Rhawn Joseph, Ph.D.
BrainMind.com
THE NEUROLOGY OF CHILDHOOD AMNESIA VERSUS AMNESIA FOR CHILDHOOD DUE TO ABUSE & TRAUMATIC STRESS
Repression, Recovered Memories, and the Right Hemisphere
Over the course of the last 100 years, it has been repeatedly established that adults, adolescents, and older children are generally unable to remember events from infancy or early childhood (e.g., Fitzgerald, 1991; Freud, 1905; Hall, 1899; Mullen, 1994; Joseph, 1999, 2003; Potwin, 1901). This condition is referred to as "childhood amnesia," and appears to have its offset at about age 3.5. (Dudycha & Dudycha, 1933; Gordon, 1928; Joseph, 2000; Waldvogel, 1948; White & Pillemer,1979). For example, in a study of college students Dudycha and Dudycha (1933), found that the mean age for first recollection among men was 3 years 8 months, and for women it was 3.6. In a later study they determined the average age at first recollection was 3.67 for adolescent males and 3.50 for females (Dudycha & Dudycha, 1941). Similarly, Gordon (1928) and Waldvogel (1948) found the average age was 3.64 for males and (respectively) 3.4 and 3.23 for females. Likewise, in a recent study based on adults from the general population, it was found that on average, males form their first recallable memories at age 3.7, whereas females recall their's from age 3.3 (Joseph, 2000).
It is noteworthy that that some individuals claim memories from before the age of two and even one (Dudycha & Dudycha, 1941; Joseph, 2000; White & Pillemer,1979). However, in one study it was also found that 2% of adults from the general population claim absolutely no memory for anything that happened before age 10 (Joseph, 2000); a prolonged period of amnesia which may be due to early childhood abuse. In fact, whereas those from the general population form their first memories around age 3.5, those with a history of severe and repetitive physical, sexual and/or emotional abuse, form their first recallable memories, on average, at about age 6.1, and 11.5% claim no memory before age 10 (Joseph, 2000). Regardless of gender or childhood history, negative and unpleasant memories are formed at a significantly later age than positive memories (Joseph, 2000). In fact, almost all investigators have found that the earliest recollections tend to be positive (Jersod, 1931; Joseph, 2003, Kihlstrom & Harackiewicz, 1982; Meltzer, 1931; Stagner, 1931; Waldvogel, 1948). Waldvogel (1948) found that pleasant memories constituted about 50% of the total with unpleasant making up 30% and neutral memories constituting the rest (see also Colgrove, 1899; Hall, 1899; Potwin, 1901), whereas in the Joseph (2000) study, 52% of male and 54% of female first memories were positive. Hence, negative and unpleasant memories do not promote memory.
It has, in fact, been repeatedly demonstrated in animal and human studies, that exceedingly negative events and highly arousing experiences are forgotten to varying degrees (Christianson, 2012; Conrad, Galea, Kuroda, & McEwen, 2006; Diamond, Bennett, Fleshner, & Rose, 2012; Diamond, Fleshner, & Rose,1994; Joseph, 2003, Kirchbaum, Wolff, May, Wippich & Hellhammer, 2006; Newcomber, Craft, Hershey, Askins, Bardgett, 1994; Yerkes & Dodson, 1908). For example, victims of rapes and physical assaults tend to provide poorer descriptions of their assailants than those who are victims of robberies (Kuehn, 1974). Briere and Conte (2013), found that over 50% of 450 men and women who had been sexually abused as children had suffered periods of total or partial amnesia for the event. In a study of individuals who had committed murder and violent crimes, over 10% had no recollection and reported amnesia for their crimes (Taylor & Kopelman, 1984; see also Christianson & Nilsson, 1989) whereas those who engage in non-violent crimes or those who are not physically assaulted, do not demonstrate a loss of memory.
Traumatic memory loss also occurs among children (Bergen, 1958; Brier, 2012; Brier & Conte, in press; Courtois, 1988; Fredriskon, 2012; Herman & Schatzow, 2015; Summit, 1983; Terr, 1988, 1990). For example, Terr (1988, 1990) in her examination of children who have been sexually abused or traumatized by the death of a parent or a severe injury found that although they were able to accurately reenact the event, their verbal memories tended to be fragmented, incomplete and quite poor. Likewise, in a study of children who had undergone a painful medical exam, Goodman and colleagues (Goodman, Bottoms, Schwartz-Kenney, & Rudy, 1991; Saywitz, Goodman, Nicholas, & Moan, 1991) found "suppressed memory," and that children up to age 7 required cues and specific questions before they were able to recall the details "In free recall and demonstration... 78% and 83% failed to report it, respectively" (Saywitz et al., 1991, p. 686). Hence, perhaps not surprisingly, adults with a history of severe childhood abuse demonstrate severe memory disturbances for early childhood (Joseph, 2000).
These findings, however, appear to conflict with those presented by Usher and Neisser (2013) who claim first memories are formed around age 2 for a negative event (hospitalization). Unfortunately, Usher and Neisser's results are likely due to sampling bias as they are based on college students who had heard family stories and/or had seen pictures of, and who earlier filled out a questionnaire documenting the events they were later supposed to remember. As noted, other studies have also found that a small percentage of subjects recall memories from age 2 or before, including 7% of those with a history of abuse vs 13% of those from the general population (Joseph, 2000).
The validity and generalizability of Usher and Neisser (2013) results are not only questionable but contradicted by another study by Neisser and Harsh (2012) on negative "flashbulb memories." Specifically, Neisser and Harsh had subjects fill out a questionnaire regarding the Challenger space craft explosion. When questioned 32-34 months later, 75% could not recall filling out the questionnaire and many had forgotten considerable detail regarding the accident. According to Neisser and Harsch (2012), "As far as we can tell, the original memories are just gone." Memory for negative events in fact decreases with decreasing age. For example, in examining memory for the deaths of President Kennedy and Robert Kennedy, Winograd and Killinger (1983) found a steep gradient of forgetting which became more profound with decreasing age. Those who were younger than 3 when these events occurred had no verbal recollections regarding information sources, context or associated events, and only approximately 50% of those who had been 4.5 years old or older could verbally recall the news and provide at least one verbal detail when questioned as adults. Likewise, Warren and Swartwood (2012) in their memory study of the Challenger explosion, found that children age 5 were less accurate and more likely to delete features and forget details as compared to older children, and that those who had been the most emotionally upset suffered the greatest degree of memory loss.
In fact, it has been found for both adults (Blaney, 1986; Rholes et al. 2015) and children (Casey 2013; Cole 1986; Saarni 1984; Strayer 2013) that even recent positive experiences are easier to verbally recall than those which are negative. Adults (Meltzer, 1931; Stagner, 1931) and children (Casey 2013; Cole 1986; Saarni 1984) also tend to forget negative memories more quickly than those which are positive. For example, in one experiment it was found that 3 year old children had much difficulty recalling a fire drill or the correct sequence of events following the alarm, whereas five year olds were much more accurate (Pillemer & White, 1989).
Young children mentally avoid the negative, and have difficulty verbally describing and identifying negative emotional expressions in themselves or others (Feshbach & Roe 1968; Mood et al. 1978; Strayer 1980), and tend to give verbal reports which misidentify these feelings as positive (Casey 2013; Cole 1986; Saarni 1984). Adults do not have these difficulties. Moreover, events which occur during positive mood states are also easier for children to verbally recall as well (Forgas et al. 1988). Conversely, they are less likely to verbally recall events which occurred when in a negative mood (Hill 1972; Masters et al. 1979; Peters 2015), or when observing adults or children behaving in a negative manner (Blugental et al. 2012; Hoffman 1983). Even extraneous contextual details which occurred in association with negative events are quickly forgotten by young children (Blugental et al. 2012). By contrast, positive memories and stimuli and events immediately following a negative incident or mood, are recalled much more correctly (Goodman et al. 1991).
Because children find it quite difficult to verbally recall, describe and identify negative vs positive experiences and memories, this may also explain why adults with a history of severe childhood abuse demonstrate a period of childhood amnesia which is twice that of the general population (Joseph, 2000). In fact, under exceedingly stress and abusive conditions, particularly if they are repetitive and prolonged, young (and even older) victims may suffer a complete dissociative amnesia (Joseph, 1998b, 1999d). Consider, for example, the well publicized trauma of "Baby Jessica" McClure who at age 18-months was trapped in a narrow hole, 22 feet below the Earth's surface, for 58 terrifying and painful hours. Although internationally televised and subject to one television movie and several books, and despite the skin grafts and amputation of one toe, 10-year old Jessica cannot remember anything of her ordeal (Babineck, 2009).
Similarly, Tara Burke was kidnapped three months shy of her 3rd birthday, held captive in a van for 10 months, and repeatedly sexually assaulted by two men; an ordeal photographed and filmed by her tormentors.
According to her parents, these men kidnapped Tara when she had been left in her parents's vehicle with her 9-year old brother. Both parents had dashed inside a store, and then moments later, a man tapped on the van window and told the boy that his mother wanted him. When the boy opened the door, the kidnapper snatched Tara and flung her over his shoulder, and then raced to a waiting car. The kidnapers transferred her to a van where she was confined, stripped naked, and repeatedly sexually assaulted by Luis "Tree Frog" Johnson, age 33, and his 17-year-old accomplice "A. C." (who, himself, had been a victim of Johnson for the previous 8 years). She was later sexually assaulted by some of their friends. The two men later abducted an 11-year old boy, "Mac" who was repeatedly raped and beaten with a rubber hose. According to Mac, Tara was also repeatedly beaten. He also observed her being sexually assaulted and molested several times a day by A. C. and Johnson who wanted to have a child by her. She was also sexually assaulted by friend's of Johnson who also liked sex with children.
