Rhawn Joseph, Ph.D.
BrainMind.com
THE CORTICOSPINAL TRACT
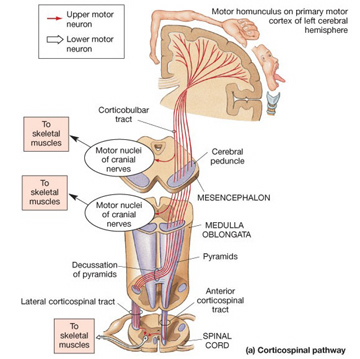
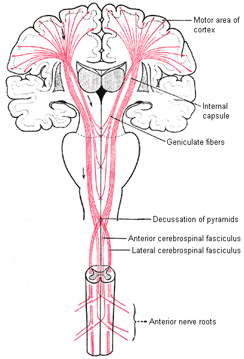
The corticospinal/pyramidal tract consists of all axonal fibers which descend from the neocortex, through the white matter, into the brainstem where some of which make contact with cranial nerve nuclei, and which then cross over longitudinally in the pyramids of the lower medulla, and then descend into the spinal cord where they make contact with sensory and spinal motor neurons (Brodal, 2001; Kuypers & Catsman-Berrevoets, 1984).
Only approximately 31% of the corticospinal tract arises from the pyramidal cells of agranular primary motor area 4, with another 30% arising from areas 6, 8, and the SMA, and another 30% from the primary and secondary/association somesthetic areas in the parietal lobe, and the remainder arising from the deep layers of the temporal, occipital, and orbital frontal lobes, as well as from pyramidal cells scattered throughout the amygdala, cingulate, and striatum. Hence, widespread areas of the neocortex control or influences movement by acting directly on the brainstem and spinal cord via the corticospinal tracts.
For example, the neocortex of the motor areas are relatively thick and "agranular" in structure. This is because internal "granular" layer IV is obscured by the increased size of layers III and V which are packed with large numbers of exceedingly large pyramidal cells including the giant cells of Betz. By contast, in somesthetic areas 3,1,2, layers III and V are much smaller, whereas layer IV is much thicker.
Within the frontal motor areas, it is layers III and V which gives rise to the corticospinal tract, and which consists of large and medium size pyramidal cells. Layer V also gives rise to the corticbulbar, corticopontine, and corticorubral fibers.
Approximately 90% of corticospinal axons range from 1 to 4 um in diameter. The largest fibers are axons from the giant Betz cells which are located in layer V of the primary motor area. About 60% of the axons of the corticospinal tract are myelinated.
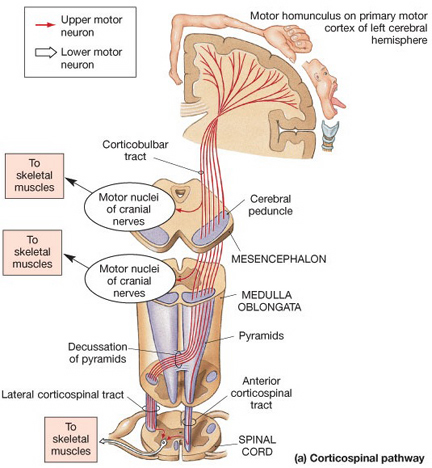
By contrast, corticospinal axons descend through the pons where they separate into tiny nerve bundles before regrouping within the medulla to form the medullary pyramid. However, at the spinal-medulla border, about three fourths of these axons cross over at the midline of the medulla to form the pyramidal decussation, with the crossed fibers forming the lateral corticospinal tracts and the remaining uncrossed axons forming the ventral corticospinal tract.
