Rhawn Gabriel Joseph, Ph.D.
Brain Research Laboratory
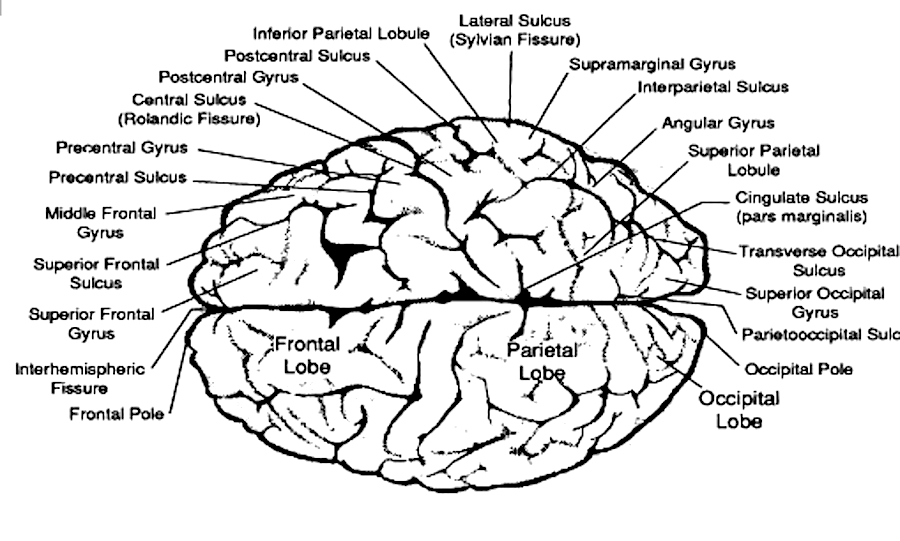
The frontal lobes are the Senior Executive of the brain and personality, and which participates in all aspects of conscious cognition including memory. Indeed, the frontal lobes receives input from the primary, secondary, association and assimilation areas, as well as from the limbic system, striatum, and brainstem
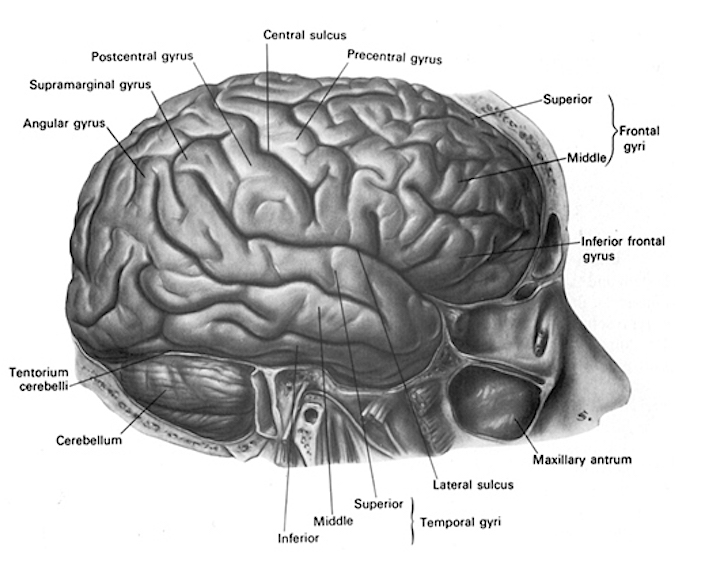
The motor area then relays this information to the lateral convexity which simultaneously receives fiber projections from the sensory association areas (Cavada, 2004; Jones et al. 1978; Jones & Powell, 1970; Pandya & Kuypers, 1969) and the inferior parietal lobule. Hence, the frontal cortex and right and left lateral convexity are "interlocked" with the posterior sensory areas via converging and reciprocal connections with the first, second, and third level of modality specific analysis, including the multimodal associational integration performed by the inferior parietal lobule. The frontal lobes, therefore able to sample activity within all cortical sensory/association regions at all levels of information analysis.
FRONTAL-THALAMIC CONTROL OVER NEOCORTICAL ACTIVITY
The role of the lateral convexity is not limited to sampling, but also involves regulation of information flow to and within the neocortex. This is accomplished, in part, via projections linking the frontal lobes with the dorsal medial thalamic nucleus--a structure which participates in the transfer of information to the neocortex and which display neuroplasticity (Jones & Pons, 2015).