And then "Mac" escaped, by slipping through the van's roof ventillator. Mac found his way to the police, whom he led back to the van. There they found Tar lying naked in a bed with A.C.. According to the police, Tara was so traumatized she didn't know her name, she couldn't tell them anything, she couldn't remember anything, and she didn't even know she was a girl. She was so traumatized that she in fact could never recall what had happened to her.
Despite intense media scrutiny and a subsequent court trial, 18-year old Tara reports that the "memory has been erased" from her mind. "It's like a story that has happened to someone else" (Joseph, 1998b). Indeed, she states that her only memories are "being set up on a bench and them doing things to me. I don't remember what." Rather, it was "Mac" who laid out the sordid details.
VISUAL AND VERBAL CHILDHOOD MEMORIES
It has been argued that the offset of childhood amnesia is directly related to the child's growing vocabulary (Freud, 1905; Waldfogel, 1948). That is, the development of verbal memory for childhood events parallels the development of verbal ability. As reported by Waldvogel (1948) when verbal "memories were plotted according to the age of their origin, it was found that there was an increment from year to year which took the form of an ogive and seemed to parallel the growth of language during childhood."
However, if the offset of childhood amnesia is purely a function of word knowledge, then it should be expected that first recallable memories would be predominantly verbal and would include statements or words uttered by the child or a friend or family member, etc. However, that is not the case.
In a study of 100 adults, aged 18 to 51, it was found that for males 80% of first recallable memories were visual-pictorial and only 20% had verbal components (i.e. verbal-visual) in which at least vague verbal statements could be recalled. As per females, 70% of first memories were visual-pictorial, and 30% were both visual and verbal (Joseph, 2000). There were no first memories that were exclusively verbal and not a single individual reported a verbal-visual (V-V) memory that was formed before a visual-pictorial (V-P) memory. Thus, recallable visual-pictorial memories are formed at a significantly earlier age than verbal-visual memories; a finding similar to those reported by other investigators (Dudycha & Dudycha, 1941; Jersod, 1931; Meltzer, 1931; Stagner, 1931; Waldvogel, 1948) . In fact, 35% of the males in this study and 10% of the females could not recall an early memory that contained explicit language or vague verbal components, and of those which did, the majority were unable to explicitly recall what had been said. Hence, it appears that first recallable memories are predominantly visual-pictorial, and are formed around 3.5 years in age and are of positive emotional events. By contrast, the first recallable verbal-visual memories are formed about 4.4 years in age. Thus, for both sexes, the first recallable V-V memories were formed, on average, nine months after the first V-P memories (female/male mean: V-P=3.3/3.7 vs V-V= 4.2/4.6).
It was also found that when considered as a group, the ability to form recallable early memories increases incrementally (Joseph, 2000, 2003). That is, more people recall memories formed at age 3 than at age 2 and so on. Moreover, although first recallable memories are predominantly visual-pictorial it appears that if considered as group and if these incremental memories are plotted according to age, they tend to parallel the functional maturation of the corpus callosum, as well as the development of those neurological substrates which subserve memory, language, and the perception of complex visual images; i.e. the temporal lobe.
Specifically, 2% of the participants from the general population claimed to recall memories from before age one, and these memories were purely visual-pictorial. Visual-pictorial processing, perception, and memory storage are associated with the right hemisphere and right temporal lobe (Andersen, 1978; Brewer, et al., 1998; Evans et al., 1995; DeRenzi; 1986; Jacobson, 1986; Joseph, 1988a; Kimura, 1963; Tranel & Hyman, 1990) which begins to mature in advance of the left (Chi, et al., 1977; Gilles et al., 1983; Joseph, 1982; Scheibel, 1991; Thatcher, 2012). Nevertheless, the neocortex of the right (and left) hemisphere is poorly myelinated and is thus exceedingly immature (Conel, 1939-1967; Flechsig, 1901). In addition, the superior temporal lobe (which in the left hemisphere encompasses Wernicke's area) and the inferior temporal lobe (which subserves memory and houses the amygdala and hippocampus), are exceedingly immature, and have grown in total surface volume to only 55% and 63% (respectively) of the adult temporal lobe (Conel, 1939-1967; Blinkov & Glezer, 1968; see also Benes, et al. 1994). As per language capabilities, infants do not begin to speak their first word until around 11 to 12 months of age (Nelson, 2004) as their initial vocalizations tend to be social emotional and produced by the limbic system rather than the neocortex (chapter 15). Presumably, the immaturity of the neocortex, temporal lobe, and hippocampus (and associated cognitive capacities) explains why 98% of the participants in this study had no memory from age one or before.
By 1.5 years of age an additional 3% of subjects report a first recallable memory. At 18 months the infant's vocabulary remains exceedingly limited consisting of between 20-40 words (Mervis & Betrand, 1995; Nelson, 2004; Thal, et al. 2009), and the surface areas of the superior and inferior temporal lobes have only marginally expanded and are (respectively) about 65% and 75% of that of the adult (Blinkov & Glezer, 1968; Conel, 1939-1967).
By two years an additional 8% of subjects report a first memory, whereas vocabulary has grown to between 186 to 310 words on average (Fenson, et al. 2013; Nelson, 2004). Likewise, at age 2 the superior and inferior temporal lobe have expanded and grown to about 80% and 85% of the adult (Blinkov & Glezer, 1968; Conel, 1939-1967).
Between 2.5 to 3 years, 40% of adult participants report a first memory, and vocabulary has expanded to up to 500 items (Fenson et al., 2013; Nelson, 2004), whereas the superior and inferior temporal lobe have reached about 84% and 86% of that of the adult (Blinkov & Glezer, 1968). Nevertheless, these first memories are predominantly visual-pictorial (and thus right hemispheric) and at age 3, the neocortex remain exceedingly immatures (Blinkov & Glezer, 1968; Conel, 1939-1967; Flechsig, 1901; Thatcher et al., 2006; Yakovlev & Lecours, 1967).
However, by age 4, the ability to process and retain and transfer information within and between the hemisphere's has improved significantly as compared to 3 year-olds (Joseph et al., 1984), the temporal lobes have continued to mature (Conel, 1939-1967; Flechsig, 1901), the child's vocabularly has grown to between 800 to 1,000 words (Aitchison, 2015), and the majority of adults are able to recall an early visual-pictorial memory from this age, whereas almost 50% can recall an early memory with verbal components. By age 5, the child's vocabularly has grown to approximately 1,500 words (Freburg & Berry, 1969). As noted, on average, the first recallable verbal-visual memories are formed about 4.4 years in age. Moreover, by age age 7, the neocortex has reached an advanced stage of maturity (Thatcher et al., 2006), and 98% of the adults from the general population remember events from before age 7.
Hence, the results detailed above, indicates that the offset of childhood amnesia parallels the maturation of the neocortex and the temporal lobes--structures which subserve learning, memory and recall (Brewer, et al., 1998; Nunn et al., 1999; Ploner et al., 1999; Wagner et al., 1998). However, given that first recallable memories are visual rather than verbal, and since the neocortex of the right hemisphere begins to mature in advance of the left, it appears that the offset of childhood amnesia is more a function of the earlier maturation of the right than the left hemisphere. Moreover, rather than being purely a function of the child's growing vocabulary, the offset of childhood amnesia instead appears to be related to the child's growing ability to process and store in long term memory non-verbal and visual-pictorial events, which in turn is due to the earlier maturation of the right temporal lobe.
On average, the first recallable verbal (verbal-visual) memories are formed 9 months after the first visual-pictorial memories (Joseph, 2000, 2004). Obviously, the ability to recall verbal memories must be related to the maturation of the left hemisphere, which begins to mature after the right. That is, it is left hemisphere immaturity and the child's limited vocabulary which contributes to the difficulty adults experience when attempting to recall early verbal memories and verbally-related events. An insufficient vocabulary limits the ability to form verbal memories.
However, lack of verbal skills should not significantly disrupt non-verbal memory per se, as non-human animals (Joseph, 1979, Joseph & Gallagher, 1980; Joseph, Hess & Birecree, 1978) and children age 2 or as young as 6 months (Hartshorn & Rovee-Collier, 2009; Perris et al., 1990; Pillemer & White, 1989; Rovee-Collier & Shyi, 2012), are clearly capable of learning, storing in long term memory, and later demonstrating non-verbally and behaviorally, the recollection of non-verbal, emotional, visual, and tactual memories weeks after they were formed; that is, if provided sufficient contextual and visual and auditory cues. In fact, neonates appear capable of remembering (or at least selectively responding to) sounds heard up to six weeks before birth, including melodies and mother's voice (DeCasper & Spence,1986). Therefore, the insufficient verbal abilities of the child, although accounting for a lack of explicit early verbal memories, cannot completely explain the failure of adults to recall early non-verbal, visual and emotional memories; though it would prevent the child, infant (or animal) from verbally coding, and verbally recalling these experiences.
SEX DIFFERENCES IN CHILDHOOD AMNESIA VERSUS AMNESIA FOR CHILDHOOD
Females form their first visual-pictorial and verbal-visual memories at an earlier age than males (female/male mean: V-P=3.3/3.7 vs V-V= 4.2/4.6); a sex difference reported by a number of investigators (Joseph, 2003, see above). Female first memories are also significantly more emotional and positive than male memories (Joseph, 2000, 2003); and other investigators have also found that female memories are more emotional (Pratt, 1985; Walker, et al. 2015; Friedman & Pines, 1991).