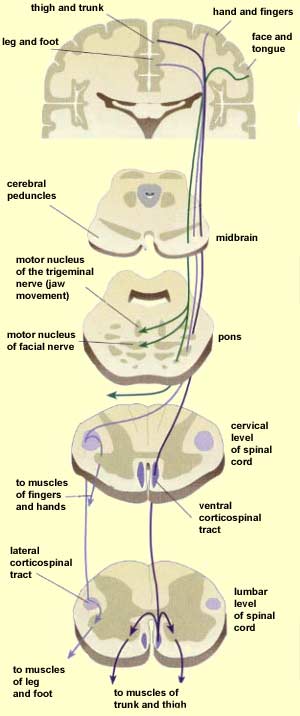
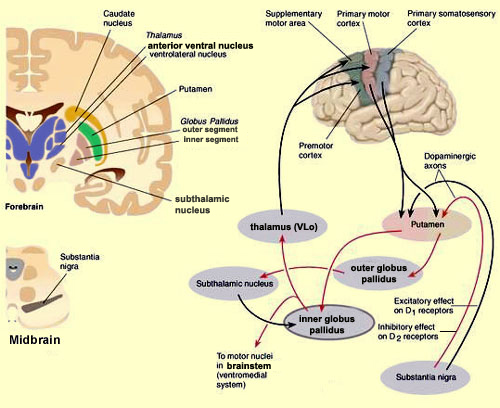
Hence, the corticospinal tract consists of two divisions, the lateral and ventral.The lateral tract projects to the lateral motor nuclei of the ventral horn and to intermediate zone interneurons, whereas the ventral tract projects bilaterally to the medial cell column which is concerned with the axial muscles. Most of the ventral (uncrossed) axons originate in Broadmans areas 4, 6 and 8, whereas the lateral tract also originates in frontal motor areas 4 and 6, and parietal somesthetic areas 3,1,2,5,7, though yet others have their source throughout the neocortex as well as in the limbic system.
Thus, these two divisions consist of a number of separate fiber bundles which originate not just within the pyramidal cells of the somatomotor areas, but within the striatum, cingulate and amygdala. These fiber bundles, therefore consist of a commingling of axons which originate throughout the forebrain, and which arise in discrete neocortical locations. Again, widspread areas of the brain contribute to purposeful movement. Moreover, although these fiber bundles descend together through the internal capsule, they innervate different brainstem targets, and are thus referred to as the corticobulbar, corticopontine, corticorubral and corticospinal tracts.
The vast majority of descending motor fibers, however, penetrate the brainstem and establish direct synaptic interconnections with spinal motor nuclei. These latter fibers are referred to as the corticospinal tracts.
Specifically, corticospinal axons descend through the pons where they separate into tiny nerve bundles before regrouping within the medulla to form the medullary pyramid. At the spinal-medulla border, about three fourths of these axons cross over at the midline to form the pyramidal decussation, with the crossed fibers forming the lateral corticospinal tracts, and the remaining uncrossed axons forming the ventral corticospinal tract. In this manner forebrain motor control is gained via neocortical pyramidal axons which innervate the motor nuclei of the brainstem and spinal cord at all levels.
LEFT FRONTAL DEVELOPMENTAL DOMINANCE FOR MOTOR FUNCTIONS
The motor cortex of the left hemisphere, however, is dominant for motor control; a function of the earlier maturation of the left cerebral motor areas and the descending corticospinal tract. In the vast majority of the population, although the nonmotor areas of the right frontal lobe appear to initially develop more rapidly than their left sided counterparts (Chi et al. 1977; Gilles et al. 1983; Scheibel, 1991, 1993), which in turn enables the right hemisphere to gain dominance over sensory limbic functions (Chiron et al., 1997; Joseph, 1982). By contrast, the primary somatomotor areas of the left frontal lobes mature at a more rapid rate than the right frontal lobes and by the first year tissues such as Broca's area overtake their right sided counterparts (Scheibel, 1991, 1993).
Hence, due to early left hemispheric motor development, the left corticospinal tract grows more quickly and descends into the brainstem, and then crosses at the pyramidal decussation, and then descends into the spinal cord in advance of those fibers from the right (Kertesz & Geschwind 1971; Yakovlev & Rakic 1966). This provides the left hemisphere and the right hand with a competitive advantage in establishing both motor control and thus right hand dominance.
Because the corticospinal tract does not reach advanced levels of myelination and does not become functionally mature until the 8th-12th postnatal month (Yakovlev & Lecours, 1968) it is only as the infant approaches their first birthday that handedness become obvious or apparent.
It is also around the 8th to 12th month that the motor and somatosensory areas become increasingly mature (Bell & Fox, 2014; Brody et al., 1987; Chugani, 2014; Hudspeth & Pribram, 1992; Huttenlocher, 2010; Schore, 2014) and the secondary motor and sensory receiving areas display increased myelination (Flechsig, 1901).
