurs. Damage to the parietal-occipital borders may result in abnormalities involving depth and form perception as well as visual neglect. Destruction of the temporal-occipital regions can give rise to visual agnosias and an inability to recognize complex objects and faces.
The occipital lobes also appear to be lateralized in regard to certain capabilities such as facial recognition. For example, destruction of the right occipital region is associated with prosopagnosia, and abnormal activity in this area is more likely to give rise to complex visual hallucinations.