Given that it has been repeatedly established that female adults and female children are better able to perceive, remember, and express emotional nuances than males (see below), and since the right hemisphere of the female appears to be more adept at processing and storing emotional data, and as females may have more neocortical space committed to perceiving and expressing affective laden information (Joseph, 2013, 1999e), these capabilities might be expected to provide females with an advantage in recalling early events.
In addition, females acquire language at an earlier age and demonstrate a variety of language superiorities compared to males (reviewed in Joseph, 1999e; Levy & Heller, 2012). As there is also some evidence to suggest that the female left hemisphere may mature more rapidly than the male left hemisphere (Shucard, et al, 1981) this may also explain why female first verbal memories are recalled from an earlier age than male verbal memories. In fact, more females are able to recall verbal memories as compared to males (Joseph, 2000). Thus, in general, females form their first recallable memories at an earlier age than males (Dudycha & Dudycha, 1933, 1941; Gordon, 1928; Joseph, 2000; Waldvogel, 1948). Female first memories are more emotional, and they tend to recall more verbal-visual memories than males, and from an earlier age.
It is noteworthy that some investigators of early memory have also found sex differences in egocentricity and the types of emotional memories recalled. It has been reported that male memories of early childhood tend to be more "egocentric" such that they have a better memory for accidents and sickness involving themselves, whereas females are more likely to recall accidents and sickness involving others (see Dudycha & Dudycha, 1941). In addition, Dudycha and Dudycha (1933) found that 37% of the male memories involved fear, whereas only 21% of the female memories were fearful. Female fear memories involved being alone and fear of strangers, whereas male fear memories concerned accidents such as falling or playing with fire, as well as fear of strangers (see Dudycha & Dudycha, 1941).
Sex differences in age of first recollection is completely reversed when comparing adults with a history of severe abuse with those from the general population. Specifically, the average age of first memory for abused women is 6.5, and for abused men, 5.7, compared with 3.3, and 3.7 years, respectively for women and men from the general population. This reversal may be due to sex differences in emotionality and the ability to cope with emotional stress.
As noted, females have a superior emotional sensitivity and a superior capacity to process, respond to and express emotional and affective-laden information (e.g., Brody, 1985; Burton and Levy, 1989; Brody, 1985; Buck, 1977, 1984; Buck et al., 1974, 1982; Card et al., 1986; Eisenberg et al., 1989; Fuchs & Thelan, 1988; Gilbert, 1969; Hall, 1978; Harackiewicz, 1982; Kemper, 1978; Lewis, 1983, Oliver, Sargent, & Weaver, 1998; Rubin, 1983; Safer, 1981; Shennum and Bugental, 1982; Soloman and Ali, 1972; Strayer, 1980; Weinberg, et al., 1999). However, because they are more emotionally sensitive, females are more easily frightened (Cantor & Reilly, 1982; Sparks, 1986; Tamborini & Stiff, 2015), and become stressed (Compas, Howell, Phares, Williams, & Ledoux, 1989; Coons 1988; Cleary, 2015; Larson, & Ham,2013) and cry easier than males, (Lombardo, Crester, Lombardo & Mathis, 1983; Oliver, 2013; Shields, 2015; Thomas, 2013), sometimes crying and becoming upset even under conditions that seem affectively neutral (Oliver, Sargent & Weaver, 1998). They are also two to three times more likely than men to develop mood disorders such as depression (Nolen-Hoeksema, 1990) and stress related abnormalities such as anxiety and phobias (DSM-IV), and appear to become more depressed than males (Cleary, 2015; Wichstrom, 1999). According to Cleary (2015, pp. 41, 49), "virtually all surveys find that women report more symptoms of distress than men," and that "women are more likely than men to experience symptoms of depression and anxiety." Females who are sexually abused are also more likely to become upset, experience stress, and display disturbed behavior patterns than males on average (reviewed in Bauserman & Rind, 2009; West, 1998); which is not to imply that males are not negatively impacted, as that is obviously not the case (Bauserman & Rind, 2009; Joseph, 2000; West, 1998).
In part, these sex differences may be due to the sexual differentiation of the amygdala (Breedlove & Cooke, 1999; Bubenik & Brown, 2004; Filipek, et al., 1994; Nishizuka & Arai, 1981) as well as the hippocampus (Filipek, et al., 1994; Woolley, et al., 1990). The female amygdala although smaller than the male amygdala (Breedlove & Cooke, 1999; Filipek, et al., 1994), has more neurons, which are more densely packed--and smaller more densely packed neurons fire more frequently and easily. Hence, as the amygdala and hippocampus are sexually differentiated, and as the amygdala subserved emotionality and emotional memory, and the hippocampus memory (chapter 14) this may contribute to sex differences in emotionality, and emotional memory, and may contribute to the greater degree of emotionality which is characteristic of females in general (see chapter 13, 15, 25).
Hence, presumably, because of her greater emotional sensitivity (and the sexual differentiation of the limbic system), females in general may be more profoundly affected by traumatic stress. Thus they are more likely to demonstrate long term disturbances in affective and non-verbal intellectual and memory functioning (Joseph, 2000). Therefore, although males are more severely affected by fetal stress, or lack of emotional stimulation in early infancy (see chapter 28), females tend to be more profoundly affected by abnormalities in caretaker-infant interactions (Mayes & Carter, 1990; Stoller & Field, 1982) and emotional extremes including the stress and trauma of severe sexual, physical, or emotional abuse (chapters 28, 30). In consequence, those females with a childhood history of severe sexual, physical, and emotional abuse, develop an amnesia for childhood which is twice as lengthy as compared to adult females from the general population.
THE NEUROLOGY OF CHILDHOOD AMNESIA
The offset of childhood amnesia, among those from the general population, appears to be directly related to the maturation of the right hemisphere including hippocampal structures (hippocampus, amygdala) which accounts for the visual-pictorial nature of first recallable memories. The later development of the first verbal memories likely reflects the maturation of the language dominant left hemisphere and the left superior and inferior temporal lobe--structures which subserve verbal memory and recall (Squire, 2012; Wagner et al., 1998). Hence, loss of memory for earlier childhood experiences is probably a function of neocortical as well as hippocampal and amygdala immaturity and may also be directly related to the child's insufficient vocabulary and an inability to verbally code and later recall non-verbal memories.
As noted, however, although the brain of the fetus, infant and young child is decidedly immature, they can still learn and remember (DeCasper & Spence,1986; Hartshorn & Rovee-Collier, 2009; Perris et al., 1990; Pillemer & White, 1989; Rovee-Collier & Shyi, 2012). The question then becomes, why are these early memories forgotten? There are several possibilities, including corpus callosum immaturity, the loss of memory-laden neurons due to programmed cell death (Joseph, 1982, 1988a, 1999d, 2000; Joseph, Gallagher, Holloway & Kahn, 1984), "synaptic overlay" (Spear, 1979), and the propensity of adults and children to rely on different cognitive, nmemonic, and "coding" strategies to process, store, and gain access to information. For example, information may be stored in multiple interconnected neocortical regions in the adult brain, whereas the same data may be stored in only a single site within the still immature brain of the child; early isolated memories which may be shed due to programmed cell death.
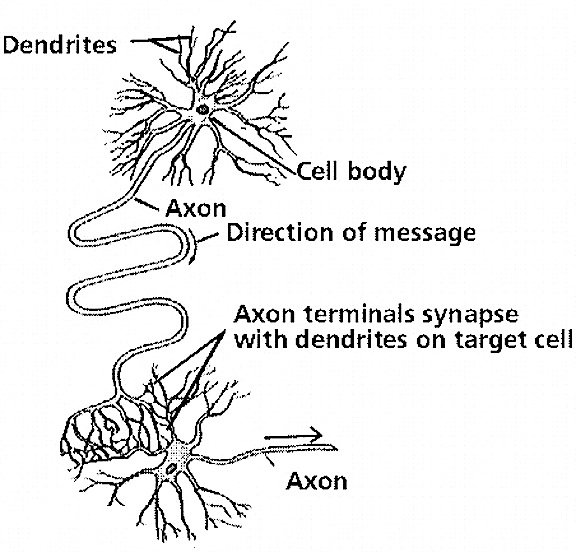
Dying neurons (left). Normal neurons (right)
PROGRAMMED CELL DEATH
As the brain matures, neurons not only continue to grow (and possibly divide) and establish new synaptic connections, but excess neurons and synaptic connections are also eliminated as initially brain cells and synapses are produced in excess and innumerable random interconnections are formed (Rakic, Bourgeois, Echenhoff, Zaecevic & Goldman-Rakic, 1986). In fact, neuronal density declines by up to 50% over the first decade (Blinkov & Glezer, 1968; Huttenlocher, 1990). Programmed cell death is an integral part of the maturational process and "fine tunes" the nervous system (Finlay & Slattery, 1983; Joseph, 1982; Oppenheim, 1981). It is through attrition, dendritic pruning, and the elimination of excessive neurons and random neural pathways, and the experience-expectant stimulation of those which are retained and stimulated to divide and grow, which fine tunes perception and selective attention, and which promotes learning, memory, cognition, personality, and emotional development. However, programmed cell death may also encompass the elimination of "excess" memory-laden neurons and synapses which may be shed by the hundreds of millions, thus erasing from the mind and brain associated early memories; a process which may contribute, over time, to an amnesia for early childhood experiences.
