Rhawn Gabriel Joseph, Ph.D.
Brain Research Laboratory
BrainMind.com
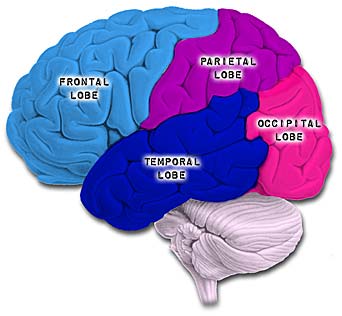

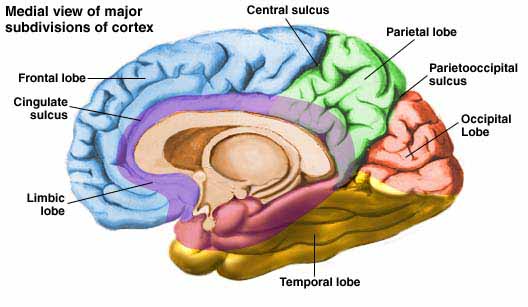
The parietal lobes are the senior executive of the physical-body-in-space and maintains one's personal image of the body, both physical and visual. The parietal lobes receives distinct sensory impressions from the entire body and can feel "pain" or a bug crawling on one's arm, leg, or face.
It is the parietal lobe which guides the movement of the body in space, coordinating body movement while running, walking, skipping, or climbing over obstacles.
The parietal lobe are also considered a "lobe of the hand" and receives sensory sensations from the bones, tendons, muscles, and skin of the hand, and guides the movement of the hand in visual-space. Therefore, the ability to reach for and manipulate a tool, open and remove the cap from a bottle and pour the contents into a glass, are made possible by the parietal lobe in association with the frontal motor areas and the visual cortex.
The parietal lobes are not a homologous tissue but consists of cells which are responsive to a variety of divergent stimuli, including movement, hand position, objects within grasping distance, audition, eye movement, pain, heat, cold, as well as complex and motivationally significant visual stimuli (Aoki et al., 2008; Cohen et al. 2014; Deibert et al., 2008; Dong et al. 2014; Lam, et al., 2008; Lebedev et al. 2014; Lin & Sessle 2014; Pred'Homme & Kalaska 2014; Remy et al., 2008; Snyder et al., 1998).
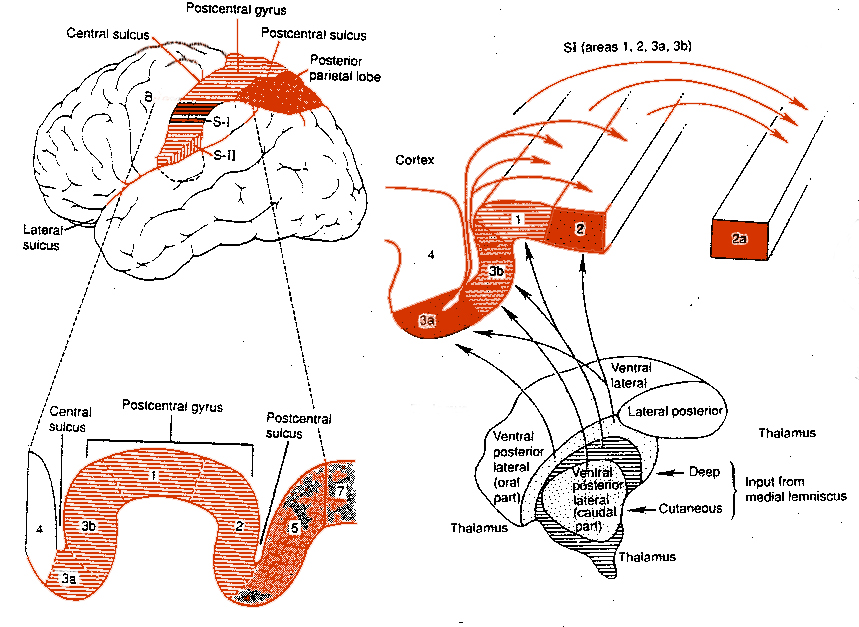
For example, area 7 of the superior parietal lobe receives considerable visual input, particularly from the lower visual fields and periphery--information which it receives not only from the visual cortex but from extra-retinal pathway. Area 7 also receives complex somesthetic stimuli regarding the hand and objects beyond and within reaching distance. Moreover, different neurons in different regions of area 7 perform somewhat different functions. Even the subareas within the primary somesthetic neocortex, areas 3ab,1, 2, respond to different stimuli and receive projections from different subregions within the thalamus, which in turn receive different forms of input from skin, joints and muscles.
Because it serves so many diverse yet related functions, damage to the parietal lobe can therefore result in a variety of disturbances. This includes abnormalities involving somesthetic and pain sensation, the body image, visual-spatial relations, temporal-sequentual motor activity, language, grammar, numerical calculation, emotion, and attention, depending on which area has been lesioned as well as the laterality of the damage.
PRE-PARIETAL PROCESSING
There is no general consensus as to peripheral receptors or the specific roles they play in somesthetic perception (Paulesu et al., 2011), with some investigators arguing that specific somatic receptors transmit modality-specific signals which provide distinct information along discrete neural pathways (Mountcastle, 1980; Turebjork, et al., 1987). Others, however, dispute this and instead argue that there are few "organized receptors" and that most "receptors are not essential for the recognition of..." for example, "cold and warmth" (Adams & Victor, 2014), and that "skin receptors have specialized properties" only "for the transduction of particular ranges of stimuli... " and do not transmit "modality-specific information" (Melzack & Wall 1962). Adams and Victor (2014) do admit, however, that "the quality of sensation depends on the type of fiber that is stimulated."
Of the different putative receptors for sensation, it has been argued that mechanoreceptors such as Pacini's and Meissner's corpuscles are rapidly adapting and and signal the onset and offset of stimulation, whereas Ruffini's and Meissner's corpuscles are slowly adapting and thus transmit information over long time periods. However, both rapid and slowly adapting receptors are found in the superficial skin (Pacini's and Ruffini's) and subcutaneous tissue (Meissner's and Meissner's).
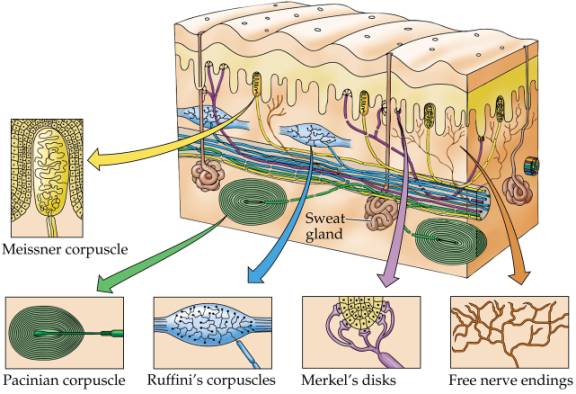
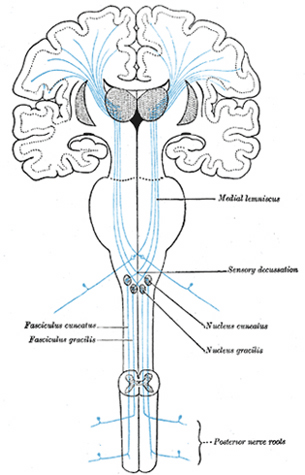
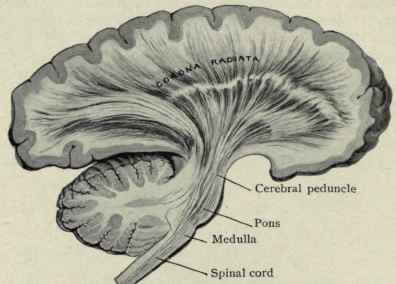
Hence, both slow and fast adapting receptors may be stimulated simultaneously and provide a wealth of information regarding external stimulation--information which is transmitted to sensory neurons located in the dorsal root ganglia which in turn transmit this data along the dorsal columns of the spinal cord where they terminate in the gracile and cuneate nucleus of the medulla. Likewise, so called "cold," "thermal" and "nociceptors" (pain) receptors are transmitted from the spinal cord, or the cranial nerves, to the medulla where considerable processing takes place (e.g. Blessing, 2011).
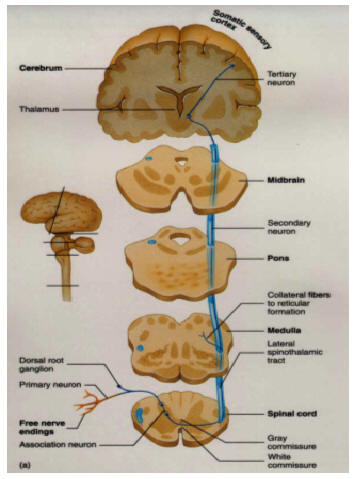
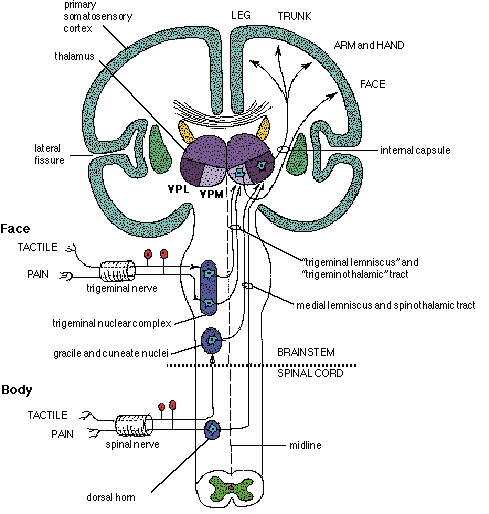
From the medulla, this data is shunted to the cerebellum (Schmahmann, 2011) and to the upper brainstem via the dorsal column pathways, projecting through through the medial lemnsical pathway where the axons carrying this information terminates in the ventral posterior nucleus of the thalamus (VPL and VPM), wherea again, considerable processing takes place (Friedman & Murray, 2006; Kaas, 2014; Ohye, 2010). These thalamic nuclei are further subdivided into subnuclei, each of which projects to specific regions within the primary and association somesthetic receiving areas, as well as to the insular region of the parietal lobe (Burton, 2006; Friedman & Murray, 2006; Kaas, 2014).
Specifically VPL and VPM act to relay impulses received from the ascending trigeminothalamic, spinothalamic, and medial lemniscus pathways to cortical layer IV. This input is somatotopically organized and the entire body surface comes to be spatially represented in the parietal neocortex. Again, however, subdivision within these thalamic nuclei (the posterior and medial ventral posterior complex) project to the association and insular regions of the parietal lobe--the latter of which is concerned with pain perception.
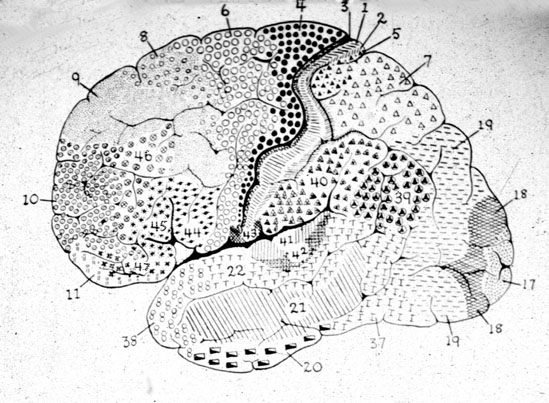
PARIETAL TOPOGRAPHY
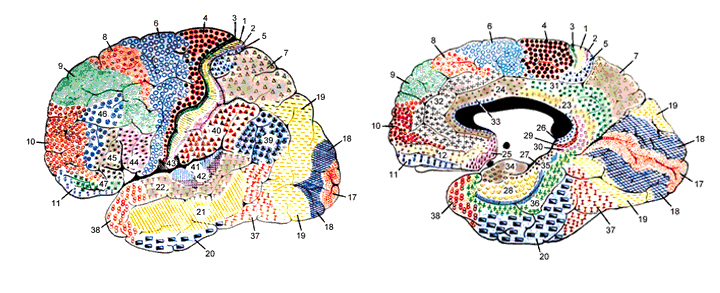
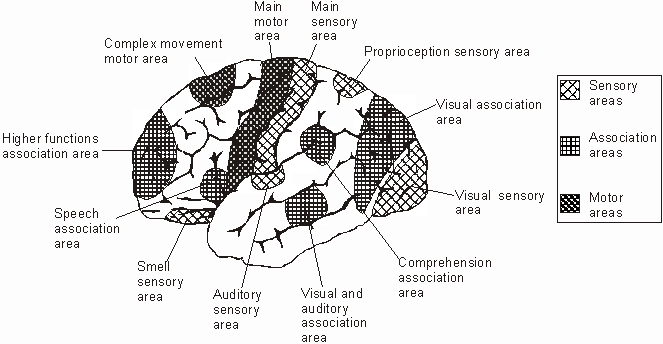
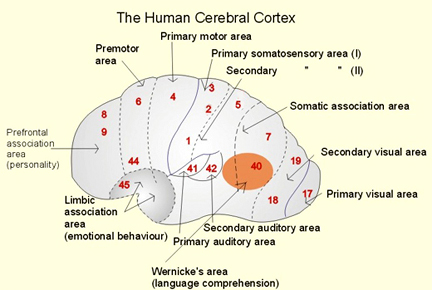
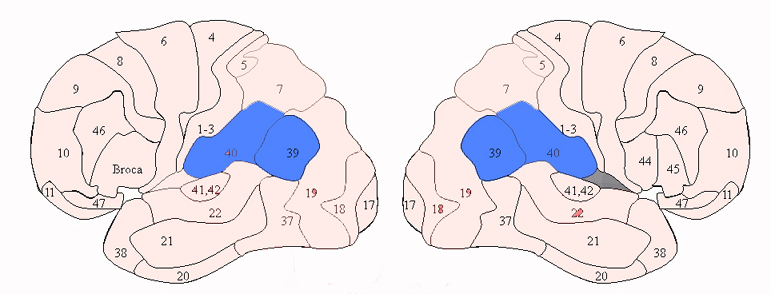
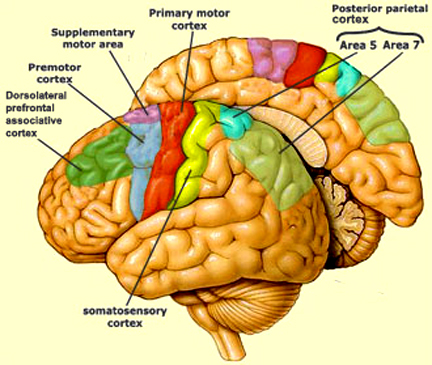
There are nine major somesthetic areas within the parietal lobe, such that the primary, association, and assimilation areas actually consist of numerous subareas. Broadly, and most generally, however, the parietal lobe may be subdivided into a primary receiving area (involving Brodmann's areas 3ab,1,2) within the post central gyrus, an immediately adjacent somesthetic association area (Brodmann's area 5ab), a polymodal (visual, motor, somesthetic) receiving area located in the superior-posterior parietal lobule (area 7ab), a granular insular area which is located in the inferior convexity and encompasses part of the marginal gyrus, and a multimodal-assimilation area within the inferior parietal lobule (areas 7, 39, 40) which encompasses the angular and supramarginal gyrus.