As based on PET scans, the frontal lobes also display increased metabolic activity from 8 to 12 months of age (Chugani, 2014), and around the end of the first year the neocortical EEG begins to increasingly resemble a more adult pattern (Kellaway, 1979; Ohtahara, 1981). In addition, around the 8th to 12th month, the corticospinal and pyramidal axons begin to approximate adult level of cellular development, myelination, and organization, and then become increasingly well myelinated over the course of the second year (Debakan, 1970; Langworthy, 1937; Yakovlev & Lecours, 1967)--a process which continues into late childhood (Paus et al., 1999).
Hence, around the 8-12th month the neocortex and frontal motor areas begin to increasingly exert hierarchical control over the limbic forebrain, brainstem, and musculature, and this process continues to develop over the course of the ensuing decade and requires considerable coordinated interaction between the parietal and motor areas of the frontal lobe as well as the striatum, limbic system, and cerebellum.
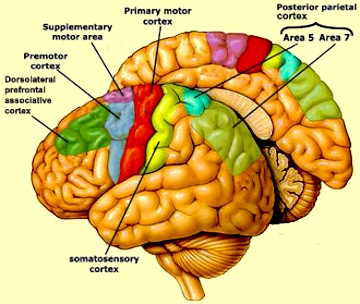
THE PRIMARY MOTOR AREA
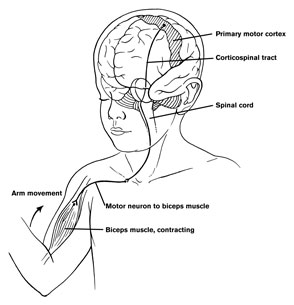
The primary motor area serves as a neocortical nodal point where impulses organized in other brain tissues are transferred for expression, as the entire body musculature is represented in this tisse and as it contributes the majority of axons (as compared to any other single brain area) that comprises the corticospinal tract.
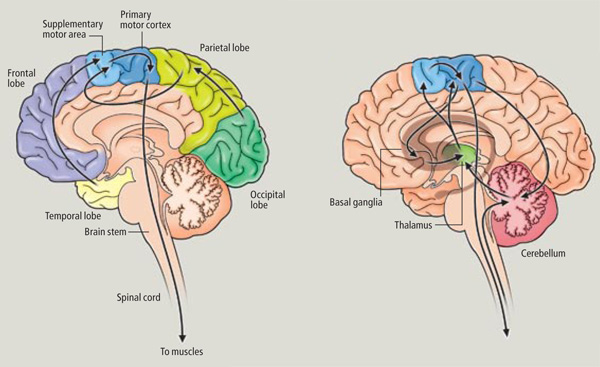
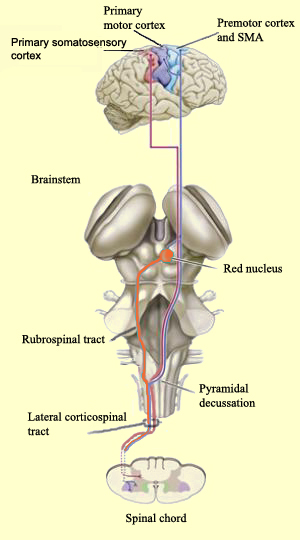
As noted, axonal projections from the motor and somesthetic regions all give rise to the massive cortico-spinal (pyramidal) tract which innervates cranial nerve and sensory and spinal motor neurons (Brodal, 1981; Kuypers & Catsman-Berrevoets, 1984). For these reasons some authors have referred to the somesthetic and motor regions as the sensorimotor cortex.
The primary motor area is concerned with the coordination and expression of gross and fine motor functioning including finger movements (Colebatch et al., 1991; Luria 1980; Rao et al. 2015; Sanes et al., 2015; Shibasaki et al. 1993; Woolsey, 1958), and serves as the final common pathway where impulses organized in other brain areas are transferred for expression, particularly those involving the fingers, thumb, lips, tongue, and hand. Indeed, the region of area 4 that represents the hand is especially prominent and in fact forms a bulge upon the surface (Yousry et al., 2015).
Electrical stimulation of discrete points within the motor cortex while at rest can trigger contractions and movements of tiny muscle groups on the opposite side of the body (Penfield & Jasper, 1954; Penfield & Rasmussen, 1950; Rothwell et al. 1987). Conversely, however, activation of one half of the body can induce bilateral activation of the motor area.