CHILDHOOD AMNESIA, EARLY MEMORY ISOLATION AND "SYNAPTIC OVERLAY"
Although random and insufficiently stimulated neurons, axons, dendrites and synapses are gradually eliminated, yet other are stimulated to grow, to myelinate, and to establish more elaborate and extensive interconnections. As various regions of the brain and neocortex mature, including the association and then the assimilation areas as well as the frontal and temporal lobe, the infant's and then the child's ability to process and retain information progressively improves. In addition, as unmyelinated axons become myelinated their ability to rapidly transmit complex stimuli significantly improves. Hence, over the course of early development, functional "islands" of semi-isolated brain tissues, become "peninsulas," and then great interconnected continents of cerebral tissue that are intimately "wired" together making instantaneous and complex forms of processing possible. For example, it is due to these multiple interconnected neocortical regions (e.g. neural networks), that if we visualize a "chair", name it, describe it, and can imagine a multitude of chairs differing in every possible dimension.
Likewise, over the course of development, islands of memories are formed which accounts for the ability of infants and young children to learn and remember. However, due to neocortical immaturity, these early memories are probably maintained by the brainstem and limbic structures, such as the still immature amygdala and hippocampus.
As the neocortex matures, however, instead of being incorporated, these early memories, including those established in the neocortex, may instead become increasingly isolated and "overlaid," due to in part to programmed cell death, and as a function of the establishment of new connections which may replace and/or overlay the old; i.e. synaptic overlay (for related discussion see Spear, 1979). These early memories then become progressively isolated as they are overlaid by new memories and the establishment of new neuronal circuits which are linked by new learning experiences which are immediately shared. That is, in the more mature and more fully "wired" brain, new memories have the advantage of being shared with vast regions of the brain. These new memories are not only shared, but in the process of being learned and formed, vast regions of the brain come to be synaptically linked together. By contrast, the earliest memories remain isolated.
In addition, because later memories have the advantage of being linked through numerous synaptic interconnections (and are also represented in multiple modalities), this increases the likelihood that many diffirent types of cues or probes might activate the memory by association. That is, in the more mature brain, a tactile or visual or auditory experience can processed in a multi-modal fashion, and can thus be stored in somesthetic memory and verbal memory and visual memory simultaneously. Moreover, the memories are easily accessed because tactile, verbal, or visual cues can trigger their recollection and as they are stored in multiple site throughout the brain. <
Infants and young children are not so well endowed. Early memories are more isolated and are not represented in multiple ways. Hence, first recallable memories tend to be overwhelmingly visual-pictorial, and only 9 months later (on average) are recallable multi-modal(verbal-visual-pictorial) memories formed.
Hence, childhood amnesia may be due to programmed cell death, the isolation and single modal processing and storage of early memories, and their eventual submersion due to "synaptic overlay," i.e. early memories become inaccessible because of new growth in the brain and synaptic morphological development (see Spear, 1979). Once an individual becomes an adult these early isolated memories are no longer accessible but come to be disconnected or increasingly isolated. Moreover, with new neural development, these early memories may also come to be grossly misinterpreted when passing through new brain regions which then attempt to process and translate them.
In this regard, the greater efficiency in adult retention may be directly related to the multiple ways in which different attributes of a memory may be stored by diverse brain regions. A single memory in the adult (vs the child) thus has multiple representations and can be triggered by a variety of cues. Younger organisms, having less developed brains, will store these experiences in fewer regions, such that later these memories are less easily activated. When coupled with synaptic overlay these early memories become increasingly isolated and/or they are shed during the course of programmed cell death.
THE "KEY" DOES NOT FIT THE LOCK: VERBAL CODES AND UNCONSCIOUS MEMORIES
As detailed above, left hemisphere immaturity and the child's limited vocabulary likely contributes to the difficulty adults experience when attempting to recall early verbal memories and verbally-related events. An insufficient vocabulary limits the ability to form verbal memories.
Conversely, the development of an extensive vocabulary, and thus the emergence of a wholly new means of coding and storing information may also contribute to early memory loss, as early memories are stored non-verbally. That is, adults are more likely to rely on language, due to their immense vocabulary whereas infants and children, being less endowed and thus less inclined to do so. In consequence, early experiences may be unrecallable for older vs younger individuals because infants use a different system of codes to store memories and are more likely to rely on emotions and feelings, whereas adults and older children use strategies, symbols and associations such as language not yet fully available to younger children (Brown 1975; Dollard & Miller, 1950; Freud 1900, 1916; Hagen et al. 1975; Joseph 1982, 1988a, 2012b; Piaget 1952, 1962, 1974; Pillemer & White, 1989; Shantz 1983; Vygotsky 1962). Much of what is experienced and committed to memory during early childhood takes place prior to the complete development of linguistic labeling ability and is based on a pre- or nonlinguistic code (Dollard & Miller 1950; Freud 1900, 1916; Joseph 1982, 1988a, 2012b; Pillemer & White, 1989; Shantz 1983).
Moreover, this transition and the change in coding, appears to occur around age 3, coinciding with the sudden spurt in language development. For example, it has been found that older children and adults are able to verbally recall a considerable amount of information about the recent birth of a sibling and surrounding events that occurred between the ages of three and four. However, if they are questioned about any siblings born before they were age 3, they had absolutely no verbal memory of the event (Sheingold & Tenney, 1982).
Therefore, since older individuals are presumably relying on more mature and sophisticated coding systems, such as language, they cannot find the right set of neural programs to open the door to early childhood memories (Dollard & Miller, 1950; Neisser, 1967; Schachtel, 1947; Underwood, 1969). The key does not fit the lock because the key has changed.
If this view is correct, then this implies that if provided with a suitable "key" these early memories may be unlocked and then remembered. However, insofar as the "key" remains verbal, then these early memories will remain hidden, "disguised" and seemingly forgotten. Of course, this also suggests that these early memories are hidden from consciousness and are thus unconscious, a theory popularized by Freud.
According to Freud (1899, 1905, 1916) it is the development of language which not only prevents access to, but which disguises all memories from the "earliest beginnings of childhood up to the sixth or eighth year." Moreover, according to Freud, because these early memories are not easily verbalized, they are subject to suggestibility and fantasy. The process of translating these non-verbal memories into language results in considerable distortion (Joseph 1982, 1988a). In fact, Freud (1916) argued that insofar as an adult is able to provide complex narratives of these early events, is proof that they are not the memories laid down by the child. The young child is too verbally immature and unsophisticated to create such complex memories. Hence, these recollected early memories are reconstructions and in this regard may be completely false, or serving as a screen (Freud, 1899) to block out of view the original, sometimes traumatic, memory, which, however, remains intact albeit safely locked away within the confines of the unconscious. The unconscious, therefore, is the depository even for those early memories which are positive and happy. However, due to the organization of the psyche, even positive early childhood memories remain hidden and locked away, that is, within the "unconscious."
As detailed in chapters 1, 2, 10, 11, although conscious-awareness can be considered a continuum, there are aspects of consciousness which rely on language--and which can be referred to as "linguistic consciousness"-- and there are aspects of consciousness which are visual, tactile, emotional, and so on. The language dependent aspects of consciousenss are also associated with the left hemisphere and are maintained and expressed by the interactions of Broca's and Wernicke's areas and the inferior parietal lobule, the "language axis." However, following severe traumatic stress or due to stroke, head injury, or other neurological disorders, consciousness can be fractured and the language axis may become disconnected from yet another region of the brain that remains intact and which subserves a different aspect of consciousness. That is, conscious awareness may be split and fragmented. However, the broken off portions of consciousness may retain the ability to engage in complex actions as well as store these experiences in memory (Bogen, 1969, 2013; Geschwind, 1965; Joseph, 1986b, 1988b; Sperry, 1966, 1982).
For example, if a patient suffers a discrete lesion in the left occipital-temporal visual associations areas, although vision is preserved and the patient can see (due to preservation of the primary visual cortex), they will be unable to verbally recognize, describe, or name objects that are shown to them, even if encouraged to guess or if provided multiple choices. This is because the language axis is disconnected from the visual association areas and cannot receive complex visual associations. It cannot name what it sees because it cannot match an auditory equivalent with the visual image due to disconnection. Instead, the language axis may confabulate and call a "glass of water" a "clock" or a "comb" a "harmonica" or "toothbrush," and so on, and they may fail to realize an error has been made (Freud, 1891; Geschwind, 1965). However, although unable to name or identify a glass of water, or explain its function or utility, once they became thirsty they might pick up the glass and drink from it (Geschwind 1965). Nevertheless if the lesion also disconnects the somesthetic areas from the language axis, they may remain unable to name the item even during the course of utilizing it (depending on the extent of disconnection); a condition referred to as "agnosia"--a term coined by Freud (1891). However, although unable to name, for example, a comb that had been placed in their hand, they can still demonstrate its use by combing their hair (Freud, 1891; Geschwind, 1965).
Thus, under these conditions, that aspect of the mind and personality that drinks from the glass or demonstrates the use of a comb, represents a broken off and disconnected fragment of the "mind" that is no longer attached to the dominant verbal stream of consciousness. However, this broken-off fragment of consciousness remains fully capable of acting independently and in a purposeful and intelligent manner. As stated by Geschwind (1965), under these conditions "we are dealing with more than one patient. The patient that speaks to you is not the patient who is perceiving--they are, in fact separate."
Similar disconnection syndromes also characterized the mental and neurological functioning of the infant and young child. As noted, due to neurological immaturity, certain areas of the brain initially function in semi-isolation. In fact, and as will be detailed below, due to neurological and corpus callosal immaturity (Yakovlev & Lecours, 1967), not just certain brain areas, but the right and left hemisphere of the child are unable to fully communicate (Deruelle & de Schonen, 1991; Galin et al., 1978; Joseph et al., 1984; O'Leary, 1980; Salamy, 1978), thererby giving rise to disconnection syndromes which are qualitatively similar to those of "split-brain" (corpus callosotomy) patients.