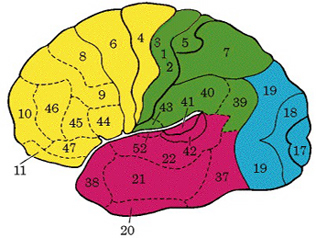
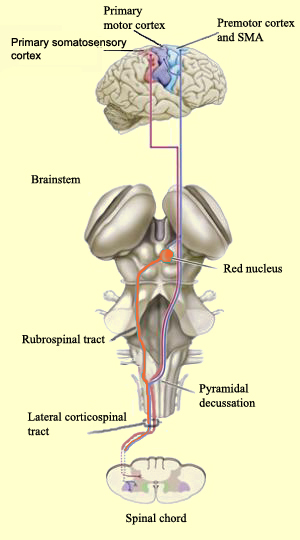
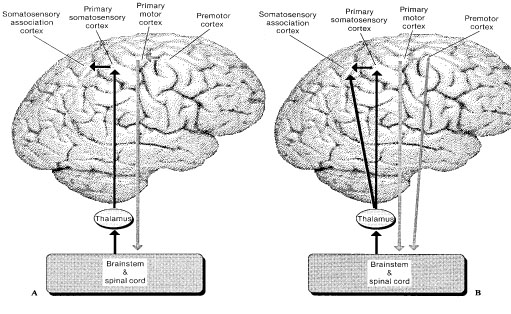
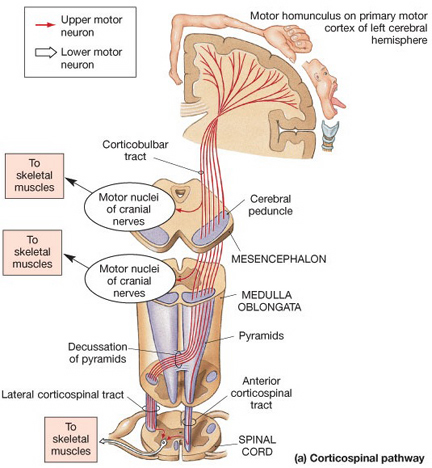
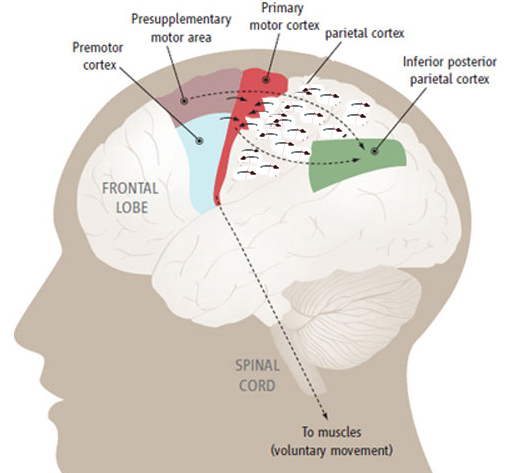
The primary somesthetic as well as portions of the association area contribute almost one third of the fibers which make up the cortical-spinal (pyramidal) tract. Hence, this region is very involved in motor functioning; e.g., the sensory and postural guidance of movement, including hand movements and the direction of gaze (Cohen et al. 2014; Dong et al. 2014; Snyder et al., 1998). Moreover, the primary motor and somesthetic association regions are richly interconnected (Jones & Powell, 1970) with the primary areas transmitting to the association areas which in turn project to the motor cortex. Indeed, in order to make motoric responses with some precision, there must be tremendous sensory feedback concerning proprioception, including data regarding the positions of the various joints and tendons, etc. --information which is provided by the somesthetic cortices (Cohen et al. 2014; Dong et al. 2014; Lebedev et al. 2014; Pred'Homme & Kalaska 2014; Snyder, et al., 1998). Together, the motor and somesthetic areas comprise a single functional unit which some have referred to as the sensorimotor cortex (Luria, 1980).
THE PRIMARY SOMESTHETIC RECEIVING AREAS
The primary somesthetic areas consists of three narrow strips of tissue (areas 3ab, 1, 2) which differ histologically, in architectural composition, and in sensory input. Moreover, each of these areas maintains a complete and independent representation of the body (Kaas, 2014).
Specifically, area 3a receives input from the muscles spindles (group IA muscle afferents) and can also signal muscle length (e.g. flexion or extension) whereas areas 3b receives cutaneous stimuli. Hence, area 3 appears to maintain cutaneous and muscular maps of the body. However, almost all of the cells in area 3ab receive input only from the contralateral half of the body. Hence, only half the body is represented.
These two maps, however, are also semi-independent, and in some respects they do not directly correspond to the location of body parts along the body surface, but instead are organized in regard to those parts which most frequently interact. That is, certain body parts more greatly represented in accordance with their sensory importance. However, in area 3b, for example, the hands, fingers, and jaw and mouth are juxtaposed--a function of the interaction of the hand and mouth when eating food (Kaas, 2014). In this way, an individual can coordinate their hand movements toward their mouth.
Information received and processed in area 3 is relayed to the immediately adjacent areas 1, and 2; each of which also contain a specialized spatial map of the body (Kaas, 2014; Kaas et al. 1981; Lin et al. 2014; Sur et al. 1982). For example, area 1 appears to maintain an overlapping cutaneous-joint body map (Evarts, 1969; Mountcastle & Powell, 1959; Schwartz et al. 1973). Area 2 maintains a map of the joint receptors and can signal the position and posture of the limbs based on input from the muscle spindles. Hence, the somesthetic cortex maintains four independent maps of the body.
Moreover, within this tiny expanse of tissue there is a sequential hierarchical convergence of input from areas 3, to 1, onto area 2. That is, information is analyzed and then passed from area 3 to 1, and from 3 and 1 onto area 2, as well as from area 3 to area 2. Therefore, a single neuron in area 2 receives multiple input from several cells in area 1 and 3, as well as vestibular input.
This organization is evident anatomically and as based on single cell recording and evoked potentials. For example, evoked potentials appear in area 1 about 5 msec, after they appear in area 3b. However, the degree of activation is also dependent on the attentional state and degree of arousal. With minimal attention to the source of input, there is minimal activation, which is why sensations from the clothing, shoes, or while sitting, lying down, and so on, can rapidly fade from consciousness.
Together these four strips of tissue comprise an interactional functional unit and are responsive to touch, texture, shape, motion, and the direction of stimulus movement, including temporal-sequential patterning, and can directly monitor the position and movement of the extremities (Cohen et al. 2014; Lebedev et al. 2014; Levitt & Levitt, 1968; Lin et al. 2014; Pred'Homme & Kalaska 2014; Mountcastle, 1957; Warren et al. 2006ab; Whitsel et al. 1972). Many cells are also responsive to changes in temperature as well as the presence of noxious stimuli applied to the skin.
Because the majority of these neurons receive input concerning pressure, light touch, vibration, the movement of joints, and muscular activity (Cohen et al. 2014; Lebedev et al. 2014; Levitt & Levitt, 1968; Prud'Homme & Kalaska 2014; Mountcastle, 1957) they can signal and determine whatever posture or position the body is in as well as the amount of force or pressure being exerted by the limbs (Jennings et al. 1983), i.e. if carrying or lifting some object. Conversely, via the reception and analysis of this input an individual can detect an insect crawling up or down their leg, the direction it is moving, as well as determine the position of their arms and legs without looking at them.
Nevertheless, predominantly elementary and simple contralateral somesthetic information is processed in this region (Lin et al. 2014; Prud'Homme & Kalaska 2014). Electrical stimulation of the primary somesthetic area gives rise to simple, albeit well localized sensations on the opposite half of the body (Penfield & Boldrey, 1937; Penfield & Jasper, 1954; Penfield & Rasmuseen, 1950) such as numbness, pressure, tingling, itching, tickling and warmth.
BODY IMAGE REPRESENTATION
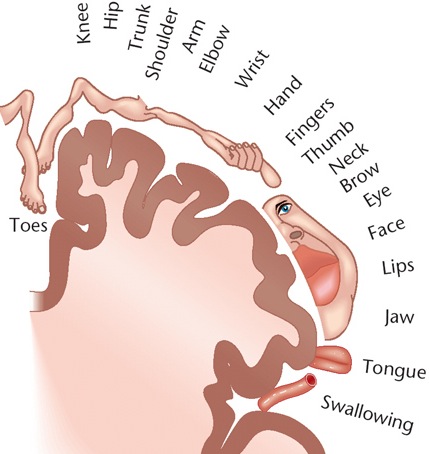
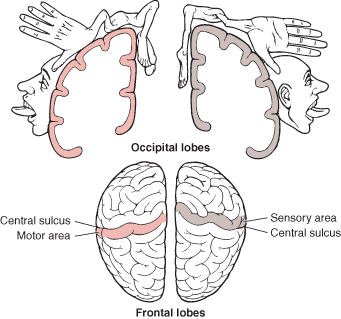
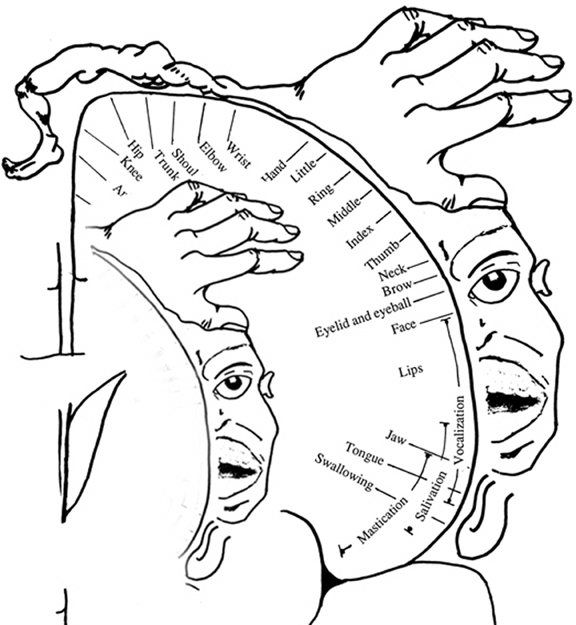
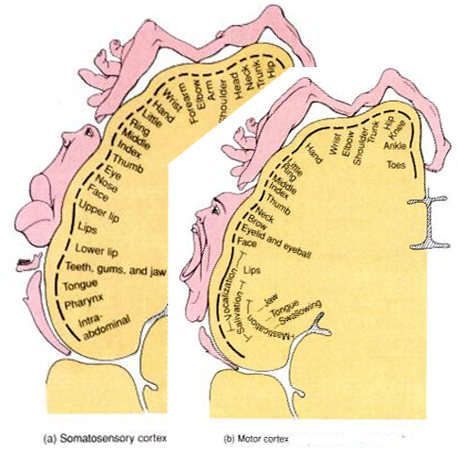
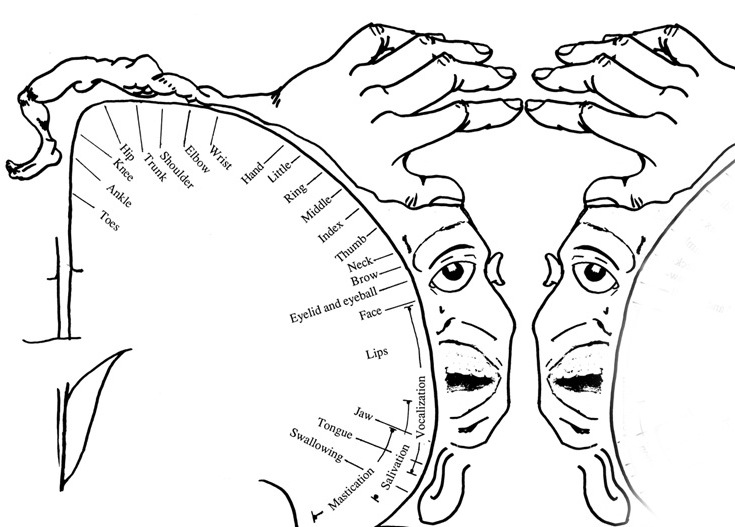
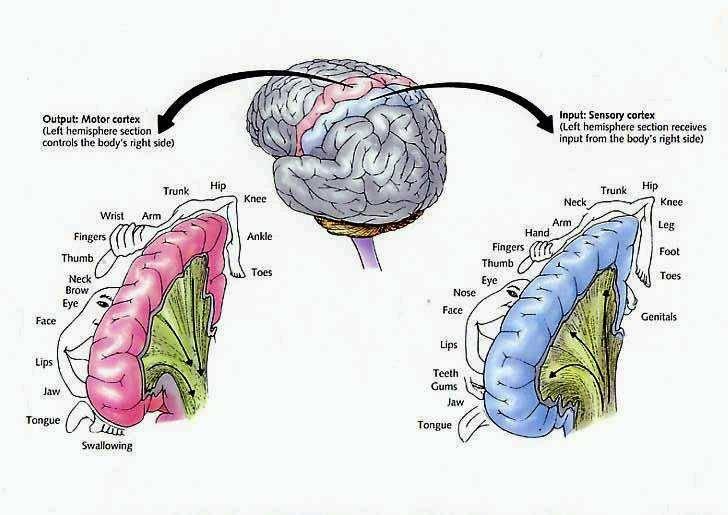
The primary receiving areas for somesthesis continues up and over the top of the hemisphere and along the medial wall where the lower half of the body is represented. Specifically the rectum, genitals, foot and calf are located along the medial wall, the leg along the superior surface of the hemisphere, and the shoulder, arm, hand and then face along the lateral convexity (Penfield & Boldrey, 1937; Penfield & Jasper, 1954; Penfield & Rasmussen, 1950).
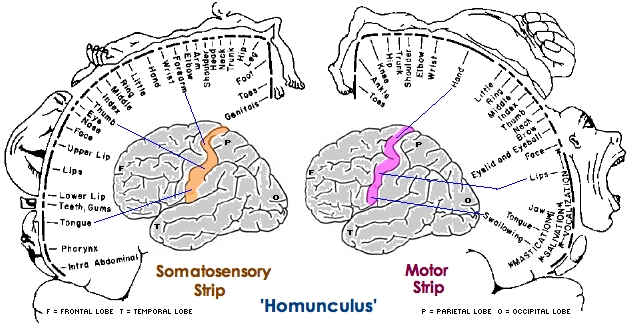
Body parts are also represented in terms of their sensory importance, i.e. how richly the skin is innervated. For example, more cortical space is devoted to the representation of the mouth, fingers and the hand than to the elbow or trunk (Warren et al. 2006). In fact, the area devoted to representation of the fingers is 100 times larger than the area devoted to the trunk. Because of this the cortical body map is very distorted. However, as noted, some areas are also juxtaposed, such as the hand and mouth area.
In summary, the primary receiving area receives very precise information regarding events occurring anywhere along the internal/external body and responds to converging inputs from muscle spindles, cutaneous and joint receptors, as well as proprioceptive and vestibular stimuli. In this manner, not only the body but the global properties of objects held in the hand can be determined (Iwamura & Tanaka, 1978); i.e. stereognosis.
FUNCTIONAL LATERALITY
As detailed in Chapter 10, there is clear evidence that the right parietal area is dominant in regard to many aspects of somesthetic information processing. Hence, neurons in this half of the brain appear to be more sensitive and more responsive and to more greatly monitor events occurring on either half of the body, but particularly the left. In fact, this relationship was noted over 150 years ago by Weber.
According to Weber (1834/1977), the left half of the body exceeds the right in regard to most forms of tactual sensitivity. The left hand and the soles of the left foot, as well as the left shoulder are more accurate in judging weight, have a more delicate sense of touch and temperature, such that "a greater sense of cold or of heat is aroused in the left hand" (p. 322). That is, the left hand judges warm substances to be hotter, and cold material to be colder as compared to the right hand, even when both hands are simultaneously stimulated.
The right half of the brain, in fact, appears to maintain multiple images of the body. Therefore, the parietal lobe of the right hemisphere appears to have more neocortical space devoted to maintaining images of the body.
SOMESTHETIC AGNOSIAS
Surgical or other forms of destruction involving the primary somesthetic receiving areas results in a complete, albeit, temporary loss of sensation from the entire half of the body. that is, with damage to the right or left parietal lobe (the right in particular) the patient may no longer feel any sensation, including pain, from the right or left half of the body. However, if the lesion involves just the hand, or leg area, then sensation only from the hand or leg will no longer be perceived.
Depending on the extent of the lesion, the effects may include elevation of sensory detection thresholds, loss of position and pressure sense, including two-point discrimination, and a greatly reduced ability to detect movement of the fingers. In addition, the capacity to determine texture, shape, temporal-sequential patterning, or to recognize objects by touch or to discriminate among different forms or their properties, e.g. size, texture, length, shape and stereognosis is significantly attenuated (Corkin et al. 1970; Curtis et al. 1972; LaMotte & Mountcastle, 1979; Randolf & Semmes, 1974). Passive, (non-movement) sensation is less impaired. In some instances, over time a remarkable recovery of somesthetic discrimination sense may be observed (Semmes, 1973).
Nevertheless, even with complete removal of the post-central gyrus, stimuli applied to the face are much better perceived as compared to the same stimuli applied to the hand. This is because more area is devoted to the face than the hand, whereas more area is devoted to the hand than to the legs.
Lesions to the parietal lobe which spare the hand area of the post-central gyrus, but which destroy the remaining tissue, will result in mild or no permanent sensory deficits when the hands are tested (with the exception of stereognosis; Semmes, 1965). The patient will continue to experience sensations from their hand, but not from the rest of the body. Sensations from across other body parts will not be perceived. Hence, when testing for parietal lobe dysfunction, not only the face and hands, but other body parts should be examined.
As noted, the parietal lobe is also highly concerned with motor functioning as it is extensively interconnected with the primary motor area and gives rise to almost 1/3 of the cortico-spinal tract. Hence, damage to this region can give rise to motor disturbances such as paresis accompanied by hypotonia, and/or produce inaccuracy and reduced speed of movement (Cole & Glees, 1954; Luria, 1980). The ability (or will) to initiate movement may also be reduced.
THE SOMESTHETIC (SUPPLEMENTARY) ASSOCIATION AREA
In addition to receiving information already analyzed in the immediately adjacent primary somesthetic cortices, some cells in the association area (Brodmann's area 5ab) also receive input from the contralateral primary zone in the opposite hemisphere (via the corpus callosum) as well as from the motor association areas (area 6) in the frontal lobes (Dong et al. 2014; Jones & Powell, 1970). These cortical areas are all linked together and in this way movement of the entire body can be coordinated in visual-physical space.
The secondary somesthetic area also received input from the thalamus the posterior, i.e. the ventral posterior complex (Burton, 2006; Friedman & Murray, 2006; Kaas, 2014).
Due in large part to the callosal interconnections linking the secondary (supplementary) sensory areas in the right and left half of the brain, both halves of the body, the trunkal area in particular (Robinson, 1973) are represented in this region. Indeed, the two halves of the body appear to be superimposed such that the body is bilaterally represented in the secondary sensory areas (Whitsel et al. 1969).
However, as based on behavioral and electrophysiological data from intact and brain damaged individuals, bilateral represention is predominantly maintained within the right half of the human brain. It is this greater and bilateral degree of representation and the more unilateral nature of left parietal representation, which explains the greater incidence of neglect (see below) and the greater degree of hemiplegia and hemianesthesia which is seen after right vs left parietal lesions (Sterzi et al., 2014).
HAND MANIPULATION CELLS
A small percentage of cells in area 5 also appear to be concerned with more complex activities such as the movement of the hand and arm and the manipulation of objects (Cohen et al. 2014; Mountcastle et al., 1975). Indeed, a detailed representation of the cutaneous surface of the body, and in particular, the hand (and face), is maintained in here (Burton, et al. 1982; Lin et al. 2014). These are referred to as "hand-manipulation cells". In fact, electrical stimulation of area 5 can result in limb movements (Hyvariene, 1982) whereas experimentally induced tactile extinction of either the right or left hand can induce bilateral reductions in activity in this region (Remy et al., 2008).