The primary motor area, however, is organized in a dual fashion in regard to the control over the body musculature. There is a one to one correspondence between neurons located in the primary motor area and specific muscles, which in turn makes fine motor functioning possible (Penfield & Jasper, 1954; Penfield & Rasmussen, 1950). However, there are also convergent connections as well, such that a single neuron innervates numerous muscle groups which in turn are innervated by yet other motor neurons (Humphrey, 1986; Shinoda, et al., 1981). In this manner, a neuron innervating a specific group of arm muscles, can also act on other muscles so as to coordinate movement.
Hence, a given muscle is also influenced by a number of motor neurons, such that a single muscle is represented by multiple neurons (Humphrey, 1986; Shinoda, et al., 1981). Multiple representation enables the motor cortex to coordinate a variety of movements, such that wide areas of the motor area become activated during even discrete movements of, for example a single finger (Colebatch et al., 1991; Sanes et al., 2015). Thus, activity is distributed over the motor area even when performing discrete movements.
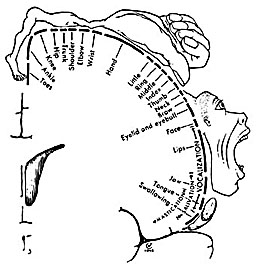
Specifically, represented deep in the inferior medial portion of area 4 and moving anterior toward the superior medial frontal lobe are the toes, ankle, knee, hip, anal sphincter and genitals respectively. Circling up and over the superior surface to the lateral convexity are the shoulder, elbow, wrist, and hand, with extensive areas of neocortex devoted to the fingers and thumb, followed by the brow, eyelids, larynx, lips, jaw, and tongue which is located at the most inferior opercular portion of the precentral gyrus. Like the somesthetic neocortex which maintains a double representation of the body surface (e.g. a double body image) the primary motor area contains multiple representations of the body's musculature (Colebatch et al., 1991; Humphrey, 1986; Sanes et al., 2015; Shinoda, et al., 1981).
As noted, based on single cell recording, activity begins in the primary motor area following activation of the premotor and SMA and becomes maximally active at the time of movement. However, as based on single cell recording and functional imaging, neurons in the primary motor area also become active just prior to movement, increase their activity during movement, and will become increasingly active in response to direction, velocity, and the amount of force required to perform the movement (Ashe & Gerogopoulos, 2014; Crutcher & Alexander, 2010; Passingham, 1997). Indeed, this has also been demonstrated with evoked potentials. Activity will begin in the primary motor area prior to movement, and will increase as the time of movement onset approaches, and increases in amplitude during the course of movement (Ikeda & Shibasaki, 1992). In addition, these neurons will increase their activity depending on the amount of force involved in the movement, and in reaction to the direction and velocity of movement (Ashe & Georgopoulus, 2014).
Moreover, the motor area is exceedingly plastic and as indicated above, is capable of learning and memorizing and remembering (e.g. Carpenter et al., 1999). If a single muscle is repeatedly activated and/or as an individual becomes increasingly skilled at performing a certain motor tasks, the cortical territory representing those muscles will significantly increase (Pascual-Leone et al., 2014).
Paralysis.
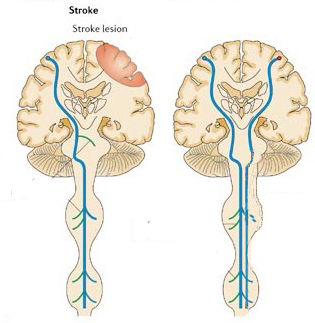
Damage to the motor areas, or to the descending cortico-spinal tract initially results in a flaccid hemiplegia such that the muscles are completely without tone contralateral to the lesion (Adams & Victor, 2014; Brodal, 1981). If the examiner were to raise and release an effected arm, it would drop in a limp rag doll fashion.
Over the course of the next several days the muscles develop increased tone and there is resistance to passive movements. Reflexes become very brisk and spasticity and hyperreflexia are manifest. With massive lesions extending into the medial regions (where the leg is represented), the leg will become permanently extended and the arm will assume a flexed position (Adams & Victor, 2014; Brodal, 1981). After several weeks or months a very limited capacity to perform gross movements reappears. Fine movements are usually permanently lost (Brodal, 1981).
REFERENCES
