RIGHT AND LEFT HEMISPHERE UNILATERAL MEMORY STORAGE
Be it adult or child, information processed and memories formed by the right hemisphere cannot also be completely transferred to the left (Berlucchi & Rizzolatti, 1968; Hicks 1974; Joseph et al., 1984; Marzi, 1986; Merriam & Gardner, 2015; Miller, 1990, 1991; Myers, 1959, 1962; Rizzolatti et al. 1971; Taylor & Heilman 1980). When inter-hemisphere transfer fails, the left hemisphere may become verbally amnesia and remain verbally amnesic regarding these right hemisphere information sources (Bures & Buresova 1960; Doty & Overman 1977; Hasegawa, et al., 1998; Joseph, 1986b, 1988b; Joseph et al., 1984; Risse & Gazzaniga, 1979). This "psychic cleavage" is a consequence of the differential functional organization of the left vs right cerebral hemisphere and the fact that some information is processed in a wholly different fashion and cannot be recognized by one vs the other half of the brain which in turn precludes complete interhemispheric transfer (Berlucchi & Rizzolatti, 1968; Hicks 1974; Joseph, 1982, 1988a; Marzi, 1986; Merriam & Gardner, 2015; Miller, 1990, 1991; Myers, 1959, 1962; Rizzolatti et al. 1971; Taylor & Heilman 1980).
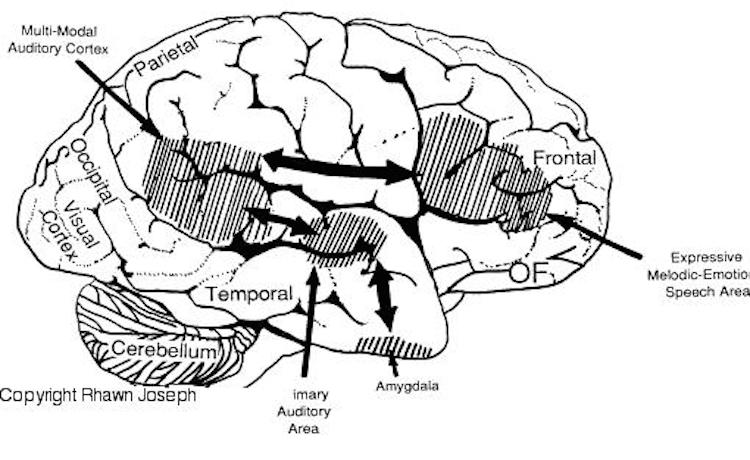
For example, the right hemisphere/temporal lobe is responsible for storing affective, social-emotional, melodic, facial, and personal information in long term memory (Cimino et al. 1991; Corkin 1965; Samson & Zatorre, 1988, 2012; Wechsler 2004; Weddell, 1989). Emotional and personal memories, including those with are negative, depressing and traumatic, are more likely to be stored within the right temporal lobe (Abrams & Taylor, 1979; Cimino, et al. 1991 Cohen, Penick & Tarter, 1974; Deglin & Nikolaenko, 1975; Shagass et al.,1979; Wexler, 2004), whereas ECT administered to the right vs left temporal lobe is more likely to alleviate depressive symptoms (Cohen, et al, 1974; Deglin & Nikolaenko, 1975).
By contrast, the left hemisphere/temporal lobe is specialized for storing words, sentences, conversations, verbal passages, written and reading material, and conversations in long term memory (Barr et al. 1990; Delaney, Rosen, Mattson & Novelly 1980; Kapur et al. 2012; Meyer & Yates,1955; Milner 1968; Milner & Teuber 1968; Samson & Zatorre, 2012; Wagner et al., 1998; Weingartner 1968).
Moreover, expressive, word-rich, grammatically complex speech, reading, writing, spelling, naming, mathematical and analytical reasoning, as well as the temporal-sequential and rhythmical aspects of consciousness, are associated with the functional integrity of the left half of the brain in the majority of the population (see chapters 2, 11). However, the left hemisphere has great difficulty correctly analyzing and recognizing social-emotional and contextual information and in fact is quite socially dense and concrete, lacking even a sense of humor (Brownell et al.1986; Cicone et al.1980; Foldi, et al. 1983; Gardner et al. 1975). The left hemisphere, in fact, tends to misperceive emotional nuances (Dimond & Farington, 1977; Dimond et al. 1976; Ostrove, Simpson, & Gardner, 1990).
The right cerebral hemisphere is specialized for perceiving, processing and expressing almost all aspects of emotionality as conveyed by the face, through body body, posture, and through melodic and affective attributes of speech including swearing and praying (Borod, 2012; Borod et al. 2012; Cancelliere & Kertesz, 1990; Heilman et al. 1985; Heilman & Bowers 2006; Joseph 1988a; Ross, 2013; Samson & Zatorre, 1988, 2012; Tucker & Frederick, 1989; Van Strien & Morpurgo, 2012). This emotional dominance extends to bilateral control over the autonomic nervous system, including heart rate, blood pressure regulation, galvanic skin conductance and the secretion of cortisol in emotionally upsetting or exciting situations (Rosen et al. 1982; Wittling, 1990; Wittling & Pfluger, 1990; Yamour et al. 1980; Zamarini et al. 1990). The right hemisphere is fully capable of determining and deducing not only what a persons feels about what he or she is saying, but why and in what context they are saying it --even in the absence of words, vocabulary and other denotative linguistic features (Blumstein & Cooper, 1974; DeUrso et al. 1986; Dwyer & Rinn 1981).
And not just adults, but infants between the ages of 10 weeks and 5 months are capable of appropriately discerning, discriminating and responding to social-emotional vocalizations conveying approval, disproval, happiness, and anger and can thus distinguish between different emotional vocalizations so as to determine the mood state and intentions of others (Fernald, 2013; Haviland & Lelwica, 2015)--nuances which are comprehended by the infant's right hemisphere and limbic system (Davidson & Fox, 1989; Joseph, 1982, 1988a, 2012a).
Infants are also exceedingly responsive to and interested in the human face (see chapter 25) and the right hemisphere is specialized not only for perceiving and recognizing faces, but perceiving facial emotion (Ley & Bryden 1979; Moreno, et al. 1990; Suberi & McKeever 1977), regardless of the emotion conveyed (Buchtel et al. 1978; Dekosky et al. 1980; Landis et al. 1979; Strauss & Moscovitch 1981; Suberi & McKeever, 1977). In fact, the the left side of the face has been found to be more emotionally expressive (Campbell 1978; Chaurasis & Goswami 1975; Moreno, Borod, Welkowitz, & Albert, 1990; Sackheim et al. 1978) and to be perceived as more intensely emotional as well (Borod & Caron 1980; Sackheim & Gur 1978).
Nevertheless, although specialized and functionally lateralized, mental unity and the continuum of conscious-awareness is mantained in the adult brain through the interactions of the frontal lobes, and via the corpus callosum, and other subcortical and intra-cortical pathways. The same is not true, however, regarding the brain and the mental functioning of the child.
CORPUS CALLOSUM IMMATURITY AND UNILATERAL MEMORY STORAGE
As detailed above, and in chapters 23-26, the brain of the infant and child is exceedingly immature; neurons and synapses continue to grow and drop out, such that initially, large expanses of cortex are not functionally developed and their synaptic, dendritic, and axonal interconnections have yet to form and/or become myelinated. Moreover, the thick axonal pathways which links the right and left hemisphere, the corpus callosum, takes over 10 years to fully myelinate (Yakovlev & Lecours, 1967), and due to corpus callosum immaturity, information transfer between the hemisphere and the exchange of tactual, auditory, and visual information is limited and incomplete (Deruelle & Schonen, 1991; Finlayson 1975; Gallagher & Joseph 1982; Galin et al. 1977, 1979; Joseph & Gallagher, 1985; Joseph et al. 1984; Kraft et al. 1980; O'Leary, 1980; Ramaekers & Njiokiktjien, 1991; Salamy, 1978). Even when some of this information is transferred to the left half of the brain, much of it is distorted and/or immediately forgotten.
For example, when complex pictures were selectively transmitted by tachistoscope to the brains of four years olds, they were able to accurately describe what had been viewed by the left hemisphere. By contrast, verbal descriptions of pictures shown to the right cerebrum were sometimes fragmented, filled with information gaps, and contaminated by confabulatory embellishments (Joseph et al. 1984). Similar gaps and recall fragmentation occur when children are asked to describe affective laden television shows (Collins 1970, 1978; Collins et al. 1978; Hayes & Casey 2012), and confabulatory tendencies are observed in a variety of situations involving memory reproduction in children (Hudson 1990; Nelson & Gruendel 1986).
Similarly, adults who have undergone complete or partial surgical sectioning of the corpus callosum (corpus callosotomy), respond with gaps and confabulatory embellishments when asked to verbally describe pictures shown only to the non-verbal half of their brain (Joseph 1986ab;1988b) as do children as old as 7 (Joseph et al. 1984).
However, in contrast to those with "split-brains," verbal, visual, and tactual memory for information transferred from the right to the left hemisphere progressively improves with age (Finlayson 1975; Galin et al. 1977, 1979; Joseph et al. 1984; O'Leary 1980; Ramaekers & Njiokiktjien, 1991; Salamy 1978) and the likelihood of confabulating or presenting with information or memory gaps correspondingly decreases (Joseph et al. 1984). Hence, initially it appears that infants and young children function as if their right and left cerebral hemispheres were in part disconnected and are unable to fully share certain types of information. In addition, the left, language dependent regions of the cerebrum respond as if it immediately forgets those details transmitted to it by the right hemisphere.