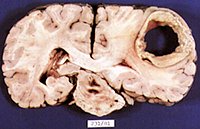
In consequence, injury to this part of the brain can interfere with hand movements. For example, a patient I examined with a right superior parietal tumor, was unable, both before and after surgery, to correctly reach for items that were located to the left or right side of his body and would invariable miss or knock the items over. He displayed stereognosis when he was asked to name (with his eyes closed) objects held in his right or left hand (worse in the left hand) also had severe difficulty with block design and puzzles from the WAIS-R, could not throw or catch with accuracy, and suffered a loss of vision in the left lower quadrant of his visual field--though there was no evidence of neglect. In fact, according to this patient, the first sign of his illness was the loss of the ability to throw with accuracy, which he noticed when he began missing the "basket ball" hoop net he had set up above the garbage can in his office. However, in this case, the tumor and resection also partly involved the superior portion of area 7.
Others neurons in area 5 are especially responsive to particular temporal-sequential patterns of sensation (LaMotte & Mountcastel, 1979) and can determine direction and rhythm of movement. It is presumably through the activity of these cells that one can "hear" via the detection of vibrations (such as reported by the deaf). In fact, auditory as well as visual simulation can induce activation of this region (Lam et al., 2008).
THE BODY IN SPACE
Signals from joint and cutaneous receptors are transmitted to association neurons (Skata & Iwanura 1978). Many association cells also receive converging input from primary neurons concerned with different body parts (Dong et al. 2014; Sakata, et al. 1973), and can combine these signals with visual information (Snyder et al., 1998) and are thus able to determine positional interrelationships.
For example, a single association neuron may receive information regarding the elbow and the shoulder, and become activated only when these two body parts are simultaneously stimulated or in motion. A considerable number of cells are especially sensitive to the posture and position of the trunk and extremities during movement (Hyvarienen, 1982). By associating this convergent input these cells are thus able to monitor, coordinate and guide limb movement (Cohen et al. 2014) as well as determine the position of the body and objects in space.
Through the integrative and associative activities of the cell assemblies within area five, an interactional image of the body is maintained. In this manner, an individual is able to ascertain the posiion of the body and the limbs at rest and in motion (Gross, et al. 1974). Therefore, an individual may run and catch a football by coordinating and integrating sensations from the body and visual-space in conjunction with the hands.
In part, this may be accomplished through comparisons with a more stable image of the body which is possibly maintained via the combined interactions of neurons in areas 3,1,2. That is a stable body image (or body image memories) are stored in these tissues. Hence, when the body has moved, this new information (received and processed in area 5) can be compared to the more stable trace (or memory maintained in the primary regions) so that the new position of the limbs and body can be ascertained. In this regard it could be argued that body-related memories are stored in the parietal lobe.
Nevertheless, to determine position, sensation per se is not sufficient. Rather, sensation must be combined with input regarding movement or positional change (Gandevia & Burke 1992). It is for this reason that in the absence of movement (and in the absence of visual cues, such as when one wakes up in the middle of the night) one usually cannot tell where or in what position their arms or legs may be in. However, with a slight movement we can immediately determine position.
TACTILE DISCRIMINATION DEFICITS & STEREOGNOSIS
Massive destruction of the somesthetic association area results in many of the same disturbances which occur following lesions of the primary region. This includes abnormalities involving two-point discrimination, position sense, and pressure sensitivity. However, detection threshold is not altered (LaMotte & Mountcastle, 1979). For example, a patient may recognize touch, but be unable to localize what part of the body has been stimulated.
For example, following a right parietal stroke one patient made gross errors of localization when I touched various regions of his body while his eyes were closed. For example, he named his elbow when I touch his leg, and his shoulder when his wrist was touched. He in fact expressed considerable astonishment when I allowed him to open his eyes after each test so as to see where the stimulation was actually being applied.
In addition, with destruction of this tissue, although a patient may be able to recognize that he is holding something in his hand he may be unable to determine what it might be (astereognosis). In these later instances, however, the cortical area representing the hand must be compromised.
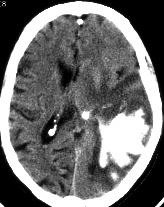
With small lesions involving only a particular part of the somesthetic cortex (e.g. the area representing the arm and shoulder), the deficit will be only manifested when the part of the body represented is examined. For example, the patient may be able to localize touch and determine the direction of a moving stimulus when it is applied to the hand, face, or leg, but be unable to do so along the shoulder or arm if this area of the cortex has been compromised.
The laterality of the lesion is also important, right sided damage having more drastic effects on somasthesis and stereognostic functioning than left parietal lesions. In addition, although lesions to either parietal lobe can give rise to astereognosis (an inability to recognize objects tactually explored), lesions to the right parietal lobe are likely to give rise to bilateral abnormalities, whereas left parietal injuries generally effect only the right hand (Hom & Reitan, 1982). Again, however, astereognostic deficits require that the somesthetic area representing the hand (see below) be comprised (Roland, 1976).
Larger lesions extending into the posterior parietal lobe, area 7, also decrease the ability to perform size, roughness, weight and shape discriminations (Blum, Chow & Pribram, 1950; Denny-Brown & Chambers, 1958; Garchas et al., 1982; Ridley & Ettlinger, 1975; Semmes & Turner, 1977).
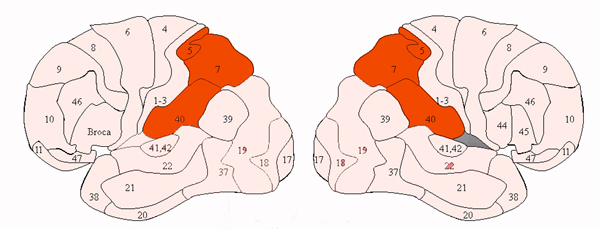
PAIN: AREAS 5, 7, & THE SUPRAMARGINAL GYRUS
As noted, neurons in area 5, as well as those located in the insula, receive direct thalamic input from the ventral and posterior portion of VPL. The ventral portion in particular, however, in addition to somesthetic information, may also convey pain sensation to the parietal lobe. In fact, Penfield and Boldrey (1937) reported that electrical stimulation of the parietal lobe resulted in the sensation of pain, albeit about 1% of the time.
Some neurons located in area 5 and 7 of the parietal lobe also demonstrate pain sensitivity, with some are 7 neurons responding exclusively to thermal and nociceptive stimuli (Dong et al. 2014) and with area 5 presumably acting to localize the source of pain. Hence, in some instances, such as when the more inferior portion of area 5, 7 or the supramarginal gyrus (Broadmann's area 40) has been destroyed, patients may demonstrate a lack of emotional responsiveness to painful stimuli, become indifferent, develop an increased pain threshold, tolerate pain for an unusually lengthy time period and fail to respond even to painful threat (Berkley & Parmer, 1974; Biemond, 1956; Geschwind, 1965; Greenspan & Winfield 1992; Hyvarinen, 1982; Schilder, 1935) --particularly with right parietal destruction (Cubelli et al 2004). However, disturbance or lack of pain sensation has been noted to occur when lesions to either hemisphere (Hecaen & Albert, 1978).
Moreover, loss of sensation or an inability to react to pain may also occur from subcortical lesions, especially within the thalamus, and less often, with surgical destruction of the anterior cingulate--the so called center of "pain and misery." In this regard, there appears to be two major cerebral pain pathways, a subcortical medial pathway involving the thalamus and cingulate, and a neocortical pathway involving the parietal lobe.
At the neocortical level, although pain responsiveness may be diminished or absent following damage to these tissues, elementary sensation remains intact and the ability to differentiate, for example, between dull and sharp is retained. The deficit is usually bilateral.
Some researchers have claimed that in order to lose pain sensitivity the lesion sometimes involves the frontal-parietal cortex (Hecaen & Albert, 1978). However, the supramarginal gyrus of the inferior parietal lobule (Geschwind, 1965; Hyvarinen, 1982; Schilder, 1935) and area 7 of the superior parietal lobule (Dong et al. 2014; Greenspan & Winfield 1992) are the most likely candidates for this condition --particularly in that a second somesthetic area is located here as well as yet another image of the human body (Penfield & Rasmussen, 1950).

In this regard, Schilder (1935), has argued that the loss of reaction to pain is due to disturbances in the image of the body. That is, the experience or threat of pain is no longer related to the body image. Geschwind, (1965), however, raises the possibility that this condition is due to disconnection from the limbic system (see Cavada & Goldman-Rakic 2009). If this were the case, somesthetic (painful) sensation would no longer be assigned emotional significance and would thus implicate the insular region of the parietal lobe, which also receives visceral as well as somesthetic information and funnels this data to the limbic system.
In fact, this same insular-limbic pathway may serve to promote tactile memory; that is, via the funneling of complex somesthetic information to the hippocampus and amygdala. Conversely, it may be this same pathway which when abnormally activated or injured, may give rise to abnormal emotional significance being attributed to bodily sensations.
PAIN AND HYSTERIA
Whereas destruction of the inferior portions of areas 7, 5, and 40 may result in loss of pain sensation, when the injury is secondary to tumor or seizure activity patients may instead report experiencing pain (Davidson & Schick, 1935; Hernandez-Peon et al. 1963; Ruff, 1980; Wilkinson, 1973; York et al. 1979). In addition patients may experience sensory distortions that concern various body parts due to abnormal activation of the parietal neocortex.
For example, one 48 year old housewife complained of diffuse, poorly localized (albeit intense) pain in her left leg, which occurred in spasms that lasted minutes. She subsequently was found to have a large tumor in the right parietal area, which, when removed, alleviated all further attacks. Head and Holmes (1911) reported a patient who suffered brief attacks of "electric shock"-like pain that radiated from his foot to the trunk; a glioma in the right parietal area subsequently was discovered.

Sometimes the pain may be related to abnormal sexual or genital sensations. For example, one 9-year-old boy seizure activity in the right parietal area experienced spontaneous attacks of intense scrotal and testicular pain (York et al.1979). A 42 year old man with a right parietal tumor complained of episodes of intense genital pain that he described as similar to blunt impact against his testicles.
Ruff (1980) reports two cases who experienced paroxsymal episodes of spontaneous and painful orgasm, which was secondary to right parietal seizure activity. In one patient the episodes began with the sensation of clitoral warmth, engorgement of the breasts, tachycardia, etc., all of which rapidly escalated to a painful climax.
It is important to note, however, that although the predominant focus for paroxysmal pain is the right hemisphere, pain also has been reported to occur with tumors or seizures activity that involves the left parietal region (Bhaskar, 1987; McFie & Zangwill, 1960).
Unfortunately, when the patient's symptoms are not considered from a neurological perspective, their complaints with regard to pain may be viewed as psychogenic in origin. This is because the sensation of pain, stiffness, engorgement, is, indeed, entirely "in their head" and based on distorted neurological perceptual functioning. Physical exam may reveal nothing wrong with the seemingly affected limb or organ. Thus such patients may be viewed as hysterical or hypochondriacle, particularly in that right hemisphere damage also disrupts emotional functioning.
AREA 7 & THE SUPERIOR-POSTERIOR PARIETAL LOBULE
POLYMODAL INFORMATION PROCESSING
Information processed and analyzed in the primary and association somesthetic areas is then transmitted to area 7 where polymodal analyses take place (Dong et al. 2014; Lam et al., 2008; Previc 2010). Jones & Powell (1970) considered area 7 to be concerned with the highest levels of somesthetic integration as it receives massive input from the visual receiving areas in the occipital and middle temporal lobe, from the motor and non-motor areas in the lateral frontal convexity as well as from the inferior parietal and temporal lobe including the parahippocampal gyrus (Cavada & Goldman-Rakic 2009; Dong et al. 2014; Jones & Powell, 1970; Previc 2010; Wall et al. 1982). Cells in area 7 also have auditory receptive capacities, including the ability to determine sound location (Hyvarinen 1982).
Hence, area 7 is heavily involved in the analysis and integration of higher order visual, auditory and somesthetic information, and single neurons often have quite divergent capabilities. Moreover, cells in area 7 process both body-referenced and world-referenced signals, which provide information for the control of gaze and navigation and movement in space (Synder et al., 2011).
THREE-DIMENSIONAL ANALYSIS OF BODY-SPATIAL INTERACTION
A single area 7 neuron via the reception of converging input from the primary and association somesthetic regions can monitor activities occuring in many different body parts simultaneously; for example, the position and movement of the arms, trunk, and legs (Leinonen et al. 1979). Via the reception of auditory, somesthetic, and visual input, area 7 is thus also able to create a three-dimensional image of the body in space (Lynch, 1980).
Moreover, cells in this area not only receive information about body part interrelationships (such as is maintained by area 5), but the interaction of the body with external objects and events in visual space (Snyder et al., 1998; Stein 1992). Indeed, many cells in this vicinty become highly active when the hand is moved toward or while reaching for and/or manipulating objects (Mountcastle et al. 1975; Robinson et al. 1978; Yin & Mountcastel, 1977). They also act to coordinate and guide gaze and whole body-positional movement through visual and auditory space (Snyder et al., 1998).

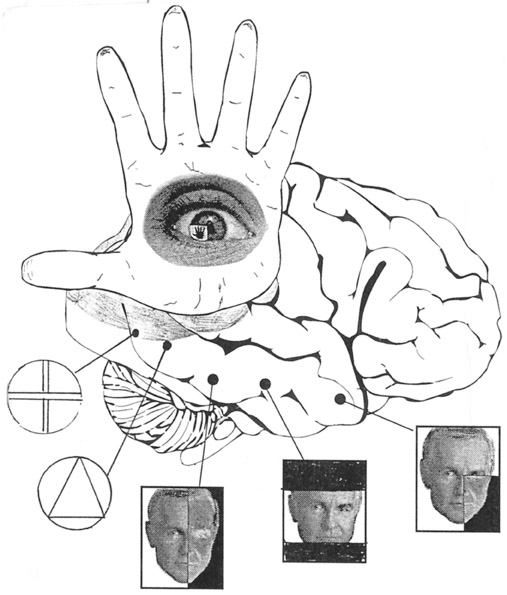
As stated by Mountcastle, Motter & Andersen, (1980), "the parietal lobe, together with the distributed system of which it is a central node, generates an internal neural construction of the immediately surrounding space, of the location and movements of objects within it in relation to body position, and of the position and movements of the body in relation to that immediately surrounding space. The region appears in general to be concerned with continually updating information regarding the relation between internal and external coordinant systems" (p.522).
VISUAL-SPATIAL PROPERTIES
As noted in chapter 5, the parietal lobe, in part, evolved from the hippocampus, and maintains rich interconnections with this nucleus via the parahippocampal gyrus. Given that the hippocampus is concerned with the body-in-space, and with the position of various objects and stimuli in visual space (Nadel, 1991; O'Keefe, 1976; Wilson and McNaughton, 2014), not surprisingly, the parietal lobe performs similar functions.