This lack of verbal memory or consciousness for right cerebral perceptions and memory in children has also been demonstrated in regard to complex cognitive processes involving spatial reasoning, non-verbal knowledge and logic (Gallagher & Joseph 1982; Joseph & Gallagher 1985; Kraft et al. 1980; Ramaekers & Njiokiktjien, 1991). For example, as has been demonstrated by Piaget (1952, 1962, 1974) and others, conservation of space, length and volume appears to require the ability to perform spatial analysis, internal spatial reversals, and the integration of an objects identity in separate and changing spatial contexts so that relational constancies may be ascertained (Gallagher & Joseph 1982; Joseph & Gallagher 1985). Young children supposedly lack these capabilities. Hence, if water is poured from one of two similarly sized and equally filled glasses into a tall and thin glass, the child will verbally state that the thin glass has more because the water volume is higher. They will do this even if a little water is first removed before it is transferred to the taller, thinner glass.
However, these abilities appear to be dependent on the intact right cerebral hemisphere (Kraft et al. 1980), which is non-verbal. Because of callosum immaturity, it is thus not surprising that they fail to demonstrate a verbal understanding of these spatial relationships. The standard test design requires verbal responses so that conservation can be demonstrated. However, if the conservation problem and question (e.g. "which has more?") it is altered so that emotional and motivational judgment are called for ("If you were thirsty and this was your favorite drink, which would you want?"), and the same paradigm is employed where a little water is first removed, the majority of supposedly non-conserving children will subsequently demonstrate a tacit understanding of these principles and will show conservation, even when verbally they continue to make the same error. In other words, when asked to make a judgment based on their feelings and emotional desires, young children were suddenly able to demonstrate knowledge of information that is not available to the speaking half of the brain (Gallagher & Joseph 1982; Joseph & Gallagher 1985). They relied on their feelings to guide their responses, rather than employing language to guess at information which is otherwise not available to the left hemisphere. As such, they pointed to the correct glass indicating that they knew which had more, despite the illusion of appearances (see also Wheldall & Poborca, 1980).
INFORMATION TRANSFER DEFICITS IN "SPLIT-BRAIN PATIENTS
Similar modes of emotionally guided information transfer has been demonstrated in individuals who have undergone complete corpus callosotomy (e.g. Sergent, 1990; Sperry et al. 1979). Corpus callosotomy involves severing the callosal fibers which in turn disconnects the two cerebral hemispheres. When the right hemisphere is shown pictures or words the left cannot verbally describe what has been viewed. However, if emotionally significant, limbic pathways (or any remaining callosal fibers) appear to allow for emotional transfer. For example, when pictures of family members, or even that of Adolf Hitler were selectively and secretly transmitted to the right half of the brain (via tachistoscope), the disconnected left half of their brain at first had no knowledge of what had been viewed, but was able to make progressively more accurate guesses (e.g. "G.I came to mind."). However, these guesses were reinforced by the emotional tone generated by the right half of the brain and cues provided by the experimenters. In these "split-brain" patients, the left half of their brains soon gained access to the images still stored within the right hemisphere and eventually came up with the correct response when provided cues and other forms of reinforcement; "Hitler," (Sperry et al. 1979).
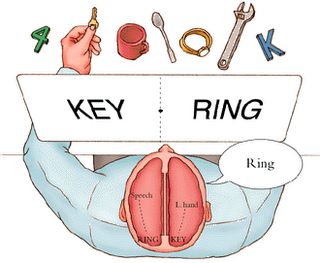
[-INSERT FIGURE 4 ABOUT HERE-]
Unfortunately, what is not originally shared, usually remains unshared even when information transfer is later greatly improved or as the commissures and the brain develops (Bures & Buresova 1960; Doty & Overman 1977; Joseph 1982, 1988a, Risse & Gazzaniga 1979). In consequence, it sometimes happens that the right half of the brain can store, recall, and even act upon its own memories much to bewilderment of the left hemisphere which is sometimes unable to even recognize much less comprehend the well kept secrets of the opposite half of the brain.
As noted, in the intact, normal brain, even non-emotional memory traces appear to be stored unilaterally rather than laid down in both hemispheres (Bures & Buresova 1960; Doty & Overman 1977; Hasegawa, et al., 1998; Levy, 1974; Risse & Gazzaniga, 1979). Presumably, to gain access to these lateralized memories, one hemisphere (perhaps via the frontal lobes) has to activate the memory banks of the other brain half via the corpus callosum (Hasegawa, et al., 1998), or anterior commissure (which links the right and left amygdala and inferior temporal lobes). Hence, when one hemisphere is selectively trained this can facilitate learning in the other hemisphere when it is trained later--a facilitation which is lost if the corpus callosum is severed (Hasegawa et al., 1998; Joseph, 1988b).
However, when one hemisphere learns, has certain experiences, and/or stores information in memory, this information is not always available to the opposing hemisphere; one hemisphere cannot always gain access to memories stored in the other half of the brain (Bures & Buresova 1960; Doty & Overman 1977; Joseph, 1982, 1988ab; Levy, 1974; Risse & Gazzaniga 1979). These learning and memory transfer deficits includes even the learning of sequential and fine motor movements (Hicks 1974; Taylor & Heilman 1980). Moreover, if the corpus callosum is prevented from transferring this information, the other half of the cerebrum will be almost completely unaware of its presence. This has been demonstrated experimentally in primates and humans.
For example, in a series of experiments performed by Doty and Overman (1977) and Hasegawa et al., (1998) one hemisphere had been trained to perform a perceptual-motor task. Once it was learned both hemispheres were able to respond correctly due to transfer of learning via the corpus callosum. Following the corpus callosotomy, only the hemisphere that originally was trained was able to perform--i.e. could recall it. The untrained hemisphere acted as though it never had been exposed to the task; its ability to retrieve the original memories was now abolished (Doty & Overman 1977; Hasegawa et al., 1998) as it was now unable to gain access to them.
In a similar study, Risse and Gazzaniga (1979) injected sodium amytal into the left carotid arteries of human patients so as to anesthetize the left cerebral hemisphere. After the left cerebrum was inactivated, the awake right hemisphere, although unable to speak, was still able to follow and behaviorally respond to commands, e.g. palpating an object with the left hand, looking at pictures.
Once the left hemisphere had recovered from the drug, as determined by the return of speech and motor functioning, none of the eight patients studied were able to verbally recall what object had been palpated with the left hand, "even after considerable probing." Although encouraged to guess most patients refused to try and insisted that they did not remember anything. However, when offered multiple choices in full field, most patients immediately raised the left hand and pointed to the correct object! Once this occurred, the patients then immediately verbally claimed to have suddenly remembered what the right half of the brain had experienced. Hence, information confined to the right half of the brain was suddenly made available to that of the left when provided sufficient cues, whereas before it had remained a "well kept secret" (Risse & Gazzaniga 1979).
According to Risse and Gazzaniga (1979), although the memory of touching and palpating the object was not accessible to the verbal (left hemisphere) memory system, it was encoded in a nonverbal form within the right hemisphere. However, it remained unavailable to the left hemisphere when normal function returned. The left (speaking) hemisphere was unable to gain access to information and memories stored within the right half of the brain. Nevertheless, the right hemisphere not only remembered, but was able to act on its memories. Similarly, memory retention can also be demonstrated in neonates, infants and young children, albeit non-verbally, and older children who are explicitly unable to remember or verbally recall, for example, the faces of early childhood classmates, are able to demonstrate "unconscious" memory retention (see also Lie & Newcombe, 1999; Newcombe & Fox, 1994).
This indicates that when exchange and transfer is not possible, is in some manner inhibited, or if for any reason the two halves of the brain become functionally disconnected and are unable to share information, the possibility of information transfer at a later time is reduced (Bures & Buresova 1960; Doty & Overman 1977; Risse & Gazzaniga 1979) -even when the ability to transfer is acquired or restored. The information may be lost to the opposite half of the cerebrum, though recognition memory can be triggered if visual and other cues are provided (Joseph, 2012b; see also Lie & Newcombe, 1999; Newcombe & Fox, 1994).
Nevertheless, although seemingly lost, these memories and attached feelings can continue to influence whole brain functioning in subtle as well as in profound ways. The right hemisphere may experience and store certain information in memory and at a later time in response to certain situations act on those memories, much to the surprise, perplexity, or chagrin of the left half of the brain; one hemisphere cannot always gain access to memories stored in the other half of the brain.
As noted, because of corpus callosal immaturity, infants (Salamy, 1978) and young children (Galin et al., 1979; Joseph et al., 1984; O'Leary, 1980) demonstrate interhemispheric transfer deficits which are qualitatively similar to the transfer deficits of "split-brain" patients. This suggests, therefore, that at least some early memories may be stored in the right hemisphere, whereas the left is unable to gain access to them. However, as the corpus callosum matures, and interhemispheric transfer increases, the left hemisphere increasingly gains access to this material, and can verbally code and later recall it. Indeed, when considered as a group, the ability to recall early memories progressively increases as children age, and by age 7, information processing within and transfer between the right and left hemisphere improves markedly and associated memory transfer deficits diminish significantly (Joseph et al., 1984). Likewise, 98% of adults from the general population (Joseph, 2000) are able to remember events before age 7. Thus, the gradual offset of childhood amnesia parallels the increasing maturity of the corpus callosum (as well as the neocortex), and thus ability of the right and left hemisphere to intercommunicate and transfer memories. These findings, therefore, are supportive of the theory that at least some of those memories which are formed prior to the functional maturation of the corpus callosum may be stored unilaterally in the right hemisphere, such that the language dominant left hemisphere becomes verbally amnesic for early experiences. >
AMNESIA FOR CHILDHOOD DUE TO TRAUMATIC STRESS
It has been found, as reported by a number of independent investigators, that first recallable memories are visual-pictorial, non-verbal, and generally of positive emotional events. In a study of 100 adults from the general population, 75% of first recallable memories were visual-pictorial. As the non-motor regions of the right hemisphere begin to mature in advance of the left (Chi, et al., 1977; Gilles et al., 1983; Joseph, 1982; Scheibel, 2013; Thatcher, 2012), and as the right hemisphere is dominant for most aspects of emotion, including the formation and recall of emotional, visual, and personal memories (Cimino et al., 1991; Evans et al., 1995; Joseph, 1982, 1988a; Levy & Heller, 2012; Wechsler, 2004), it thus appears that matuation of this half of the brain plays a significant role in the offset of childhood amnesia.
In fact, when adults who have been traumatized recall traumatic imagery, the right frontal lobe displays increased activity and the left orbital displays reduced activity (Shin et al., 1999). Moreover, when adults with PTSD were asked to recall and imagine personal traumatic events or when they were shown pictures of combat-related photographs, there are significant blood flow increases in the right amygdala, anterior cingulate, anterior temporal lobe, and right frontal lobe, but reductions in the left inferior frontal lobe (Rauch et al., 2006; Shin et al., 2007). Similarly negative (as well as positive) stimuli can evoke increased right frontal activation (Teasdale, et al., 2009;s).
However, as the right hemisphere is also dominant for the stress response (Wittling, 1990; Wittling & Pfluger, 1990), as well as most aspects of emotion, this half of the brain is also more greatly effected by emotional and stress-related disturbances (Joseph, 1982, 2000). That is, severe abuse and emotional trauma experienced during early childhood more greatly effects and injures the right hemisphere (as well as the amygdala and hippocampus) and disrupts its ability to form first memories, thus leading to a more extensive period of childhood amnesia (Joseph, 20000).
As detailed in chapter 30, it is well established that stress can damage the brain including structures implicated in memory, e.g. the amygdala and hippocampus (Joseph, 2006, 1998, 1999a,b, Lupien & McEwen, 2009; Sapolsky, 2006). Moreover, hippocampal atrophy has been documented among adults sexually abused as children (Bremner et al., 1995; Stein et al., 1995), and adults stressed from front line combat (Bremner, Randall, & Scott, 1995; Gurvits, Shenton, Hikama, Ohta, Lasko, & Gilbterson, 2006). Right (and left) hippocampal reductions have also been demonstrated in deprived rats (Diamond, 1985, 1991). The non-human amygdala (Cain, 2012; Henriksen, Bloom, & McCoy, 1978; Kraemer, 2012), and the right amygdala in particular (Diamond, 1985, 1991) has also been shown to become reduced in size, and/or to develop abnormal activity in response to severe stress including deprived rearing conditions. Rats reared under deprived conditions also display significant disturbances in immediate and long term visual memory, and females are more severely affected (Joseph, 1979, Joseph & Gallagher, 1980) as is the case with women with a history of severe childhood abuse (Joseph, 2000).
Functional deficits associated with the right hemisphere and right amygdala and hippocampus have also been demonstrated as based on the performance of abused adults on neuropsychological tests. Specifically, adults with a childhood history of severe and repetitive instances of sexual, physical, and/or emotional abuse, demonstrated significant reduction in the performance IQ (PIQ) of the Wechsler Adult Intelligence Scale-revised as well as disturbances in short-term memory for visual-pictorial stimuli, including faces (Joseph, 2000). The PIQ is directly associated with the functional integrity of the right hemisphere (Chase, Fedio, Foster, Brooks, Di Chiro, & Mansi, 1984; Matarazzo, 1972), whereas visual-pictorial memory and memory for faces is directly associated with the functional integrity of the hippocampus and amygdala (Andersen, 1978; Evans et al., 1995; DeRenzi; 1986; Jacobson, 1986; Kimura, 1963; Tranel & Hyman, 1990); and adults with a history of abuse display significant deficits on these measures. Moreover, these results are consistent with the findings from numerous behavioral, neuropsychological, neurophysiological, and neuroanatomical studies which have demonstrated that stress can injure the brain, the hippocampus and amygdala in particular, and can thus result in significant disturbances of memory (chapter 30).
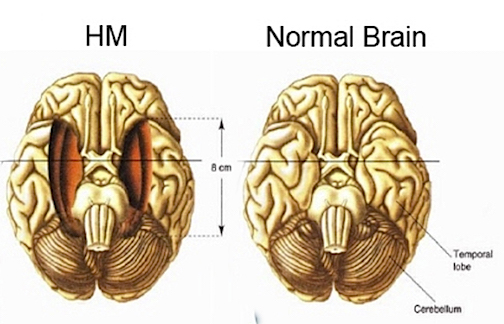
The attribution of these memory deficits to these limbic structures is not merely hypothetical for it has been repeatedly demonstrated that injuries to and abnormal activity involving the amygdala (Andersen, 20015; Chapman, 1958; Chapman, et al 2017; Jasper & Rasmussen, 1958; Jacobson, 1986; Tranel & Hyman, 1990) or the hippocampus (Brazier, 2006; Chapman et al., 1967; Eichenbaum et al., 1994; Frisk & Milner, 1990; Milner, 1970; Reed & Squire, 2007), directly disrupts different aspects of long term memory retention for visual-pictorial, verbal, and emotional stimuli. Consider, for example, the classic case of H.M., who underwent bilateral hippocampal and amygdala removal, and who demonstrates a profound loss of memory even for emotional events that occurred years before the surgery such as the death of his favorite uncle (Milner, 1970). Brenda Milner has worked with H.M. for over 25 years, yet she is an utter stranger to him.
As detailed in chapter 30, it has also been repeatedly experimentally demonstrated that highly arousing conditions, or repetitive and prolonged episodes of extreme stress, can injure the hippocampus and amygdala which may suffer profound dendritic death and atrophy, or develop seizure-like activity (Cain, 2012; Gahwiler 1983; Goelet & Kandel, 1986; Henriksen, et al 2018; Kraemer, 2012; Lupien & McEwen, 2009; Sapolsky, 2016; Uno et al., 1989). Moreover, it has been demonstrated that severe stress, including the secretion of stress-hormones and neurotransmitters such as cortisol, significantly impacts and disrupts learning and memory (Diamond et al., 2012, 1994; Dubrovsky, Liquornik, Noble, & Gijsbers, 2007; Kovacs, et al 2016; Lupien & McEwen, 2009; Shors et al., 1989; Spoont, 2012; Warembourg, 1975), including memory retrieval (de Quevain, et al, 2018) recognition memory (Wolkowitz, et al 20100; Wolkowitz, Weignartner, Rubinow, et al. 2013) and the ability to discriminate between relevant and irrelevant stimuli (Lupien & McEwen, 2009).
Presumably, because repeated instances of severe sexual, physical, and/or emotional abuse, directly impacts the right hemisphere and amygdala and hippocampus, adults who characterized their childhoods as "miserable," "nightmarish" and so on not only suffer form non-verbal memory and intellectual deficits, but display a period of amnesia for childhood which is twice that of adults from the general population, i.e., 3.5 vs 61. (Joseph, 2000). In fact, 11.5% of those with a history of severe and chronic abuse claim no memory before age 10, which is in contrast to the general population in which only 2% claimed no memories from before this age. Although 7.2% of those reporting abuse have first memories that were formed by age 2, 13% of those from the general population claim to recall memories from this age or before. Hence, although there are exceptions, clearly, those who claim to have suffered severe and repeated instances of childhood sexual, physical, and emotional abuse and horrible childhoods demonstrate, on average, a much lengthier amnesia for childhood which in some cases may encompass the entire first decade.
However, although in some or perhaps most cases, these early traumatic memories have probably been erased and forgotten, in some instances, there is evidence to suggest that at least some of these trauma-memories may remain "well kept secrets of the right hemisphere."
REPRESSED MEMORIES AND HEMISPHERIC LATERALITY
On average, the first verbal-visual memories appear around age 4.4 (Joseph, 1999, 2003). This is the age at which verbal capabilities begin to rapidly develop and is a stage of life marked by an emotional-verbal transition in psychological and cognitive functioning. Hence, adults and older chlidren can verbally recall some early memories, but not many, and those that occurred prior to the onset of verbal language development, and those which are stored non-verbally, and which may have never been transferred to the left hemisphere due to corpus callosum immaturity, appear to be wholly forgotten. However, adults with a history of severe abuse form their first recallable memories at a significantly later age, 6.1, which raises the possibility that some of these seemingly forgotten early trauma-memories may also be stored non-verbally, possibly as "well kept secrets" of the right half of the brain. Hence, regardless of if one has a history of abuse, at least some early memories may be stored non-verbally, in the right hemisphere, but cannot be accessed due to corpus callosum immaturity and the inability of the left hemisphere to gain verbal access to them. If that is indeed the case, these early memories could be described as "repressed."
As noted, it is the development of the language which Freud (1916) believed acted in part to disguise early memories. Freud (1905) made a distinction, however, between childhood amnesia, which he believed hid the "earliest beginnings of childhood up to the sixth or eighth year," and repression. Amnesia for early childhood experiences was a passive force, he argued, whereas repression is active and prevents conscious realization of unpleasant and emotionally traumatic experiences which are sometimes of a sexual or threatening nature. Indeed, Freud (1896) at one time argued that repression and female hysteria, was a consequence of having been sexually molested during childhood. Although he later downplayed the idea as a product of female fantasy (Freud, 1909) he continued to note that incestuous relations were quite common as well as subject to repression (Freud, 1916, 1931, 1937).
Freud (1899), also argued that those early events which are subject to emotional disorganization and not easily verbalized, may also come to be subject to repressive forces and that suggestibility and fantasy may play a role in their disguise, misinterpetation, and distortion. The process of translating these non-verbal memories into language results in considerable distortion.