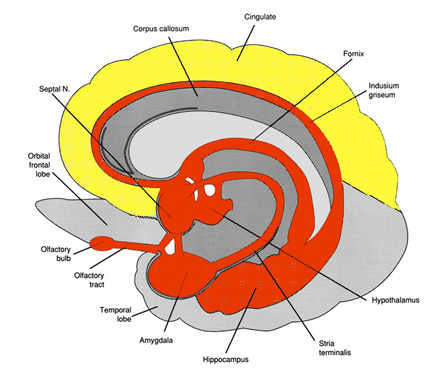

Although cells in area 7 receive and transmit data to the hippocampus via the parahippocampal gyrus, these neurons also accomplished these functions via the convergence of somesthetic information from area 5, visual input from the visual association areas, as well as via the reception of midbrain and vestibular signals (Cavada & Goldman-Rakic 2009; Gross & Graziano 2013; Hyvarinene, 1982; Kawano & Sasaki, 2004; Previc 2010). Like some hippocampal neurons, single parietal neurons are also involved in the representation of space (Gross & Graziano 2013; Stein 1992) as well as the body in space (Synder et al., 2008) including topographic learning.
Area 7 neurons also have quite large visual receptive fields, sometimes occupying a whole quadrant, hemifield, or the entire visual field (Robinson et al., 1978). However, the receptive visual fields of these neurons do not usually include the fovea and are more sensitive to objects in the periphery and lower visual fields (Motter & Mountcastle, 1981; Previc 2010); i.e. where the hands, feet, and ground are more likely to be viewed. In this regard, many of these cells are not concerned with the identification of form but rather place, position, and reaching distance as well as depth perception and the coordination of the body as it moves through space. To accomplish this, however, requires that this area of the brain also participate in memory and place this information in short-term memory so that comparison can be made and information retained.
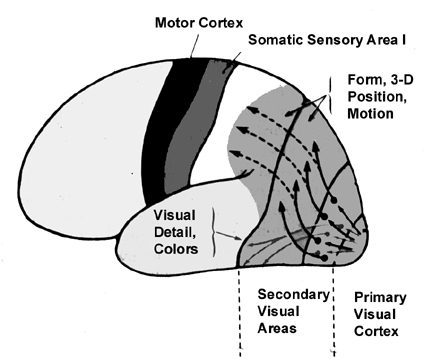
Because many of the neurons in area 7 receive highly processed visual (as well as limbic) input, including information regarding ocular movement and direction of gaze (Snyder et al., 1998), they are also responsive to and can determine a variety of visual-object qualities and interrelationships, such as motivational significance, direction of movement, distance, spatial location, figure-ground relationships, and depth, including the discrimination and determination of an objects 3-dimensional position in space, (Andersen et al. 2005; Gross & Graziano 2013; Kawano,et al., 2004; Lynch, 1980; Previc 2010; Sakata et al. 1978,1980; Snyder et al., 1998; Stein 1992). They are largely insensitive to velocity or speed of movement. In addition, many cells respond most to stimuli which are within grasping distance, whereas others respond most to stimuli which are just beyond arms reach.
Neurons in this supramodal region are able to accomplish this by responding to somesthetic positional information provided by area 5, visual input from areas 18,19, and the inferior and middle temporal lobe and hippocampus, as well as from extraretinal signals regarding convergence and accomodation of the eyes, and the position and movement of the eyes while tracking (Gross & Graziano 2013; Previc 2010). Indeed, electrical sitmulation of this area elicits eye movements as well as convergence, accomodation, and pupil dilation (Jampel, 1960). By integrating these signals these cells are able to monitor and mediate eye movement and visual fixation, map out the three-dimensional positions of various objects in visual space, and determine the relation of these objects to the body and to other objects.
Thus the visual analysis performed by many of these cells is largely concerned with visual spatial functions (Robinson et al., 1978; Sakata et al., 1980). Moreover, this area maintains extensive interconnections with two other regions highly concerned with visual functions and eye movements, i.e. the frontal eye fields and superior colliculus (Jones & Powell, 1970; Kawamura et al. 1974; Knuenzle & AKert, 1977; Pandya & Kuypers, 1969; Previc 2010) as well as visual areas 18 and 19 (Jones & Powell, 1970).
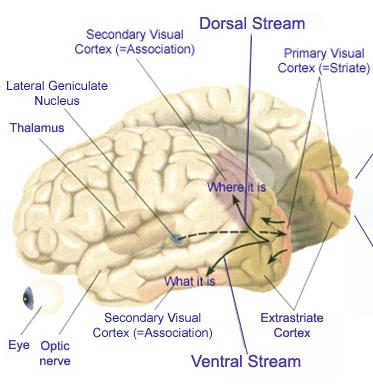
Conversely, when this area is damaged, depth perception, figure-ground analysis, and the ability to tract objects or to correctly manipulate objects in space (e.g. constructional and manipulospatial skills) becomes compromised.
VISUAL ATTENTION
Many neurons in area 7 can act so as to increase or decrease visual fixation, direct attention to objects of motivational significance, and promote the maintanance of visual grasp such that a moving object continues to be visually scanned and followed (Lynch et al., 1977; Previc 2010). Electrical stimulation of this area induces lateral eye movements due to interconnections maintained with the frontal eye fields, visual cortex, and subcortical visual centers.
In contrast, lesions to this area often disrupt attentional functioning, such that in the extreme (for example, following a right parietal lesion) patients fail to attend to the left half of not only visual space, but the left half of the body; i.e. neglect. Neglect is most common with right cerebral lesions but in some instances may be seen initially following massive left cerebral destruction (Chapter 10; see also Umilta 2013).
As detailed below, with circumscribed parietal lesions involving area 7, neglect is more pronounced for the lower visual fields. The lower visual fields, in turn are a predominant sensory domain of the parietal lobes, for this is the area of visual space in which the hands and feet are most likely to view -therefore making movement of the body in space more efficient. The lower visual field is also the area in which objects are most likely to be physically explored by the hand, which in turn are guided by the parietal lobe.
MOTIVATIONAL & GRASPING FUNCTIONS
A number of cells in area 7 have been described as exerting "Command" functions (Mountcastle, 1976; Mountcastle et al., 1980), especially those located along the inferior lateral convexity. These cells are motivationally responsive, can direct visual attention, become excited when certain objects are within grasping distance and can motivate and guide hand movements including the grasping and manipulation of specific objects (Hyvarinene & Poranene, 1974; Lynch et al., 1977; Mountcastle, 1976). Most of these latter cells cease to fire when the object fixated upon is actually grasped, suggesting that they may be exerting some type of driving force or at least an alerting function so that objects of desire or of general (vs. specific) interest will be attended to (Rolls et al. 1979).
It has been argued that many neurons in area 7 actually execute a matching function between the internal drive state of the subject and the object which is being attended. That is, by responding to signals transmitted from the limbic system (Cavada & Goldman-Rakic 1999; Mesulam et al. 1977), e.g. the cingulate gyrus as well as the middle and inferior temporal lobe, these cells in turn direct visual attention to objects of potential interest, and when detected, act so as to maintain visual grasp (Lynch et al., 1977).
In other words, when an object is recognized as being of motivational significance (determined by the limbic system and visual form recognition neurons in the temporal lobe), this information is relayed to neurons in area 7. Although not concerned with form recognition, these cells will guide as well as monitor eye movement so that the object of interest is fixated upon. These cells then exert motor command functions so that the hand is guided toward the object until it is grasped.
THE RIGHT & LEFT PARIETAL LOBE: LESIONS & LATERALITY
ATTENTION & VISUAL SPACE
Lesions involving the superior, as well as the inferior parietal lobule (of which area 7 is part) and the parietal-occipital junction can greatly disturb the ability to make eye movements, maintain or shift visual attention, visually follow moving objects, and in the extreme result in oculomotor paralysis (Hecaen & De Ajuriaguerra, 1954; Previc 2010). Right parietal lesions are associated with deficiencies involving depth perception and stereopsis, including the abilIty to determine location, distance, spatial orientation and object size (Benton & Hecaen, 1970; Ratcliff & Davies-Jones, 1972). Visual constructional abilities may also be compromised (see Cowey, 1981; Critchley, 1953) and many patients suffer from visual-spatial disorientation and appear clumsy.
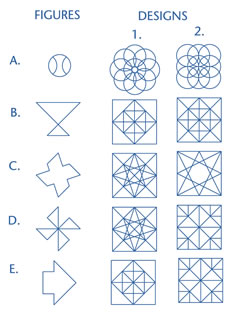
Individuals with right parietal lesions show defective performance on line orientation tasks (Warrington & Rabin, 1970; Benton et al. 1975), maze learning (Newcombe & Russell, 1969), the ability to discriminate between unfamiliar faces (Milner, 1968), or to select from the visual envrionment stimuli which are of importance is also a consequence of right parietal lesions (Critchley, 1953), whereas those with right parietal-occipital damage are deficient on tasks requiring detection of imbedded figures (Russo & Vignolo, 1967).
Others may also have severe problems with dressing (e.g. dressing apraxia) and may become easily lost or disoriented even in their own homes. One patient I examined with a gun shot wound involving predominantly the right superior posterior parietal area was unable to find his way to and from his hospital room (although he had been an inpatient for over 3 months) and on several occasions had difficulty finding his way out of the bathroom. Indeed, in one instance he was discovered feeling his way along the walls in his attempt to find the door.
Commonly right parietal injuries can result in a complete neglect of the left half of visual space. By contrast, left parietal injuries result in only minimal right sided neglect. Again, this is because the right parietal lobe not only attends to both halves of the body, but both halves of visual space (Joseph, 2006ab, 1988a).
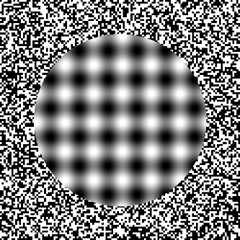
LOCALIZATION OF OBJECTS IN SPACE
Patients with right parietal lesions perform defectively on visual localization tasks (Hannay, Varney, & Benson, 1976). However, Ratcliff and Davies-Jones (1976) found that the localization of stimuli within grasping distance is disrupted equally by lesions to the posterior region of either hemisphere. Hence, the right parietal lobe appears to play an important role in generalized localization, whereas the left exerts influences in regard to objects which may be directly grasped and manipulated.
APRAXIA
The parietal lobes mediate hand, arm and body movements in space, as well as temporal-sequencing (Joseph 2014; Kimura 2014; Lynch et al. 1977). Hence, damage to this vicinity can also result in apraxia such that individuals have difficulty controlling or temporally sequencing the extremities. That is, they have difficulty performing tasks requiring a sequence of steps. For example, taking a bottle, screwing off the cap, taking a cup, and pouring the liquid into the cup, drinking from the cup. They may get the order confused and be unable to perform simple tasks.
This is especially evident with left rather than right parietal injuries. There can also result gross inaccuracies as well as clumsiness when making reaching movements or when attempting to pick up small objects in visual space (Kimura 2014; Lynch, 1980). Tendon reflexes may be slowed, and hypotonia coupled with a paucity of and/or a slowness in movement initiation may result (Denny-Brown & Chambers, 1958; LaMotte & Acunam, 1978; Lynch, 1980). With left parietal damage there may result difficulty visually recognizing objects (agnosia), and left-right orientation may be grossly deficient.
EMOTION
Some of the effects of lesions in this region also include altered emotional-motivational functioning, body and visual-spacial neglect, as well as clumsiness and visual-spatial disorganization. With massive right parietal lesions involving area 7, many patients are often initially hypokinetic and seem very passive, inattentive, unresponsive and take very little interest in their environment (Critchley, 1953; Heilman & Watson, 1977). Moreover, when their disabilities are pointed out (e.g. paresis, paralysis), they may seem indifferent or conversely euphoric (Critchley, 1953).
Area 7, as well as 5 (and the inferior parietal lobule) receive auditory information (Cavada & Goldman-Rakic 1999; Hyvarinen, 1982; Lam et al., 2008; Roland et al., 1977) and are capable of discerning the emotional-motivational significance of this input as well as differentiating between different vocal-emotional characteristics --especially right parietal neurons. Hence, when the right parietal region is damaged, patients not only seem unconcerned about their disability, but they may have difficulty perceiving and differentiating between different forms of emotional speech (Tucker, Watson & Heilman, 1977).
THE INFERIOR PARIETAL LOBULE
Developmentally, of all cortical regions, the inferior parietal lobule is one of the last to functionally and anatomically mature (Blinkov & Glezer, 1968; Flechsig, 1901; Conel, 1937-1943; Joseph & Gallagher, 2005, Joseph et al., 2004). Hence, many capacities mediated by this area (e.g. reading, calculation, the performance of reversible operations in space) are late to develop appearing between the ages of 5-8.
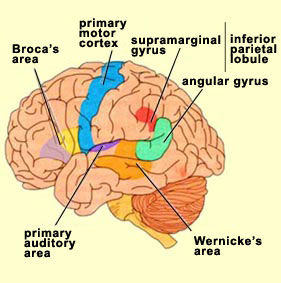
Sitting at the junction of the temporal, parietal, and occipital lobes, the inferior region (which includes the angular and supramarginal gyri) has no strict anatomical boundaries, is partly coextensive with the posterior-superior temporal gyrus, and includes part of area 7 as well as area 37. It maintains rich interconnections with the visual, auditory, and somesthetic associations areas including the middle (basal) temporal lobe, the superior colliculus via the pulvinar, the lateral geniculate nucleus of the thalamus, and massive interconnections with the frontal lobes, inferior temporal region, and other higher order assimilation areas throughout the neocortex (Bruce, Desimone & Gross, 2006;Burton & Jones, 1976; Geschwind, 1965; Jones & Powell, 1970; Seltzer & Pandya, 1978; Zeki, 1974).
THE MULTI-MODAL ASSIMILATION AREA
As noted in chapters 5 and 12, over the course of evolution the amygdala, hippocampus, and medial temporal lobe began to balloon outward and upward, giving rise to superior temporal lobe, and then continuing to expand in a posterior direction, forming part of the angular and marginal gyrus. Hence, this portion of the inferior parietal lobule has auditory and thus (in the left hemisphere) language capabilities. However, with the evolution of the thumb and the capability of utilizing a precision grasp coupled with tool making and related temporal-sequential tasks, the superior parietal lobule also expanded, thereby also giving rise to inferior parietal neocortical tissue.
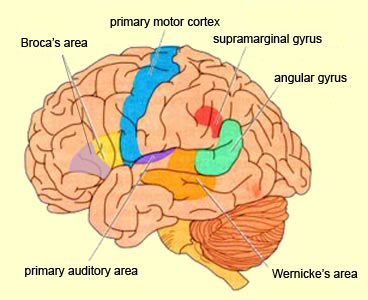
Given its location at the border regions of the somesthetic, auditory, and visual neocortices, and containing neurons and receiving input from these modalities, as the inferior parietal lobule evolved it became increasingly multimodally responsive; a single neuron simultaneously receiving highly processed somesthetic, visual, auditory and movement related input from the various association areas. Hence, many of the neurons in this area are multi-specialized for simultaneously analyzing auditory, somesthetic, and spatial-visual associations, and have visual receptive properties which encompass almost the entire visual field, with some cells responding to visual stimuli of almost any size, shape, or form (Bruce et al. 1982, 2006; Hyvaerinene & Shelepin, 1979).
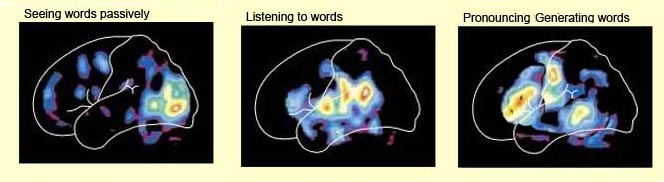
Inferior parietal neurons are involved in the assimilation and creation of cross modal associations and act to increase the capacity for the organization, labeling and multiple categorization of sensory-motor and conceptual events (Geschwind, 1965; Joseph 1982). One can thus create visual, somesthetic, or auditory equalivalents of objects, actions, feelings, and ideas, simultaneously. For example, conceptualizing a "chair" as a word, visual object, or in regard to sensation, usage, and even price. That is, the IPL is directly involved in naming--as demonstrated by functional imaging (Price, 2011). The left IPL becomes activated when reading ( Bookheimer, et al., 2013; Menard, et al., 2012; Price, 2011 Price, et al., 2012; Vandenberghe, et al., 2012) during semantic processing (Price, 2011), and when generating words (Shaywitz, et al., 2013; Warburton, et al., 2012) or when making syllable judgements (Price, 2011). Indeed, the IPL appears to act as a pholological storehouse that becomes activated during short-term memory and word retrieval (Demonet, et al., 2014; Paulesu, et al., 2014; Price, 2011) and becomes highly active when retrieving the meaning of words during semantic processing and semnatic decision tasks (Price, 2011).
LANGUAGE CAPABILITIES
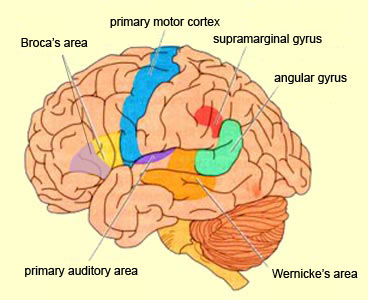
Because of it's involvement in functions such as those described above, one side-effect of damage to the left angular gyrus, is a condition called anomia, i.e. severe word finding and confrontive naming difficulty. These individuals have difficulty naming objects, describing, pictures, etc. Moroever, lesions involving the angular gyrus, or when damage occurs between the fiber pathways linking the left inferior parietal lobule with the visual cortex, there can also result Pure Word Blindness. This is due to an inability to receive visual input from the left and right visual cortex and to transmit this information to Wernicke's area so that auditory equivalents may be called up. Such patients are thus unable to read and suffer from alexia.
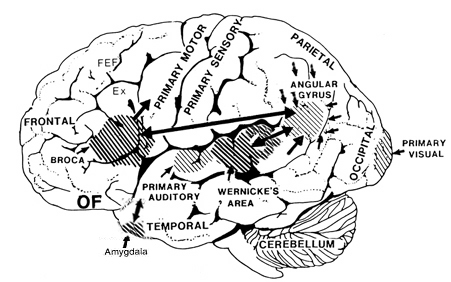
Because the inferior parietal lobule also acts as a relay center where information from Wernickes region can be transmitted, via the arcuate fasciculus, to Broca's area (for expression) destructive lesions, particularly to the supramarginal gyrus of the left cerebral hemisphere can result in conduction aphasia (see chapter 11). Although comprehension would be intact and a patient would know what she wanted to say, she would be unable to say it. Nor would she be able to repeat simple statements, read out loud, or write to dictation. This is because Broca's area is disconnected from the posterior language zones.
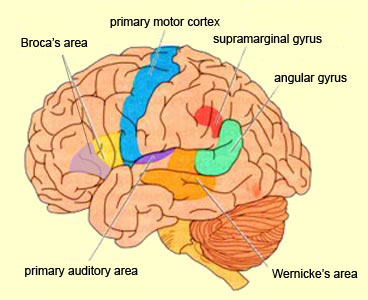
The IPL (which includes the angular and supramarginal gyrus) assimilates associations received from yet other areas of the left and right hemisphere (including the amygdala and cingulate gyrus), fills any gaps with relevant associations, and then injects the resulting verbal associations into the stream of language and thought via the arcuate and longitudinal fasciculus which interlinks the language areas. Hence, the concept of a "language axis" (Joseph, 1982, 2009e,f).
This parallel and localizationist convergence model of language is based on lesion studies and human brain dissection, has been confirmed by functional imaging studies, and has recently been adapted by other theorists (reviewed in Joseph, 2009f). For example, as based on functional imaging studies, it has been demonstrated that speech processing, reading, subvocal vocalization, and innerspeech activates the left frontal lobe (Buchel et al., 2008; Demonet, et al., 1994; Paulesu, et al., 2013; Peterson et al., 1988). And, left frontal activation increases as word length increases, and in response to unfamiliar words (Price, 2007).
In addition, reading and speaking activates the Wernicke's area and the left posterior temporal lobe (Bookheimer, et al., 2005; Howard et al., 1996), including the supramarginal gyrus (Bookheimer, et al., 2005), angular gyrus of (Price, 2007) and (when reading) the left medial extrastriate visual cortex (Peterson et al., 1990).
Moreover, when making semantic decisions (involving reading words with similar meanings), there is increased activity in the left posterior temporal lobe at the junction of the IPL (Price, 2007).
Moreover, like the left frontal lobes, the left temporal areas are activated during word generation (Shaywitz, et al., 2005; Warburton, et al., 1996), and sentence comprehension tasks (Bottini, et al., 1994; Fletcher et al., 2005), whereas the IPL in general appears to serve as a phonological storehouse that becomes activated while reading, listening, and when engaged in a variety of languages tasks including word retrieval (Bookheimer, et al., 2005; Demonet, et al., 1994; Menard, et al., 1996; Paulesu, et al., 2013; Price, 2007; Vandenberghe, et al., 1996). Moreover, activation in the IPL as well as the left frontal and left temporal lobe increases as word length increases and for long and umfamiliar words (Price, 2007).
THE INFERIOR PARIETAL LOBE AND LANGUAGE
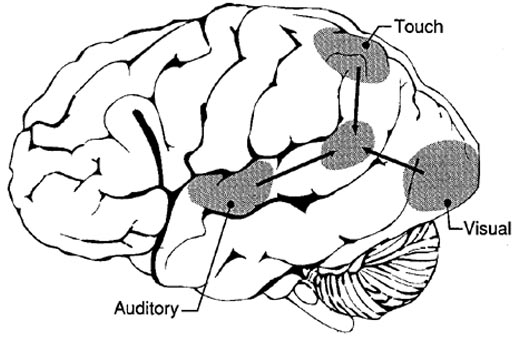
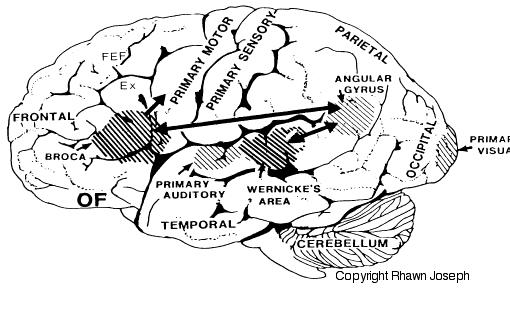
However, the inferior parietal lobule (the angular and supramarginal gyrus) is not just a "hand" area but a multi-modal language area which acts to sequence language as well as inject words and categories into the stream of language and thought (Joseph, 1982). The role of the IPL in language is not only evident as based on lesion studies (as will be discussed) but functional imaging. For example, as based on functional imaging, it appears that the supramarginal gyrus may act as a phonological storehouse that becomes activated during short-term memory and word retrieval (Demonet, et al., 1994; Paulesu, et al., 2013; Price, 2007); whereas conversely, deficits in phonological processing are the most common correlate of reading disability (Brady & Shankweiler, 2001). Simply looking at words will activate the left supramarginal gyrus (Bookheimer, et al., 2005; Vandenberghe, et al., 1996; Menard, et al., 1996; Price, 2007) which also becomes active when performing syllable judgements (Price, 2007), and when reading (Bookheimer, et al., 2005; Menard, et al., 1996; Price, et al., 1996). Likewise, the IPL become highly active when retrieving the meaning of words during semantic processing and semantic decision tasks and activation increases as word length increases (Price, 2007).
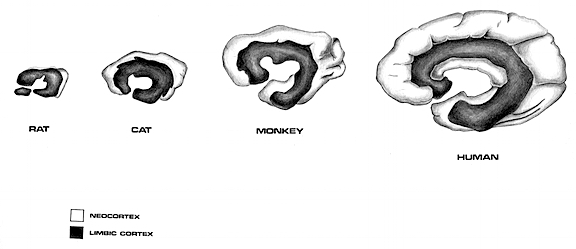
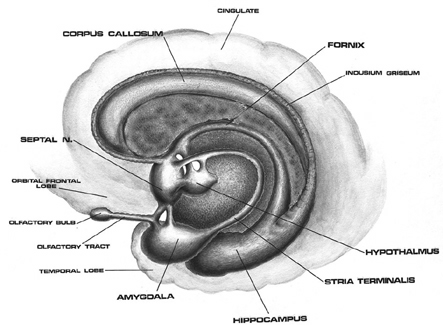
As detailed below and in chapters 6, with the evolution of the IPL, what had been limbic language became yoked to the neocortex. Briefly, the lateral frontal convexity, including Broca's area, may have evolved from the supplementary motor areas and medial frontal lobe which in turn evolved from and is richly interconnected with the anterior cingulate (e.g. Sanides 1964). The amygdala (and hippocampus) gave rise to the medial and inferior temporal lobes, the insula (see Sanides 1964), followed by the superior temporal lobe, Wernickes area, and by extention, portions of the inferior parietal lobule. The IPL, however, is also an evolutionary derivative of superior parietal tissue which expanded in accordance with and as represented by temporal sequential hand use and fine motor function involving the fingers and the thumb.
The evolution of the IPL (that is, the angular gyrus), therefore, may have served as a nexus, interlocking, at a neocortical level, the cingulate-Broca pathways, and the amygdala-Wernicke's pathway, thereby enabling limbic language impulses to become hierarchically represented as well as subject to temporal sequencing by neocortical neurons (Joseph 2013, 2009e,f). Prior to the evolution of the IPL/angular gyrus, Broca's area presumably was unable to receive sufficient input from primary auditory receiving and Wernicke's areas (and the amygdala), and language thus remained by and large, limbic and controlled by the anterior cingulate gyrus.
The impetus for inferior parietal and frontal lobe and Broca's area evolutionary development, however, appears to be two-fold, being in part limbic derivatives (amygdala-hippocampus, amygdala-cingulate) and a function of the evolution of fine motor control involving the facial-oral musculature, vocalization, and especially the establishment of handedness. Given that the human left corticospinal tract matures earlier and crosses the medullary pyramids at an earlier age than fibers from the right (Kertesz & Geschwind 1971; Yakovlev & Rakic 1966), thereby presumably establishing synaptic control over the spinal and cranial motor nuclei in advance of the right as well, dominance for hand control and temporal sequential processing became the province of the left hemisphere (Joseph 1982). With motor dominance, the left amygdala, cingulate gyrus, superior temporal lobe, inferior parietal and frontal were reorganized accordingly.
It is in part due to the interrelationship between sensory feedback, motor control and gesture, that hand control and gesture are greatly dependent on the parietal area, the left IPL in particular.
In addition, the motor engrams that make possible temporal and sequential motor acts (e.g. making a cup of coffee, fashioning a tool) appear to be localized within the inferior parietal lobe (Heilman et al. 1982; Kimura, 2013; Strub & Geschwind, 2005). It is the IPL which enables humans to engage in complex activities involving a series of related steps, create and utilize tools, produce and comprehend complex gestures, such as American Sign Language (ASL), and express and perceive grammatical relationships (Joseph, 2013, Kimura, 2013; see also Corina et al. 1992). Hence, when the left IPL has been injured patients may be afflicted with apraxia (Heilman et al. 1982) and have difficulty with tasks requiring complex motor sequencing.
Broca and Wernicke's areas and thus left cerebral linguistic functioning are exceedingly dependent on the IPL and it's capacity to impose rhythmic temporal sequences on auditory associations and motoric actions (Geschwind, 1966; Goodglass & Kaplan, 1982; Joseph, 2013, 2009e; Heilman et al. 1982; Kimura, 2013; Strub & Geschwind, 2005), including vocalizations which arise from the limbic system.
Presumably when the inferior parietal lobule and the angular gyrus fully evolved, humans acquired the capacity to segment incoming sounds and to hierarchically represent and punctuate social-emotional, limbic vocalizations so as to vocally express themselves in temporal and grammatical sequences. Thus social-emotional vocalizations came to be governed by grammatical rules of organization, thus producing "modern" human language.
MULTI-MODAL PROPERTIES
In humans, the left IPL being an indirect product of temporal lobe and superior parietal evolution (see chapter 6) is capable of multimodal processing of auditory, visual, as well as tactile impressions, and then naming this material by forming verbal associations. The IPL then injects this material into the stream of language and thought. For example, as based on functional imaging, the left IPL becomes highly active when looking at words and reading, and when engaged in word retrieval (Bookheimer, et al., 2005; Vandenberghe, et al., 1996; Menard, et al., 1996; Price, 2007). Indeed, because of its unique position at the juncture of the auditory, visual, somesthetic, and motor neocortex, it has gained the capability of analyzing, associating, and assimilating this divergent data in order to create multiple categories of visual, auditory, and tactile imagery and meaning.