Thus, Freud (1916) argued that insofar as an adult is able to provide complex narratives of these early events, is proof that they are not the memories laid down by the child. The young child is too verbally immature and unsophisticated to create such complex memories. Hence, these recollected early memories are reconstructions and in this regard may be completely false, or serving as a screen (Freud, 1899) to block out of view the original, sometimes traumatic, memory.
Of course, one might ask, "why should one aspect of the psyche attempt to hide information so as to deceive yet a different region of the mind?" As noted, it is the functional specialization of the right and left hemisphere which appears to be responsible for this psychic cleavage. However, as to forgotten trauma memories, this information is probably hidden for the purposes of protection, so as to not disrupt the psychological and cognitive functioning of that aspect of the mind responsible for language and rational, verbal thought, the left cerebral hemisphere.
Although in part this "psychic cleavage" is a consequence of the differential functional organization of the left vs right cerebral hemisphere (which is more concerned with visual, tactual and social-emotional processing) and the fact that some information is processed in a wholly different fashion and cannot be recognized by one vs the other half of the brain which in turn precludes complete interhemispheric transfer (Berlucchi & Rizzolatti, 1968; Hicks 1974; Joseph, 1982, 1988a; Marzi, 1986; Merriam & Gardner, 2015; Miller, 1990, 1991; Myers, 1959, 1962; Rizzolatti et al. 1971; Taylor & Heilman 1980), traumatic memories are also formed and possibly maintained by the limbic system. As detailed in chapter 30, trauma and repetitive stress not only activates various limbic nuclei (and the right hemisphere), it may damage them, particularly the hippocampus (see below), thus resulting in subsequent hippocampal retrieval failure and thus a verbal and hippocampal amnesia. Damage and atrophy, however, is usually secondary to exceedingly high levels or prolonged and or repetitive instances of traumatic stress. Likewise, numerous investigators have reported that it is repetitive, painful, life threatening and traumatic sexual and physical memories which are the most resistant to immediate verbal recollection in both children and adults (Briere & Conte, in press, cited by Batterman-Faunce & Goodman, 2013; Courtois, 1988; Christianson & Nilsson, 1989; Donaldson & Gardner, 1985; Fisher, 1982; Fredrickson, 2012; Grinker & Spiegel, 1945; Herman & Schatzow, 2015; Kuehn, 2004; Parson, 1988; Southard, 1919; Summit, 1983; Williams, 2012).
The right hemisphere (and limbic system) is directly implicated in stress-induced loss or repression of trauma-memories, as this half of the brain not only stores affective information in longer term memory, but it becomes differentially and highly active in response to sexual, physical, emotional stress. By contrast, the left hemisphere may remain at a lower level of activation and/or it may even become functionally suppressed, thus resulting in transfer failure and left hemisphere verbal amnesia.
For example, the right hemisphere becomes highly aroused when emotionally aroused, or the body is physically or painfully stimulated or engaged in sexual activity, and is responsible for the maintenance of the somesthetic body image (Cohen, Rosen, & Goldstein, 2006; Cubelli et al. 2004; Desmedt, 2007; Freemon & Nevis, 1969; Haslam, 1970; Murray & Hagan, 2004; Joseph, 1988a; Penfield & Rasmussen, 1950, p. 27; Remillard, et al. 1983; Ruff, 1980; Spencer et al. 1983; York, et al., 1979). When this occurs, there sometimes results a disconnection syndrome such that the left hemishere, being at a lower level of arousal, is unable to fully participate or monitor the events being processed and learned by the right hemisphere (Chapter 10). In consequence, the individual may appear to be verbally amnesic as associated memories cannot be verbally accessed. Instead they appear to be repressed. AMNESIA AND UNCONSCIOUS MEMORIES
It has been repeatedly demonstrated in split-brain patients, that although the left hemisphere may be denied access to the memories and information maintained in the right half of the brain, and thus appears to be amnesic, the right hemisphere can also act on those memories, sometimes to the bewilderment and even embarrassment of the left hemisphere. The same principle appears to hold true for some individuals who become verbally amnesic following a severe emotional trauma--which selectively activates the right half of the brain and which is likely stored in the right half of the brain which can then act on its memories.
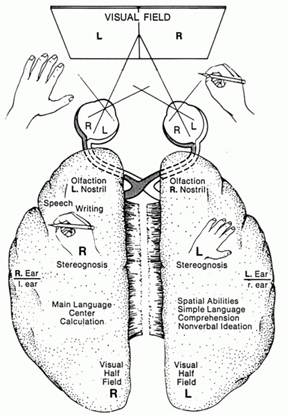
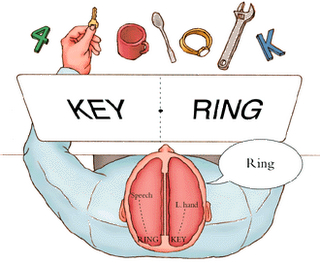
Consider for example, a child who has witnessed a murder (Bergen, 1958) or who has been sexually molested. Although she may never talk about it, and may deny it if asked, she may incoporate the traumatic event into her play (Bergen 1958), or go to school and pull up her dress, pull down her panties, play with the genitals of her friends, or allow them to play with hers or act out sexually with dolls, stuffed, animals, or their friends, or even adults in a highly aggressive fashion (Conte & Schuerman, 2015; Joseph, 2012b; Pynoos & Eth, 1985; Terr, 1988, 1990). In other words, she acts out the memories and experiences selectively stored within and recalled by the right half of her brain. Because her right hemisphere cannot talk about what has happened, it acts it out instead. Sometimes, however, these traumas are played out emotionally within the privacy of an individuals own head (Joseph 2012b). There have been numerous examples of individuals who although suffering from verbal amnesia are nevertheless able to demonstrate the possession of knowledge and information which they in fact denied possessing (Claparede, 1911; Crovitz, et al. 1981; Meudell & Mayes, 1981; Schacter, Harbluk & McLachlan, 1984), including details from a short story read to them previously as well as names and addresses or little known facts provided during their amnesic period. Similarly individuals suffering from profound amnesia are able to demonstrate recognition memory involving identification of visual objects and "picture puzzles," such that recognition time is reduced by over 100% on day 2 vs day one of training, although they deny having seen the material before (Crovitz, et al. 1981; Meudell & Mayes, 1981). This has been referred to this as "source amnesia." Nevertheless, because of findings such as these, some have argued that amnesia is a reversible, task dependent retrieval deficit (Warrington & Weiskrantz, 1970; Weiskrantz, 1978).
UNCONSCIOUS MEMORIES, AMNESIA AND TRANCE STATES
As detailed in chapters 2 and 30, those who are severely traumatized may enter trance-like states, dissociate and experience an out-of-body hallucination, and then suffer memory loss. Likewise, individuals who have been hypnotized may dissociate such that a second "hidden observer" is created Moreover, post hypnotic amnesia is often a hall mark of the hypnotic experience. According to Kihlstrom and Evans (1979; p. 179-180) "The experience is ... similar to the difficulty that people generally have in remembering their dreams. In some respects, post-hypnotic amnesia also parallels the 'state-dependent' learning. And hystorically posthypnotic amnesia has been viewed as similar to the memory disturbances observed in clinical cases of hysteria, fugue, and multiple personality. In all these instances, there is a compelling disturbance of memory that particularly affects personal experiences and material with a strong autobiographical content..."
However, hypnotized subjects with loss of memory will also experience memory recovery. Moreover, like those who have been traumatized and who experience an initial loss of memory followed by memory recovery, their recollections tend to be disorganized and overwhelmingly random. However, if the experimental provides the subject with a prearranged cue, the critical memories will appear to "flood back" into memory (Kihlstrom & Evans, 1979).
Post hypnotic amnesia is also similar to "source amnesia." Although specific memories appear to be maintained non-verbally and unconsciously, they can nevertheless effect conscious behavior. For example, if provided with specific historical or geographic information while hypnotized, and if asked relevant questions following the ending of the session, subjects will readily supply the answer (that they had been provided while hypnotized) but will have no idea how they obtained the information. According to Evans (1979), approximately 11% of subjects who are hypnotized demonstrate source amnesia, whereas about 2% will demonstrate both source and recall amnesia.
These and related findings indicate that knowledge and information can be present and influence behavior although the individual verbally denies knowledge of its presence, for this is exactly what occurs with amnesiacs (see Cermak, Lewis, Butters & Goodglass, 2004; Corkin, 1965; Milner, Corkin, & Teuber, 1968). For example, in one study an amnesic patient was told some very unusual stories about various pictures. When he was later shown the same pictures, he claimed to have no recollection of having seen them or hearing the story. However, when asked to pick a title for each picture, he picked those which mirrored the theme that he had been told (Schacter & Moscovitch, 1984).
In another instances, an amnesic patient was introduced to and shook hands with a a doctor who had hidden a pin in his hand, sticking them in the process. Later, when the doctor had left and then returned, although the patient claimed to have no memory of having met them before, they refused to shake hands. When asked why, the patient stated that sometimes people hid pins in their hands (Claparede, 1911).
Hence, although unable to verbally report these memories and actively denying having had certain experiences, this information can nevertheless be attended to, stored, and demonstrated even when the patient has no verbal recollection of having heard or seen it before. In this regard, even in amnesia secondary to obvious brain damage, it appears that loss of memory may not due to a storage deficit per se, but to a failure or inability to gain access to these memories secondary to disconnection. That is, the individual is unable to gain verbal access to the memory, due in part, to a disconnection and access failure secondary to brain damage (see chapters 2 and 30).
REFERENCES
