Hence, because the IPL receives multi-modal input, one can feel an object while blindfolded and know what it would look like and be able to name it as well.
One can also integrate and assimilate these diverse sensory signals so as to abstract, classify and produce multiple overlapping categories of experience and cross modal associations (Geschwind, 1966; Joseph, 1982, 1986a 2013; Joseph et al., 1984).
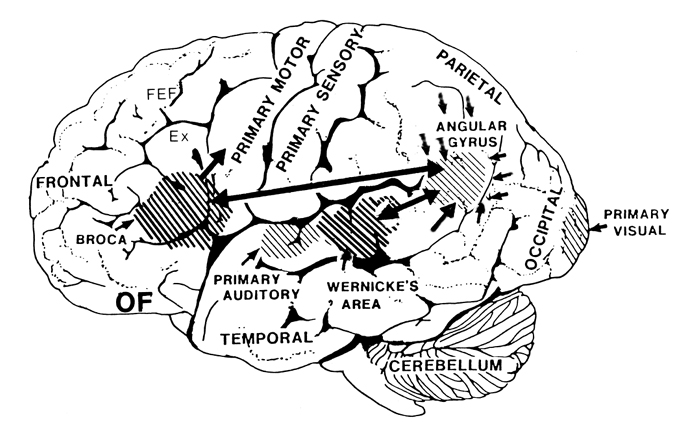
The primary sensory receiving areas for vision, audition, and somesthesis are located in the occipital, temporal and parietal lobe respectively. Adjacent to each primary zone is a secondary-association neocortical region where higher level information processing occurs and where complex associations are formed. Wernicke's region is one such zone, as is the middle-inferior (basal) temporal and the superior parietal lobe. Moreover, there are complex third order association areas such as the middle-inferior temporal lobe (Brodmann's area 37).
Area 37 is located between the visual cortex and the anterior temporal cortex and becomes activated during a variety of language tasks, including reading and object and letter naming (Price, 2007)-- as demonstrated by functional imaging (Buchel et al., 2008; Price, 2007), direct cortical recoding (Nobre et al., 1994), and electrical stimulation (Luders et al., 1986). In fact, both normal, cognitally blind, and late-blind subject display activity in the medial temporal area (Buchel et al., 2008). Moreover, similar to injuries in the IPL, if the middle-inferior temporal lobe is injured, patients may suffer from reading and naming deficits (Rapcsak, et al., 1987); a condition referred to as phonological alexia. As noted, deficits in phonological processing are the most common correlate of reading disability (Brady & Shankweiler, 2001).
The IPL (which includes the angular and supramarginal gyri) is located at the junction where the all secondary and multi-modal association areas meet and overlap, and receives converging visual-linguistic input from the basal-lateral (middle-inferior) temporal lobe. In this regard, the inferior parietal region receives converging higher order information from each sensory modality and all association areas and in fact makes possible the formation of multiple associations based on the assimilation of this divergent sensory input (Geschwind, 1965, Joseph, 1982, 2013). One can thus feel an object while blindfolded and know what it would look like and be able to name it as well.
Through its involvement in the construction of cross-modal associations, this region acts so as to increase the capacity for abstraction, categorization, differentiation, and the verbal as well as visual labeling of sensory-motor experience. One is thus able to classify a single stimulus or event in multiple ways. In part this is made possible because the inferior parietal lobule is the recipient of the simple and complex associations already performed in the primary and association cortices via the ten billion axonal interconnections that occur in this region.
STIMULUS ANCHORS AND THE TRAIN OF THOUGHT
The left IPL of which it is part, makes possible the assimilation of complex associations which have been constructed elsewhere so that multiple classifications, categorizations, and descriptions are possible. The IPL also acts to integrate and arrange them according to preestablished (gestural) temporal sequences and the requirements of what needs to be communicated.
Moreover, via rich interconnections with Wernicke's area and the middle temporal lobe, the IPL it is able to associate auditory/verbal labels with other sensory experiences such that we can describe things as "sticky, sweet, moist, red, lumpy," as well as use single word descriptions, e.g. "jelly." This capability is particularly important in regard to reading and naming as described in further detail in chapters 20, 21. For instance, when a word is read, the pattern of visual input is transmitted from the visual areas in the occipital and temporal lobes to the left IPL (which is coextensive with Wernicke's area) and which then performs an auditory visual matching function. That is, it calls for and integrates the auditory equivalent of what is viewed or read so that we can name animals, objects, words and letters and know what the name sounds like. If this area were damaged, reading ability would be lost, a function in part, of disconnection between the IPL and the middle-inferior temporal lobe.
The Train of Associations.
As noted, the left IPL (including the left posterior-superior temporal lobe become more active when reading (Bookheimer, et al., 2005; Menard, et al., 1996; Price, 2007 Price, et al., 1996; Vandenberghe, et al., 1996) and becomes active during semantic processing (Price, 2007), and when making semantic decisions, such as when reading words with similar meanings (Price, 2007). These same areas are activated during word generation (Shaywitz, et al., 2005; Warburton, et al., 1996), and sentence comprehension tasks (Bottini, et al., 1994; Fletcher et al., 2005).
In most instances in which the IPL is activated via internal or external sources of stimulation, multiple trains of inquiry are initiated via the numerous interconnections this areas maintains. Impressions, memories, ideas, and feelings which are in any manner associated with the initial stimulus probe, are aroused in response.
If a student is asked: "What did you do in school today?" a number of verbal and memory associations and association areas are aroused in parallel and integrated within the Language Axis, all of which are related in some manner to each element of the eliciting stimulus. Finally, in the process of associational linkage, those associations with the strongest stimulus value and which most closely match each element of the question in terms of internal and external appropriateness and thus with the highest probability of being the most relevant, rapidly take a place in a hierarchical and sequential, grammatical arrangement that is being organized in a form suitable for expressing a reply.

To return to the question regarding "school," each speech segment and sound unit become triggers which first activate and then, like a magnet, draws associations accordingly. All aroused forms of mental imagery, verbal associations and so on which are received in the IPL are then arranged, individually matched and group matched such that the associations which correspond to all sources of relevant input with sufficient value of probability then act as templates of excitation that stimulate and attract other relevant ideas and associations. These in turn are assimilated and associated or are subsequently deactivated due to their low probability in contrast to the association already organized.
Moreover, because the strength and value of closely linked associations change in correspondence to the developing sequential hierarchy (or the initial parallel hierarchies), previously aroused and assimilated material may subsequently come to have a now lower value of probability or appropriateness within the the matrix of overall activity and may be deactivated (Joseph, 1982, 1986a, 2013).
Consider the question: "What is furry, small, loves milk and makes the sound Meoww?" At the level of the neocortex, each word, "furry," "small," "milk" and "meoww," acts to trigger associations (e.g. "furry = coat-animal-...," "milk = liquid-white-cow-..."). The grammatical linkage of these words also acts to trigger certain associations (e.g. "furry-milk-meoww = animal-cat-...") while deactivating others (e.g. "cow"). Following the analysis and comprehension of these sounds and words in Wernicke's area, the angular gyrus, and the middle temporal lobe, the IPL continues to call forth associations so that a reply to the question can be generated.
So that the animal can be named, the IPL via its interactions with the temporal lobe, activates the necessary phonemic elements (e.g. "k-a-t"), and then transfers this information to Broca's area and the question is answered: "Cat." If instead the individuals replies "tak" this would indicate a problem in organizing the correct phonemic elements once they were activated (see chapter 21 for an extended and detailed discussion).
The final product of this hierarchical, highly grammatical arrangement of mutually determining and parallel associational linkages is the train of thought or a temporal-sequential stream of auditory associations in the form of words and sentences. However, before this occurs, these verbal associations must receive a final temporal-sequential grammatical stamp which is a consequence of the organization imposed on this material as it passes from Broca's area to the oral-speech musculature.
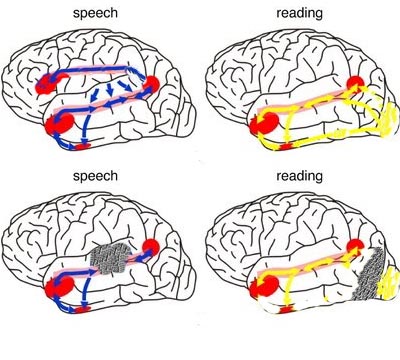
Following massive lesions of a brain area with which it normally communicates, the language axis sometimes begins to invent an answer or reply to questions based on the information available despite the gaps in that data or the incongruent nature of what is being reported. Consider, for example, denial of blindness (following massive injuries to the visual neocortex) or denial or neglect of the left extremity which may also be paralyzed (due to massive right cerebral injuries involving the motor and parietal neocortex). Patients will claim to have sight although they bump into objects or fall, or they may claim that their paralyzed left arm belongs to the doctor or a person in the next room (chapter 10).
To be informed about the left leg or left arm, the Language Axis must be able to communicate with the neocortical area which is responsible for perceiving and analyzing information about the extremities. For example, since the right parietal area maintains the somesthetic body-image, as well the storage site for body-image memories, when that areas is destroyed, the left half of the "body-image" all associated memories and essentially "erased" -as if they never existed.
When no message is received by the Language Axis, due to destruction of the neocortical area responsible for that message or memory, and when the Language Axis is not informed that no messages are being received (because the brain area which would alert them is no longer functioning), the language zones instead rely on some other source even when that source provides erroneous input (Joseph 1982, 1986a). Substitute material is assimilated and expressed and corrections cannot be made (due to loss of input from the relevant knowledge source) and the patient begins to confabulate (see chapters 10, 19). That is, the Language Axis fills the "gap" with erroneous material.
DISORDERS OF LANGUAGE & THOUGHT
THE LANGUAGE AXIS
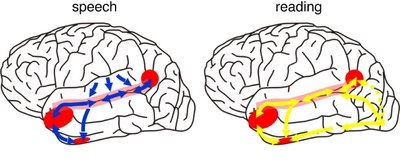
When listening to someone speak the information is transferred from the brainstem to the inferior colliculus and medial geniculate of the thalamus, and transferred to the amygdala and the primary auditory cortex where the data is extensively analyzed (see chapter 21). These auditory signals are then transferred to Wernicke's area (which merges with the angular gyrus) where the temporal-sequential, semantic and related linguistic features are stabilized, extracted, analyzed and labled.
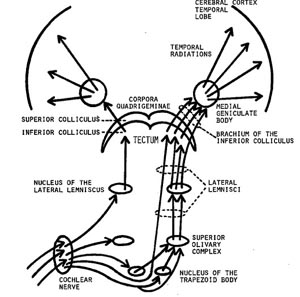
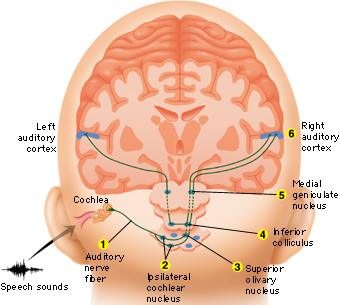
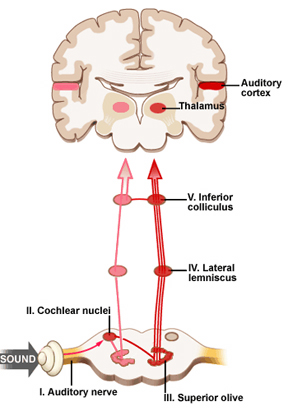
If it is a question which has been asked, the message is transferred from Wernicke's to the inferior parietal lobule where various ideational associations are aroused and organized via it's vast interconnections with other cortical regions. Presumably a series of interactions continue to occur between Wernicke's and the inferior parietal lobe (as these areas are coextensive) until a reply is formulated and properly organized for possible expression at which point it is transferred to Broca's area (Geschwind, 1965; Joseph, 1982)--as also demonstrated through functional imaging (Buchel et al., 2008; Demonet, et al., 1994; Paulesu, et al., 2013; Peterson et al., 1988; Price, 2007).
Specifically, the semantically correct and suitably chosen reply is transferred from Wernicke's region and the inferior parietal lobe, via the interlining axonal bundle, the arcuate fasciculus, to Broca's area which then programs the speech musculature and neocortical motor areas so that the reply can expressed.
AGRAPHIA
It has been argued that the sensory motor engrams necessary for the production and perception of written language are stored within the parietal lobule of the left hemisphere (Strub & Geschwind, 1983). In fact, given that the parietal lobes are concerned with the hands and lower visual fields, they not only guide and observe hand movements, but learn and memorize these actions, including those involved in writing.
Hence, when lesioned, patients sometimes have difficulty writing and forming letters due to an inability to access these engrams (Strub & Geschwind, 1983; Vignolo, 1983); i.e. they suffer from agraphia, an inability to write (see chapter 11). Writing samples may be characterized by misspellings, letter omissions, distortions, temporal-sequential misplacements, and inversions (Kinsbourn & Warrington, 1964). Sometimes agraphia is accompanied by alexia; inability to read (Benson & Geschwind, 1969; Hecaen & Kremin, 1977).
LATERALIZED TEMPORAL-SEQUENTIAL FUNCTIONS
Because it is a recipient of so much information and aids the rest of the brain in various forms of analysis, one function of the inferior parietal lobe is to maintain track of input/output so that information may be organized appropriately in either a sequential (i.e. first, middle, last), or spatial framework. Hence, another side effect of lesions localized to the inferior parietal lobule is a disruption of visual-spatial functioning, temporal-sequencing ability (e.g. apraxia), as well as logic, grammar, and the capacity to perform calculations; depending on which hemisphere is compromised.
Individuals with lesions involving the inferior-parietal-occipital border of either hemisphere may have difficulty carrying out spatial-sequential tasks. For example, drawing "a square beneath a circle and a triangle beneath a square" (Luria, 1980). Often they may draw the objects in the order described (i.e. square, circle, triangle, square). That is, they have difficulty in conceptualizing how to place the objects in relation to each other.
Those with left inferior parietal lesions have trouble with more obvious sequential-grammatical relationships (Luria, 1980). For example, they may be unable to understand the question: "John is taller than Jim but shorter that Pete. Who is taller?" In part, this is not only a function of left parietal dysfunction but the right hemispheres difficulty in dealing with temporal-sequential and grammatical relations.
Because the right brain does not understand grammatical relationships, a sentence that starts which the name "John" is interpreted by the right parietal area as all about "John". i.e. the first word of the sentence is undertood by the right brain as the "agent" regardless of semantics or grammar (Chernigovaskaya & Deglin, 2006). In this manner, if presented with the sentence, "give me the book after you give me the pencil", the right brain would respond to the order of presentation rather than their grammatical relationship and would thus present the book then the pencil. When left parietal input is abolished, proper temporal-sequential and grammatical programming/comprehension thus suffers.
APRAXIA
THE SENSORY GUIDANCE OF MOVEMENT
The parietal lobe is highly concerned with the mediation of movement. As noted in chapter 19, the primary motor cortex extends well beyond area 4 and includes portions of the somatosensory regions which in turn contribute almost one third of the fibers which make up the pyramidal tract. These areas are in fact richly interconnected (Jones & Powell, 1970). Together, the motor and somesthetic regions comprise a single functional unit, i.e. the sensorimotor cortex.
Nevertheless, it is important to emphasize, as pointed out by Luria (1980) that every voluntary movement is in fact comprised of a series of movements which are spatially organized in accordance with successively changing input from other modalities (Barrett, et al., 1998; Buxbaum et al., 1998; Kimura 2014). That is, in order for a movement to be correctly planned and carried out signals must be directed to the right muscle groups as based on efferent streams of visual, somesthetic, as well as auditory input. This includes information regarding the position of the body and limbs in space. Indeed, movement becomes extremely difficulty without sensory feedback and guidance. Because of this, parietal lesions can result in unilateral paresis and even wasting (i.e. parietal wasting). Hence, the somesthetic cortex is very important in the guidance of movement, and in fact some neurons fire prior to making a movement (Lebedev et al. 2014).

Although the entire parietal lobule makes important contributions , the superior and inferior parietal lobule of the left hemisphere appears to be the central region of concern in regard to the performance of skilled temporal-sequential motor acts. This is because the motor engrams for performing these acts appear to be stored in the left angular and supramarginal gyri (Geschwind, 1965; Heilman, 2014) -a consequence, in part of its unique ability to guide, visually observe, and thus selectively learn hand movements, gestures, and complex temporal sequential actions such as involving tool construction (Joseph 2014, 2008e). Related memories are therefore stored in this cortex.
Conversely, these hand movement related memories assist in the programing of the motor frontal cortex where the actions are actually executed (e.g. Deibert et al., 2008). However, the inferior parietal lobule in turn is dependent on input from the primary and association somesthetic areas.
There is some evidence of laterality in regard to all of the above, the right half of the brain may be more concerned with movement of the trunk and the lower extremities. This would include navigational movement through space, running, certain types of dancing, and actions requiring analysis of depth and balance. The left cerebral hemisphere exerts specialized influences on the upper extremities including the control of certain types of complex, sequenced motor acts such as those requiring alterations in the orientation and position of the upper limbs (Haaland & Harrington 2014; Kimura, 1982, 2014).
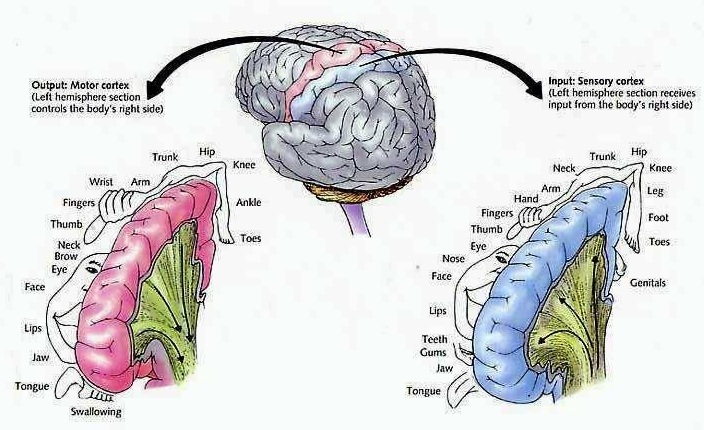
APRAXIA
If the left inferior parietal region is destroyed the patient loses the ability to perform actions in an appropriate temporal-sequence or to even appreciate when they have been performed incorrectly. They may also be impaired in their ability to acquire or perform tasks involving sequential changes in the hand or upper musculature (Kimura, 1979, 1982, 2014), including well learned, skilled, and even stereotyped motor tasks such as lighting a cigarette or using a key.
Apraxia is a disorder of skilled movement in the absence of impaired motor functioning or paralysis (Barrett et al., 2008; Buxbaum et al., 2008). Usually apraxic patients show the correct intent but perform the movements in a clumsy fashion. Like many other types of disturbances, patients and their families may not notice or complain of apraxic abnormalities. This is particularly true if they're aphasic or paralyzed on the right side. That is, clumsiness with either extremity may not seem significant. Hence, this is something that requires direct evaluation.
Performance deteriorates the most when required to imitate or pantomime certain actions including the correct usage of some object (McDonald et al. 2014). For example, the patient may be asked to show the examiner, "how you would use a key to open a door", or "hammar a nail into a piece of wood". In many cases the patient may use the body, i.e. a finger, as an object (e.g. a key), rather than the finger and thumb holding the key. Although performance usually improves when they use the real objects (Geschwind, 1965; Goodglass & Kaplan, 1972), a rare few may show the disturbance when using the real object as well (Heilman, 2014; Kimura 2014).
In addition, patient's with apraxia may demonstrate difficulty properly sequencing their actions (Barrett et al., 1998). For example, they may pretend to stir a cup of coffee, then pretend to pour the coffee into the cup, and then take a sip. However, the individual acts may be performed accurately.
Broadly speaking, there are several forms of apraxia, which like many of the disturbances already discussed may be due to a number of causes or anatomical lesions. These include, ideational apraxia, ideomotor apraxia, bucal facial apraxia, constructional apraxia and dressing apraxia. With the exception of dressing and constructional apraxia, apraxic abnormalities are usually secondary to left hemisphere damage, in particular, injuries involving the the left frontal and inferior parietal lobes.

For example, Kimura (1982, 2014) found that the ability to perform meaningless oral or hand movements was related to the frontal or posterior nature of the lesion, such that those with frontal lesions were impaired on oral whereas those with parietal lesions had the most difficulty making hand postures or complex movements of the extremities (Kolb & Milner, 1981). Thus, apraxic abnormalities secondary to left cerebral lesions tend to either involve destruction of the inferior parietal lobule (IFP) or lesions resulting in disconnection of the frontal motor areas (or the right cerebral hemisphere) from this more posterior region of the brain.
If the inferior parietal region is destroyed the patient loses the ability to appreciate when they have performed an action incorrectly. If the motor region is destroyed, although the act is still performed inaccurately (due to disconnection from the IFP), the patient is able to recognize the difference (Heilman, 2014; Kimura 2014).
IDEOMOTOR APRAXIA
Ideomotor apraxia is usually associated with lesions within the inferior parietal lobe of the left hemisphere. Rather than problems with temporal-sequencing of motor acts per se, these individuals tend to be very clumsy when performing an act, and/or they may perseverate and erroneously perform a previous movement. These individuals also tend to be very deficient when attempting to perform an action via pantomime or when engaged in meaningful imitation, meaningless imitation, and the meaningful use or meaningless use of actual objects (Goodlass & Kaplan, 1972; Heilman, 1973; Kimura & Archibald, 1974; McDonald et al. 2014). This is presumably due to destruction of the engrams important in motor performance. Patients will demonstrate apraxic abnormalities in both the right and left hand.
In addition, patients with ideomotor apraxia tend to have difficulty with simple versus complex movements, although various elements within a complex action may be performed somewhat abnormal (Hecaen & Albert, 1978; McDonald et al. 2014). Hence, actions such as waving goodbye, throwing a kiss, making the "sign of the cross", may be performed deficiently. Moreover, many patients tend to uncontrollably comment on their actions; i.e. "verbal overflow". That is, when asked to "wave goodbye", they may say "goodbye" while waving even when instructed to say nothing.
It has been suggested that ideomotor apraxia can occur in the absence of ideational apraxia, but that the converse is not true (Hecaen & Albert, 1978). In this regard, ideomotor apraxia may be a less severe form of ideational apraxia (Kimura 2014).
IDEATIONAL APRAXIA
This form of apraxia is usually due to severe disturbances in the temporal sequencing of motor acts (Buxbaum et al., 1998). That is, the separate chain of links which constitute an entire movement become dissociated, such that the overriding idea of the movement in it's entirety is lost. Hence, these individuals commit a number of temporal and spatial errors when making skilled movements, although the individual elements, in isolation, may be preserved and performed accurately (Hecaen & Albert, 1978; Luria, 1980). For example a patient (via pantomime) may rotate their hand before inserting the key, drink from a cup before filling it from a pitcher of water, or puff from a cigarette and then lighting it. Thus they incorrectly sequence a series of acts. Both hands are effected.
Because of conceptual, ideational abnormalities, they may also have difficulty using actual objects correctly. During pantomime they may use a body part as object such as an index finger for a key. Even so, their actions are out of temporal sequence. Hence, these patients seem to be unable to access the motor engrams (or "memories") which would allow them to perform appropriately (Luria, 1980). In this regard, patients are sometimes hesistent to perform a task as they have difficulty understanding what has been asked of them. However, they can often describe verbally what they are unable to perform (Heilman, 2014).
LEFT SIDED OR UNILATERAL APRAXIA (also called Callosal & frontal apraxia)
Patients with unilateral (callosal/frontal) apraxia are unable to imitate or perform certain movements with their left (but not right) hand and are clumsy in their use of objects. Left sided apraxia is sometimes due to a lesion of the anterior corpus callosum or left frontal motor cortex. This is because lesions of the corpus callosum or premotor and motor region of the left hemisphere can result in a disconnection syndrome; i.e. the motor areas of the right hemisphere cannot gain access to the motor engrams stored within the left inferior parietal lobe. Thus, with a left frontal lesion there results an apraxia of the left hand and paralysis of the right. Often this is secondary to strokes within the distribution of the anterior cerebral artery such that the anterior portion of the corpus callsom is destroyed (Geschwind, 1965).
It is noteworthy that patients also may show deficient finger tapping performance in the left hand due to apraxic abnormalities secondary to left hemisphere injury (Heilman, 1975). In these instances, reduced finger tapping is bilateral.
Dressing Apraxia
Dressing apraxia is usually secondary to right hemisphere lesions involving the inferior parietal region, and as the name implies, the patient has difficulty putting on their clothes. For example, a patient may attempt to put a shirt on upside down, then inside-out, and then backwards. Severe spatial-perceptual abnormalities as well as body image disturbances are usually contributing factors.

Aphasia & Apraxia
Many patients who are aphasic also appear apraxic because they have severe difficulty comprehending language and understanding motor commands. That is, a patient may fail to peform a particular action because he doesn't comprehend what is being asked.
To distinguish between receptive aphasic abnormalities and apraxia one must ask "yes" and "no" questions ("are you in a hospital?"); require them to perform certain actions via pantomime ("show me how you would throw a ball" or "show me how a soldier salutes"); as well require pointing response ("point to the lamp"). If they can answer appropriately "yes" or "no" or point to objects named but cannot execute commands they have apraxia. It is important to note that in severe cases apraxic patients may have difficulty even with pointing.
PANTOMIME RECOGNITION
Individuals with damage involving the left parietal lobule not only make errors when performing motor acts but comprehending, recognizing and discriminating between different types of motor acts such as demonstrated via pantomime (Heilman et al. 1982; McDonald et al. 2014). Moreover, individuals with lesions in the left inferior occipital lobe have also been shown to have difficulty verbally understanding, describing or differentiating between pantomimes (Rothi et al. 2006). That is, in the extreme, if one were to pantomime the pouring of water into a glass vs. lighting and smoking a cigarette, these indviduals have problems describing what they have viewed or in choosing which was which.

Deficits in pantomime recognition occur frequently among individuals with aphasia (Varney, 1978). Moreover, this disturbance is also significantly correlated with reading comprehension (Gainotti & Lemmo, 1976; Varney, 1978, 1982). In this regard, individuals with alexia frequently suffer from pantomime recognition deficits as well. Because of this relationship it has been suggested that the ability to read may be based on or derived from the abiity to understand gestural communcation, i.e. the reading of signs (Joseph 2014; Varney, 1982).
Wang and Goodglass (1992) and Kimura (2014) argue that pantomime imitation and production are related to both apraxia and the ability to interpret purposeful movements; the engrams for which are located in the inferior parietal lobule (Heilman et al. 1982; Joseph 2014; Wang & Goodglass, 1992). By contrast to left anterior lesions which may impair motor functioning and the capacity to imitate the ability to comprehend pantomime is retained (Heilman et al. 1982).
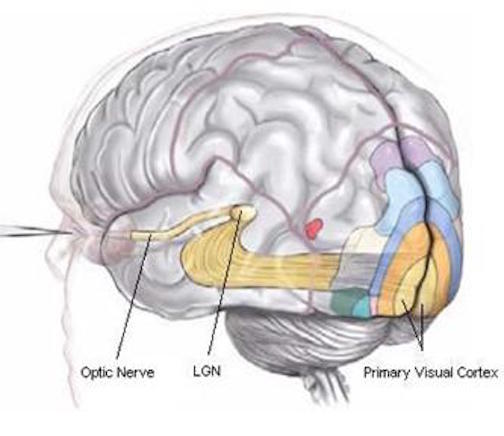

As noted, the inferior and superior parietal lobule receives considerable visual input, particularly from the periphery and lower visual field -the area in which the hands are most likely to be viewed. Hence, this area of the brain views, manipulates, guides and mediates hand-object coordination and reaching movements, including the comprehension of hand movements; i.e. gestures (Joseph, 2014). Hence, when the left superior and inferior parietal lobule is destroyed gestural comprehension, including the understanding of (as well as the capacity to execute) complex gestures, including ASL and the capacity to engage in complex temporal sequential acts is significantly impacted. If the right parietal area is destroyed, these deficits may also include constructional apraxia (chapter 10).

CONSTRUCTIONAL APRAXIA
Constructional apraxia is by no means a unitary disorder (Benton, 1969; Benson & Barton, 1970) and can may be expressed in a number of ways. On a drawing or copying task this may include the addition of unnecessary/non-existent details or parts, misalignment or inattention to details, disruptions of the horizontal and vertical axis with reversals or slight rotations in reproduction, and scattering of parts. For example, in performing the Block Design subtest from the WAIS-R, the patient may correctly reproduce the model but angle it incorrectly. In drawing or copying figures, the patient may neglect the left half, draw over the model, and misalign details.
Moreover, although constructional deficits are more severe after right hemisphere damage (Arrigoni & DeRenzi, 1964; Black & Strub, 1976; Benson & Barton, 1970; Critchley, 1953; Hier et al., 1983; Joseph, 1988a; Kimura 2014; Piercy et al. 1960), disturbances involving constructional and manipulo-spatial functioning can occur with lesions to either half of the brain (Arrigoni & DeRenzi, 1964; Mehta et al., 1987; Piercy et al., 1960). Hence, depending on the laterality, as well as the extent and site of the lesion, the deficit may also take different forms. For example, following posterior right cerebral lesions, rather than apraxic, the patient is spatially-agnosic, i.e. suffering from constructional agnosia and a failure to perceive and recognize visual-spatial and object interrelationships. In other cases, such as following left cerebral injury, the disturbance may be secondary to a loss of control over motor programming (Kimura 2014; Warrington et al., 1966; Warrington, 1969).
Although visual motor deficits can result from lesions in either hemisphere (Arrigoni & DeRenzi, 1964; Piercy et al., 1960; Kimura 2014), visual-perceptual disturbances are more likely to result from right hemisphere damage. In contrast, lesions to the left half of the brain may leave the perceptual aspects undisturbed whereas visual motor functioning and selective organization may be compromised (Kim et al. 2004; Mehta et al., 1987; Poeck et al. 1973). As such the patient is likely to recognize that errors have been made.
In general, the size and sometimes the location of the lesion within the right hemisphere has little or no correlation with the extent of the visual-spatial or constructional deficits demonstrated, although right parietal lesions tend to be worst of all. With right parietal involvement patients tend to have trouble with the general shape and overall organization, the correct alignment and closure of details, and there may be a variable tendency to ignore the left half of the figure or to not fully attend to all details. Moreover the ability to perceive (or care) that errors have been made is usually compromised.
Conversely, constructional disturbances associated with left hemisphere damage are positively correlated with lesion size, and left anterior lesions are worse than left posterior (Benson & Barton, 1970; Black & Bernard, 2004; Black & Strub, 1976; Kimura 2014; Lansdell, 1970). This is because the capacity to control and program the motor system has been compromised. The larger the lesion, the more extensive the deficit.
Moreover, because the left hemisphere is concerned with the analysis of parts or details and engages in temporal- sequential motor manipulations, lesions result in oversimplification and a lack of detail although the general outline or shape may be retained (Gardner, 1975, Levy, 1974). However, in some cases, when drawing, there may be a tendency to more greatly distort the right half of the figure with some preservation of left sided details.
GERSTMANN'S SYNDROME:
FINGER AGNOSIA, ACALCULI, AGRAPHIA, LEFT-RIGHT CONFUSION THE KNOWING HAND
It has been said that the parietal lobule is an organ of the hand. As noted, the hand appears to be more extensively represented than any other body part, parietal neurons are responsive to hand movements and manipulations, mediate and visually observe temporal-sequential hand movements, and become highly activated in response to objects which are within grasping distance. Indeed, given that the parietal lobe receives lower visual field input, the area in which the hands are most likely to be viewed, it is therefore highly sensitive to and concerned with the somesthetic as well as the visual guidance of hand (and lower extremity) movements.
It is also via the hand that the parietal lobe gathers information regarding various objects, i.e. stereognosis, and about the Self and the World, so that things and body parts come to be known, named, and identified. Ontogenetically, the hand is in fact primary in this regard. That is, the infant first uses the hand to grasp various objects so they may be placed in the mouth and orally explored. As the child develops, rather than mouthing, more reliance is placed solely on the hand (as well as the visual system) so that information may be gathered through touch, manipulation and visual inspection.
As the child and it's brain matures, instead of predominantly touching, grasping, and holding, the fingers of the hand are used for pointing and then naming -hand activities that are most likely to be viewed (and later remembered) by the parietal lobe. It is these same fingers which are later used for counting and the development of temporal-sequential reasoning; i.e. the child learns to count on his or her fingers, then to count (or name) by pointing at objects in space.




In this regard, counting, naming, object identification, finger utilization, and hand control are ontogenetically linked and seem to rely on the same neural substrates for their expression; i.e. the left inferior and superior parietal lobule. In this regard, the parietal lobe, due to its control over the hand, and its chronic surveillance of the lower visual field, is therefore the unique recipient and observer of specialized hand-related activities and has thus become organized accordingly in regard to the guidance, as well as the learning and memory of hand and finger related activities.
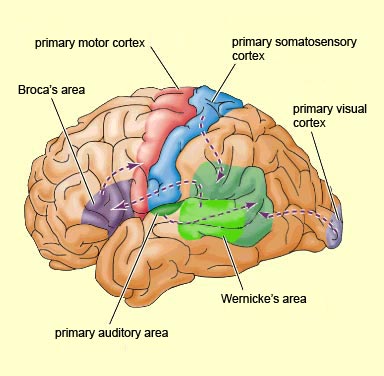
Hence, when the more posterior portions of the left hemisphere are damaged, naming (anomia), object and finger identification (agnosia), arithmetical abilities (acalculia), and temporal-sequential control over the hands (apraxia) are frequently compromised.
A variety of symptoms are therefore associated with left inferior parietal lobe damage, some of which occur as a related constellation of disturbances, i.e. finger agnosia, acalculia, agraphia, and left-right disorientation: Gerstmann's syndrome (Gerstmann, 1930, 1944; Strub & Geschwind, 1983). Gerstmann's symptom complex is most often associated with lesions in the area of the supramarginal gyrus and superior parietal lobule (Hrbek, 1977; Strub & Geschwind, 1983). However, because this symptom complex does not always occur together, some authors have argued that Gerstmann's syndrome, per se, does not exist.
We will not take issue, pro or con on this controversy but instead will focus on those aspects of Gerstmann's syndrome which have not yet been discussed (finger agnosia, acalculia, left-right disorientation).
FINGER AGNOSIA
Finger agnosia is not a form of finger blindness, as the name suggests. Rather, the difficulty involves naming and differentiating among the fingers of either hand as well as the hands of others (Gerstmann, 1940). This includes pointing to fingers named by the examiner, or moving or indicating a particular finger on one hand when the same finger is stimulated on the opposite hand.
For example, if you touch their finger while their eyes are closed, and ask them to touch the same finger they may have difficulty. If the disturbances is subtle the examiner may wish to stimulated two fingers and then ask the patient to indicate, in order, the same fingers. Many patients have difficulty on these tests regardless of there being administered in a verbal (naming) or non-verbal (touching) format (Kinsbourne & Warrington, 1962). In general, it is the middle three fingers which are hardest to recognize and the angosia is demonstrable in both hands.
Although finger agnosia is only rarely shown with those who have right hemisphere lesions (Hecaen, 1962) many patients also demonstrate some visual-constructive disability (Kinsbourne & Warrington, 1962). Hence, in testing for this disorder both verbal vs. non-verbal forms are of assistance in determing the side of lesion.
Often patients who have difficulty identifying fingers by name or simply differentiating between them non-verbally also suffer from receptive language abnormalities (Sanquet et al., 1971). Nevertheless, this disorder is not merely a manifestation of aphasia because finger angosia may appear in the absence of language abnormalities (Strub & Geschwind, 1983).
In part, a good way of determining if the disorder is secondary to a right vs. left hemisphere injury is by noting if patients have more problems recognizing fingers on the right vs. left hand, or in transferring from the right to left hand (or vice versa); that is, by stimulating a finger (or fingers) on the right hand (while it is out of sight) and then having the patient indicate the same fingers on the left hand. One must rule out deficient attentional functioning in making this diagnoses. Of course, one should not diagnose brain damage based merely on poor performance of this one index but should look at the overall pattern of deficiency.

THE EVOLUTION OF GEOMETRY & MATH
Three hundred thousand years ago a someone took a piece of red ocher pigment and sharpened it presumably so as to mark something (Pfeiffer 2005). On what surface did it draw and what the nature of the composition may have been, we do not know. We can only guess that it served some symbolic purpose, or it may have merely served only to make a mark. Three hundred thousand years ago someone took the rib of an ox and carved a series of geometric double arches on it (Pfeiffer 2005). Was he or she just doodling, or was this a common form of artistic expression even in those lost days and forgotten nights? Again, we do not know.
Sixty thousand years ago Neanderthals were painting their caves red and by time they were overun by the Cro-Magnon people twenty thousand years later, geometric patterns, designs and doodles soon graced many a wall and cavern (Leroi-Gourhan 1964; Prideaux 1973). However, it was not until about twenty thousand years ago that people began leaving marks on rocks and walls that suggested that may have been keeping track of, or counting something. Perhaps the phases of the moon, or the number of animals killed? No one knows.
Just as we have no idea when the first complex sentence was spoken, or the first words were written, the point at which human beings first began to count or to measure the geometric properties of the land or the universe surrounding remains a mystery.
Geometry and the first forms of spoken and written pictorial language appear to be naturally related to the functional integrity of the right half of the brain (see chapter 10) and the parietal lobe which is exceedingly concerned with visual-spatial relations. It is likely, however, that the first (temporal-sequential) mathematical concepts were promulgated by the left cerebral hemisphere and parietal lobe, and like writing, were related to hand use (Joseph 2014). That is, one first counts on his or her fingers, and then they learn to count by pointing with their fingers at objects which they wish to add together, and then later they grasp a pen or pencil and make marks and signs which indicate the numbers they used and their summations; actions which are guided and observed by the parietal lobes.

The decimal system is clearly an outgrowth on this reliance on the fingers for counting, for this system is based on the concept of tens. Even the decimal systems employed by the ancients of Meso-America was digitalized, with the exception that they used a base of 20 as they apparently counted their toes.
It has been postulated that human beings first became concerned with geometry and numbers with the advent of agriculture (around ten thousand years ago, after the last great flood), in order to count their crops as well as survey their fields. In addition, geometry may well have first been employed to survey the heavens: visual-space. It was due to geometric-heavenly concerns that many of the ancients considered geometry to be the math of the gods and of divine origin. Perhaps this is why almost all ancient temples and buildings were not only constructed according to complex geometric principles, but oriented in regard to certain celestial configurations, including the temples of ancient Egypt and Sumer. The Sumerians and Egyptians were exceedingly knowledgable about complex geometric principles (Kramer 1981; Wooley 1965), as were the Egyptians (Breasted 1909; Gardiner 1961; Wilson 1951).
Although it is apparent that the Sumerians were also familiar with and utilized a decimal system, and by 4000 years ago the Babylonians had developed the fundamental laws of mathematics, both cultures nevertheless, relied on a sexagesimal system for their complicated calculations because it was far superior to the decimal (Chiera 1966; Kramer 1981; Wooley 1965). For example, whereas the decimal system can be factored by 2,5,10, the 60 unit sexagesimal system could be factored by 2,3,4,5,6,9,10,12,15, and so on.

sexagesimal System, Babylon, 19th Century B.C.
The sexagesimal system is also clearly related to the geometry of space and the partition of what they considered the cosmic, or divine circle; an activity that many ancient and recent cultures have indulged in and also considered divine. Thus, when the cosmic circle or the heavens are equally divided into four quadrants, e.g. North, East, West, South, this forms the sign of a "cross." This is the same cross that most cultures have also deemed to be divine and celestial in origin (chapter 9).
However, the creation of the "divine" four, or three, represents only the most rudimentary features of the sexagesimal system. For example, a circle can be divided into 360 degrees and so on, and these principles can be applied not just to surveys of the heavens but architecture. Via the complicated permutations made possible via the sexagesimal system, the Sumerians, the Babylonians, the Egyptians, the Greeks, and those living in ancient Meso-America were able to make very precise calculations of angular, object, and mathematical relations, and to create temples and buildings, the likes of which today could only be designed, built and fitted together using extremely precise tools and advanced, computerized measuring devices
It is this same sexagesimal system that is employed in the measurement of time; i.e. 60 seconds and 60 minutes. Similarly, the first calendars were created in the same manner, the Sumerians dividing the circle into 12 parts in accordance with their beliefs regarding the sacred celestial nature of the number "12."
That is, the Sumerians, Egyptians and the Babylonians were well aware that the sun, and not the Earth, was at the center of the solar system. They also realized that the Earth was one of several planets and that all traveled around the sun. They postulated the presence of 10 planets (one of which may have been a moon), plus the moon that circles the Earth, which, when coupled with the sun, equalled the sum of 12 (Sitchin 2010). However, even more apparent are the 12 constellations, which circle around our Earth, sun, and solar system, like a clock. Thus we have the 12 disciples of Jesus, the 12 Tribe of Israel, the 12 gods of Olympus, and so on.
It is this same Sumerian "12" which makes the 12 hours of the day and the night (the 24 hour day), and is retained in the form of the 12 months and the 12 houses of the Zodiac (Kramer 1981; Wooley 1965). The ancient Egyptians essentially adapted this system for designing their own calendar, and in Meso-America an almost identical calendar system was devised by the Mayas.

However, the Babylonians (and probably the Sumerians before them) took the decimal and 60 unit sexagesimal system one step further and invented a way to write these numbers in a temporal sequence, the grammatical order of which revealed the value of the sum (Kramer 1981; Wooley 1965). In this manner, thanks to the Sumerian-Babylonians, when one writes 4254, it is clear that the first "4" is a thousand times greater than the last "4". Finally, when the ancient Hindu's appeared on the scene the concept of "nothing" was formulated and thus "zero" came into being.
Just as written language soon came to be organized in a non-pictorial series of temporal sequences, so to did the understanding of the cosmos, geometry, time, and numbers. These tremendous intellectual and creative achievements, however, like language, were dependent on the functional integrity of the inferior parietal lobe (as well as other neural structures such as located within the frontal and temporal lobe and the thalamus), for with the destruction of this tissue, one's sense of space, geometry, written language, and math may be abolished.
ACALCULIA
As noted in chapters 10, 11, there are different aspect of mathematical thinking which in turn depend on different regions of the brain. There are those aspects which are language dependent, and those which are more "intuitive" and which involve the right and left parietal lobe. As based on functional imaging, Dehaene, et al., (1998) determined that an aspect of math they refer to as "exact arithmetic" is language dependent and involves linguistic processing in order to store tables of arithmetical information. Yet another form of math, "approximate arithmetic" is non-verbal, visual-spatial and is more dependent on the right as well as left parietal lobe. It is thus interesting to note that whereas the IPL is directly implicated in mathematical and geometric thinking, that Einstein's IPL was in fact larger than normal by 15% (Witelson et al., 2008), and that the Sylivan fissure stopped well short of his IPL which in turn may have allowed more neurons in this area to flourish and grow and to establish interconnections. Damage to the IPL, in turn can disrupt the ability to perform math or geometrical problems.
Problems working with numbers or performing arithmetical operations, however, can be secondary to a number of causes and may result from injuries involving different regions of the brain. For example, an individual may suffer an alexia/agnosia for numbers, an amnesia for arithmetical facts (Cohen & Dehaene 2014) or they may have difficulties with spatial-perceptual functioning and thus misalign numbers when adding or subtracting (referred to as spatial acalculia). Hence, in many instances a patient may appear to have difficulty performing math problems when in fact the basic ability to calculate per se is intact. That is, the apparent difficulty may in fact be due to spatial, linguistic, agnosic or alexic abnormalities.
However, in many instances, patients who are no longer able to perform calculations demonstrate a number of deficiencies. They may erroneously substitute one operation for another, i.e. misreading the sign "+" as "x", such that they multiply rather than add. Or they may reverse numbers i.e. "16" as "61",substitute counting for calculation, i.e. 21 + 6 = 22, or inapprorpiately group: 32 + 5 = 325.
On the other hand, with left posterior lesions localized to the vicinity of the inferior parietal lobe, patients may have severe difficulty performing even simple calculations, e.g. carrying, stepwise computation, borrowing (Boller & Grafman, 1983; Hecaen & Albert, 1978). When this occurs in the absence of alexia, aphasia, or visual-spatial abnormalities, and is accompanied by finger agnosia, agraphia, and right-left orientation, it is considered part of Gerstmann's syndrome (Gerstmann, 1930). It has also been referred to as "anarithmetria" (Hecaen & Albert, 1978), or pure acalculia when not accompanied by other abnormalities.

PURE ACALCULIA/ANARITHMETRIA
Acalculia is an isolated impairment of calculation in the absence of alexia or agraphic or spatial organization problems and involves a disturbance of basic math processes, e.g. carrying, stepwise computation, borrowing (Boller & Grafman, 1983; Levin, 1979). It is manifested as an impairment in the ability to maintain order, to correctly plan in sequence, and to appropriately manipulate numbers, and/or it may be related to an amnesia for arithmetical facts (Cohen & DeHaene 2014).
Indeed, one patient, a former accountant, who had suffered a small circumscribed left parietal subdural hematoma in an auto accident, was unable to add past 10 (e.g. 7+4), although able to read, write, speak appropriately, and recognize objects. However, she did demonstrate severe finger agnosia, and in fact the finger agnosia appeared to be directly related to her inability to perform calculations. In this regard, I was able to work with this patient for a number of months and by emphasizing finger recognition tasks, including finger calculations (e.g. taking two fingers on her right hand and four on her left, and saying "two times four is?), was able to raise her math ability to the high school level. Of course, the normal tendency to partially recover lost functions certainly played a part.
ALEXIA/AGNOSIA FOR NUMBERS
Alexia for numbers and digits is found in over 80% of individuals with left temporal-occipital lesions, and in less than 10% of those with right hemisphere lesions. As the name implies, the patient is unable to recognize numbers. Usually these individuals also suffer from generalized or literal alexia (Hecaen, 1962); i.e. an inability to recognize letters.
ALEXIA/AGRAPHIA FOR NUMBERS
In some cases acalculia may be associated with an alexia and/or an agraphia for numbers, as well as aphasic abnormalities (referred to as aphasic acalculia (Benson & Weir, 1972). Individuals with this disorder are unable to recognize or properly produce numbers in written form. For example, they may be unable to write out or point to the number "4" vs the number "7" or the letter "B". The lesion is usually in the left inferior parietal lobule and localized within the angular gyrus. Not all patients are aphasic however (Levin, 2014).
SPATIAL ACALCULIA
As described in Chapter 10, the right cerebral hemisphere is quite proficient in performing geometrical analysis. However, it's (i.e. the isolated right hemispheres) basic arithmetical abilities are limited to the performance of addition, subtraction and multiplication of simple sums and numbers below 10 (Levy-Agresti, 1968; Sperry, 1968). However, it can also visually recognize correct answers, for small sums, when given a visual choice (Dimond & Beaumont, 1974). Nevertheless, problems with arithmetical reasoning per se, are not usually due to right hemisphere lesions.
However, when the right hemisphere is damaged difficulties in calculation may result due to visual-spatial disturbances (Hecaen & Albert, 1978). For example, figures and digits may not be properly aligned, arranged or organized on the page when writing out a problem. Or, the patient may ignore the left half of numbers when adding, subtracting, etc. If given the opportunity to perform the same calculation verbally, frequently little difficulty is demonstrated indicating that the basic ability to calculate is intact.
In general, the majority of individuals with spatial acalculia have right hemisphere lesions. However, bilateral disturbances may be present (Boller & Grafman, 1983; Hecaen & Albert, 1978).
RIGHT-LEFT DISORIENTATION.
Right-left disorientation (e.g. "show me your right hand") is usually associated with left hemisphere and left parieto-occipital damage. It occurs only extremely rarely among individuals with right cerebral injuries (Gerstamann, 1930; McFie & Zangwill, 1960; Sauguet et al.1971).
In general, these patients have difficulty differentiating between the right and left halves of their body or the bodies of others. This may be demonstrated by asking the patient to touch or point to the side named by the examiner, e.g. "Touch your left cheek", or, "Point to my right ear"; to point on their own body to the body part the examiner has pointed to on his/her body; or in performing crossed commands, "Touch your left ear with your right hand". In mild cases only the crossed comands may be performed deficiently (Strub & Geschwind, 1983). Nevertheless, individuals with aphasic disorders generally perform most poorly of all brain damaged groups (Sauguet et. al., 1971).
Interestingly, among presumably neurologically intact adults, approximately 18% of females and 9% of men perform deficiently on right-left orientation tasks (Wolf, 1973).
In part, it seems somewhat odd that right-left spatial disorientation is more associated with left rather than right cerebral injuries, given the tremendous involvement the right half of the brain has in spatial synthesis and geometrical analysis. However, orientation to the left or right transcends geometric space as it relies on language. That is, "left" and "right" are designated by words and defined linguistically. In this regard, left and right become subordinated to language usage and organization (Luria, 1980). Hence, left-right confusion is strongly related to problems integrating spatial coordinates within a linguistic framework.

ATTENTION & NEGLECT
Data from a variety of studies have indicated that the parietal lobes are heavily involved in directing and maintaining various aspects of motoric, visual and somesthetic attentional functioning (reviewed above). This includes the maintainence of visual fixation, the guidance of hand and manipulatory activities, or the detection and monitoring of a stimulus moving across the body surface. In part, this is accomplished via interconnections with the various association areas, frontal lobes as well as subcortical structures such as the superior colliculus and the reticular formation --all of which are heavily involved in attention and/or arousal.
In general, however, the parietal lobes of the right and left cerebral hemisphere do not exert identical or equal influences. For example, although many neurons in the secondary somesthetic areas receive contralateral and ipsilateral input whereas many visual neurons in area 7 are responsive to both halves of the visual field, bilateral responsiveness appears to be more characteristic of the right parietal region. The right seems to contain a greater number of bilateral cells.
Thus, visual and somesthetic stimuli exert greater EEG evoked responses over the right half of the brain (Beck et al. 1969; Schenkenberg et al. 1971), and the right cerebral hemisphere becomes activated by stimuli applied to the right or left half of the body (Desmedt, 1977; Heilman & Van Den Abell, 1980). Conversely, the left hemisphere becomes aroused predominantly in response to unilateral (right sided) input.
Reaction times to visual stimuli are also more greatly reduced following right vs. left cerebral injuries (Howes & Boller, 1975). Similarly, among split-brain patients, the right cerebral hemisphere is able to maintain attention for appreciably longer time periods (Dimond, 1976, 1979), whereas the left hemisphere tends to demonstrate attentional lapses as well as unilateral spatial-conceptual neglect (Joseph, 1988b).
Due perhaps the greater right cerebral monitoring ability, across tasks requiring sustained motor-visual attention performance in the left half of space (with either hand) is superior to performance in the right half of space (Heilman et al. 1987).
As is well known, lesions, or even surgical removal of the right parietal lobe (particularly the inferior regions extending into the second occipital convolution or the frontal lobe), can results in unilateral neglect of the left half of visual, somesthetic and auditory space (Critchley, 1953; Hecaen et al. 1956; Heilman & Valenstein, 1972; Heilman 1991; Heilman et al. 2014; Joseph, 2006a, 1988a; Nielson, 1937; Roth, 1944, 1949; Umilta 2014). In the extreme, these patients may fail to become consciously aware that half the body is in some way dysfunctional, or even that it exists (Bisiach, et al. 1979; Critchley, 1953; Denny-Brown et al. 1952; Gold et al. 2014; Heilman 1991; Joseph, 2006a, 1988a; Levine 2010; Roth, 1944, 1949; Sandifer, 1946; Schilder, 1935). They may dress or groom only the right half of their body, eat only off the right half of their plates, etc. Indeed, the left half of the environment is ignored even when the body is aligned in the verticle axis (Calvanio et al. 1987); or, when conjuring up mental imagery, i.e. the left half of the image disappears.


As attention may be directed to at least three dimensions of visual space, radial, vertical, horizontal, including near and far peripersonal space (see Mennimeir et al. 1992), effected idividuals may show neglect only the lower quadrants -the domain of the parietal lobes- or in the upper quadrants -the domain of the temporal lobe (Shelton et al. 2010). Patients may also selectively neglect stimuli oriented toward the body (Shelton et al. 2010). However, depending on the extent of the lesion, the neglect may involve multiple frames of reference.
For example, patients with bilateral parietal-occipital lesions have been shown to neglect ventricle (inferior) and near (radial) peripersonal space, and in both the tactile and visual modalities (Rapcsak et al. 1988). Mennemeir et al. (1992) have reported similar findings (see also Umilta 2013).
Neglect may take the form of hypoarousal or inattentiveness and may involve different modalities, e.g. touch vs vision (see Umilta 2013). For example, the patient may not completely ignore the left half of space, but instead, may fail to respond to left sided stimuli only under certain conditions such as when the patient is fatigued or if simultaneously stimulated bilaterally (e.g. to the left and right half of the face, or the right ear and left hand, etc.), i.e., extinction.
In addition, rather than the left half of visual-somesthetic space, neglect may encompass the left half of an object, even when the entire object falls to the right (Bisiach et al., 1979). For example, if the word "toothbrush" is presented well to the patient's right, she may report only seeing the word "brush". If presented to her left, she may state that she sees "nothing". This is probably because these patients begin scanning from the right and proceed only a short distance leftward before they stop or are pulled back toward the right.
Not surprisingly, individuals with neglect may also display visual recognition deficits, such as an inability to recognize faces or complex objects (Young et al. 1992). However, this should not be taken as evidence that the parietal lobe contains facial recognition neurons. Rather, with parietal lesions the ability to search and explore visual, tactile, and even motoric aspects of the environment is disrupted such that only half of a face or tactile stimulus, may be explored.
Similarly, patients may neglect the left half of images which they consciously conjure up (Bisiach & Luzzati, 1978; Umilta 2013) or the left half of words or sentences when reading (Kinsbourne & Warrington, 1962; Barbut & Gazzaniga, 1987). This suggests that neglect is for both internal and external events, and greatly invovles the ability to internally generate bilateral perceptions.
Neglect, however, encompasses more than internal and external inattention, but a failure to attend to gravitational influences as well (Gazzaniga & Ladavas, 1987). As noted, the parietal lobule receives and processes information concerning body-positional relationships and integrates this information with visual input regarding objects in space. Via the analysis of proproceptive and other forms of input, the parietal lobule also takes into account gravitational influences; the position of the body in space.
As pointed out by Gazzaniga and Ladavas, (1987), when the eyes are looking straight forward and the head is in an upright position, the visual frame of reference coincides with the gravitational frame. However, if the head is turned to the side, the left and right side of the gravitational field (which is invariant) no longer coincide with the visual reference; what lies to the right may be to the ground, and that to the left may be skyward. If the head is tilted to the right, the left visual field encompasses that which is up, and the right visual field that which is below. When the head is tilted, however, individuals with parietal damage and neglect ignore not only the left side of visual space, but the left side of gravitational space as well (Gazzniga & Ladavas, 1987).
For example, if the head is tilted to the left, the patient would ignore everything which is downward as well as everything falling to the verticle left. Only the right upper quadrant would continue to be perceived and responded to.
Neglect from parietal lesions is probably also secondary to a disconnection of this region from the frontal lobes. That is, the frontal lobes failing to receive input cease to exert activational influences (via its connections with the thalamus, reticular formation) such that information normally processed by the damaged parietal lobe are no longer activated and attended to.
LEFT HEMISPHERE NEGLECT
Although not as common nor as severe, inattention and neglect has also been shown to occur following left sided lesions (Albert, 1973; Ogden, 1987). However, whereas right hemisphere induced neglect is more profound, attentional disturbances following left cerebral damage tend to be more subtle, e.g. failing to attend to small figures on the right. Or, neglect may only be manifested under procedures employing extinction --when stimulated simultaneously on the right and left halves of the body. Similarly, the right half of drawings, although not neglected per se, may tend to be more distorted and incomplete.
Neglect, following left cerebral injuries, however, is more likely to occur with left anterior rather than left parietal injuries (Kimura 2014; Ogden, 1987). Sometimes, however, this is due to motoric abnormalities, such as gaze paralysis, or difficulty moving the head or right arm toward the right; i.e. a hypokinesis. That is, patients may seem to not fully attend to the right half of space because of damage to motor neurons and thus an inability to motorically respond. Moreover, because of a loss of counterbalancing influences, the right hemisphere acting unopposed also causes such individuals to favor the left half of space. In addition, if verbal report is required, the left hemisphere being damaged is necessarily at a disadvantage such that what appears to be neglect may in fact be impaired language functioning.
DELUSIONAL DENIAL
Frequently, patients with right parietal lesions, when confronted with their unused or immobile limbs may (at least initially) deny that it belongs to them or swear there is nothing wrong (Gold et al. 2014; Heilman 1991; Joseph, 2006a, 1988a). More often, however, they tend to ignore their left half. In some cases, however, patients may perceive the left half of their body but refer to it using ego-alien language, such as "my little sister", "my better half", "my friend Tommy" , "my brother-in-law", "spirits", etc.
For example, Gerstmann (1942) describes a patient with left-sided hemiplegia who "did not realized and on being questioned denied, that she was paralyzed on the left side of the body, did not recognize her left limbs as her own, ignored them as if they had not existed, and entertained confabulatory and delusional ideas in regard to her left extremities. She said another person was in bed with her, a little Negro girl, whose arm had slipped into the patient's sleeve" (p. 894). Another declared, (speaking of her left limbs), "That's an old man. He stays in bed all the time."
One such patient engaged in peculiar erotic behavior with his "absent" left limbs which he believed belonged to a woman. A patient described by Bisiach and Berti (1987, p. 185) "would become perplexed and silent whenever the conversation touch upon the left half of his body; even attempts to evoke memories of it were unsuccessful". Moreover, although "acknowledging that all people have a right and a left side, he could not apply the notion to himself. He would affirm that a woman was lying on his left side; he would utter witty remarks about this and sometimes caress his left arm".
Some patients may develop a dislike for their left limbs, try to throw them away, become agitated when they are referred to, entertain persecutory delusions regarding them, and even complain of of strange people sleeping in their beds due to their experience of bumping into their left limbs during the night (Bisiach & Berti, 1987; Critchley, 1953; Gerstmann, 1942). One patient complained that the person tried to push her out of the bed and then insisted that if it happened again she would sue the hospital. Another complained about "a hospital that makes people sleep together". A female patient expressed not only anger but concern least her husband should find out; she was convinced it was a man in her bed.
DISCONNECTION, CONFABULATION & GAP FILLING
In some respects it seems quiet puzzling that individuals with right parietal injuries may deny what is visually apparent and what they should easily be able to remember, i.e. the presence of the left half of their body. However, it is possible that when the language dominant left hemisphere denies ownerhip of the left extremity it is in fact telling the truth. That is, the left arm belongs to the right not the left hemisphere. Indeed, in that the parietal lobe is in part an evolutionary derivative of the hippocampus (which also contains neurons that code for the position of the body and objects in space), not just a body image and the body in space, but the memory of the body is maintained in this tissue.
Moreover, as discussed in chapters 10, 29, memories are sometimes unilaterally stored; i.e. the left hemisphere maintains a somesthetic-memory image of the right half of the body, the right cerebrum maintaining perhaps bilateral representations. In this regard, the left brain may in fact have no memory regarding the left half of the body.
Of course, all this seems preposterous, and yet, patients (i.e. the speaking half of their brain) will deny what is obvious, i.e. the existence or ownership of the left half of their body.
Inevitably, in order for an individual to confabulate, erroneous information must become integrated in some fashion so that the confabulated response can be expressed. When the frontal lobes are compromised there is much flooding of the association and assimilation areas with tangential and irrelevant information much of which is amplified completely out of proportion to more salient details (Fischer et al. 2013; Joseph, 2006a, 1988a, 2014; Stuss et al. 1978). Consequently, salient and irrelevant, highly arousing and fanciful information are expressed indiscriminantly. The normal filtering process is disrupted. However, when the parietal lobes are compromised, rather than flooding, there results a disconnection and information received in the Language Axis is incomplete and riddled with gaps (Joseph, 2006a).
As noted, assimilation of input from diverse sources is a major feature of left and right parietal (i.e. inferior parietal) activity. Hence, when this area is damaged errors abound in the assimilation of perceptions and ideas as the language axis can no longer access all necessary information.
That is, when the language axis is functionally isolated from a particular source of information about which the patient is questioned (such as in cases of denial), it begins to make up a response based on the information available. To be informed about the left leg or left arm, it must be able to communicate with the cortical area (i.e. the parietal lobe) which is responsible for perceiving and analyzing information regarding the extremities. When no message is received and when the language axis is not informed that no messages are being transmitted, the language zones instead relie on some other source even when that source provides erroneous input (Joseph, 2006a); substitute material is assimilated and expressed and corrections cannot be made (due to loss of input from the relevant knowledge source). The patient begins to confabulate. According to Geschwind (1965), when the speech area is disconnected from a site of perception, then the speech area will be unable to describe what is going on at that site. This is because "the patient who speaks to you is not the 'patient' who is perceiving- they are in fact, separate".
In these instances delusions and confabulatory responses occur as a result of an attempt by the Language Axis to fill the gaps in the information received with associations and ideas which are in some manner related to the fragments available (Joseph, 1982, 2006a, Joseph et al. 2004; Talland, 1961). In this regard, confabulatory-delusional statements although erroneous, can contain some accurate elements around which erroneous, albeit related, ideations are anchored. Hence, a patient may see his left leg or arm and then state it belongs to the doctor. In general, these disturbances occur most frequently when the right frontal or right parietal lobe is damage. However, neglect, denial, and delusional confabulation may also infrequently result from left parietal injuries.
DELUSIONAL PLAYMATES & EGOCENTRIC SPEECH
Young children not only produce ego-centric speech, in which they comment upon, explain and describe their own behavior, but sometimes describe their behavior to others (such as their parents) using ego-alien language. They may claim not to know whey they performed certain behaviors, may claim that someone else actually performed the deed for which they are accused, as well as develop imaginary friends with whom they share secrets, play games, or upon whom they may place the blame for some untoward incident. These same "imaginary" friends sometimes urge them to commit certain acts or explain or inform them regarding the actions and motives of others.
Not all children, of course, develop elaborate imaginary friends. However, all (or almost all) children develop egocentric speech and at times employ ego-alien descriptions when confronted with their own disagreeable behavior. Largely, much of this is secondary to corpus callosal immaturity and a reaction of the left hemisphere is response to impulses and behaviors initiated by the right cerebrum and/or limbic system; specifically hyperactivation of the hippocampus and adjoining tissue (which in turn has contributed to the evolution of the parietal lobe). However, among this same age group (up to age 7 and even 10), the parietal lobes are also quite immature, the inferior parietal area in particular.
Following destruction of the inferior parietal lobe and adjoining tissue, patients sometimes uncontrollably comment upon their actions, e.g. "Now I'm waving goodbye" (such as when given a "command" by their physician: "Wave your hand"), whereas in other instances they may claim that the person performing the action in someone other than themselves. As noted they may in fact completely deny that the left half of their body is their own and claim it belongs to another -which also occurs in some cases of emotional trauma induced injury to hippocampus (Joseph, 1998b, 2008d); a structure which has contributed to the evolution of the parietal lobe.
Although a lesioned parietal lobe is not the same as parietal lobe immaturity (particularly in that immaturity is bilateral and damage is usually unilateral), there remains a curious similarity in the behavior of these adults and children, including those who suffer dissociative states secondary to hippocampus-amygdala hyperactivation (chapter 30).
Perhaps this is because the ego and Self is first identified with the body whereas the image of the body is maintained by the parietal lobe. When one parietal lobe (due to damage or immaturity) is unable to communicate with the other half of the brain, the "normal" brain half is unable to recognize a continuity of Self. Consequently, behaviors initiated by the opposite (usually right) half of the brain, or the half of the body controlled by right brain are recognize by the speaking half of the cerebrum only from a disconnected (i.e. alien, dissociated) perspective. When the speaking (i.e. left) hemisphere is questioned about this behavior, or about it's left limbs, they are described as initiated or as belonging to someone else, such as "an old man", "my brother-in-law", or an imaginary friend.
SUMMARY & OVERVIEW
The parietal lobes, although commonly associated with the mediation of somesthetic stimuli, are also concerned with motor and attentional functioning, the perception of spatial relations, including depth, orientation, location, and the identification of motivationally significant auditory, somesthetic, and visual stimuli. It is via the integrative interaction of the parietal lobe that an individual can feel an object and not only determine its physical qualities, (e.g. shape, size, weight, texture), but can visualize, verbally lable, and even write out it's name. It is also via the interaction of these various cell assemblies that an individual can attend to specific objects in space as well as reach out and manipulate them.
Indeed, the parietal lobe is very important for mediating movement in visual space. That is, in order for a movement to be correctly planned and carried out signals must be directed to the right muscle groups as based on efferent visual, somesthetic, as well as auditory input. Moreover, one requires sensory information as to the position of the body and limbs in space, otherwise movements will be clumsy. This is accomplished through the dense interconnections linking the primary somesthetic with the motor areas in the frontal lobe and via impulses transmitted down the cortocal-spinal tract. Moreover, parietal neurons appear to guide and monitor movements as they occur in visual space.
Although the entire parietal lobule makes important contributions to movement, the inferior parietal lobule of the left hemisphere appears to be the central region of concern in regard to the performance of skilled temporal-sequential motor acts. . These engrams assist in the programing of the motor frontal cortex where the actions are actually executed.
If the inferior parietal region is destroyed the patient loses the ability to perform actions in an appropriate temporal-sequence or to even appreciate when they have performed an action incorrectly. This condition is referred to as apraxia.
In contrast, right parietal injuries are associated with severe disturbances of emotion, constructional deficiencies, as well as a host of visual-spatial perceptual abnormalities including left sided inattention and neglect. For example, with severe lesions in this area patients may demonstrate a profound inattention to all forms of stimuli falling to their left. When drawing pictures they may fail to draw the left half of an object, when writing or reading they may ignore the left half of words or the left half of the page, and they may fail to perceive and respond to individuals standing to their left, or even the left half of their own body. Largely this condition is secondary to a destruction of neurons which are sensitive to various forms of visual and somesthetic input.
Nevertheless, it is important to emphasize that lesions to the parietal lobe (or anywhere within the brain) are seldom localized to one particular quadrant (e.g. inferior, superior), or even restricted to the parietal lobe. That is, damage may be parietal-occipital, parietal-temporal, frontal-parietal, or even bilateral (such as due to cerebrovascular disease or compression from a unilateral tumor). Therefore, an afflicted individual may display agraphia but normal reading, stereognosis in the absence of apraxia, or conversely a wide mixture of seemingly unrelated symptoms.