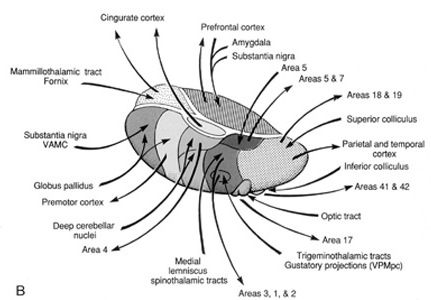
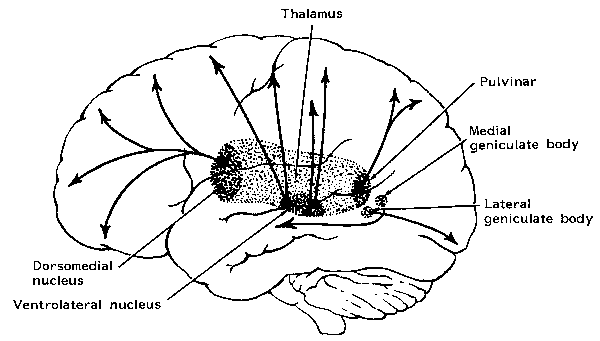
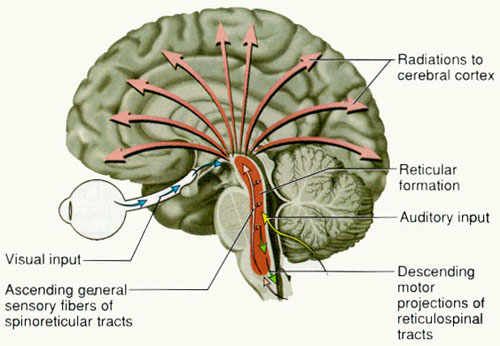
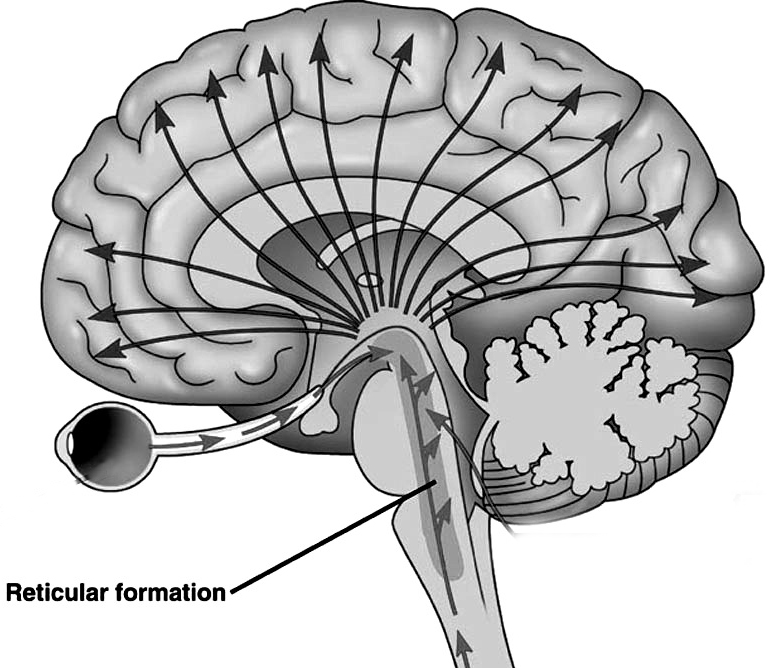
The reticular thalamus is controlled by the lateral convexity of the frontal lobes, and the lateral portion of the dorsal medial thalamus with which it maintains dense interconnections (Skinner & Yingling, 1977; Yingling & Skinner, 1977). The convexity and lateral dorsal medial nucleus (LDM) are also richly interconnected and together exert significant steering influences on the reticular thalamus. That is, the lateral frontal convexity appears to exert specific influences on the LDM so as to promote or diminish the flow of information to the cortex and thus modulate specific perceptual and cognitive activities occuring within the neocortex --activity which it is simultaneously sampling. This is in contrast to the orbital region with its connections to the reticular formation and the medial magnocellular segment of the dorsal medial thalamus, and its influences on generalized arousal and limbic activation/inhibition.
To recapitulate, the lateral frontal system is able to influence cognitive/perceptual cortical functioning via the sampling of activity occurring throughout the neocortex at all levels of informational analysis, and via its modulating influences on the lateral portion of the dorsal medial and reticular thalamic nuclei. The lateral frontal region is thus able to act at any stage of processing, from initial reception to motor expression so as to facilitate or inhibit further analysis, selectively acting to determine exactly what type of processing occurs throughout the neocortex.
Via integration and inhibitory action and through its neocortical and thalamic links the lateral convexity it is able to coordinate interactions between various regions of the neuroaxis so as to organize, mobilize, and direct overall cortical and behavioral activity and to minimize conflicting demands, impulses, distractions and/or the processing of irrelevant information.
Hence, in summary, the oribtal region exerts modulating influences on subcortical and geralized limbic arousal. By contrast, the lateral convexity of the frontal lobes are "interlocked" with the the sensory receiving areas (Cavada 2004; Fuster 2013; Jones et al. 1978; Jones and Powell 1970; Joseph, 2015a; Pandya and Kuypers 1969) and maintains rich interconnections with the reticular and dorsal medial nucleus of the thalamus (Skinner and Yingling 1977; Yingling and Skinner 1977) which relays sensory impressions to the neocortex.
The lateral frontal lobes, therefore are able to sample perceptual input as it is received in the thalamus, and thus prior to and after it has been transferred to the neocortical receiving areas. Through its interconnections with the primary and association areas, the frontal lobes can also censor, inhibit, and thus control the processing of this data, and in this manner can control attention as well as facilitate or inhibit further analysis and thus information processing throughout the neocortex. These frontal capabilities include information storage and retrieval at the neocortical level; i.e. memory (Brewer et al., 2005; Carpenter et al., 2016; Hasegawa et al., 2012; Koechlin et al., 2013; Wagner et al., 2015).
In consequence, when the lateral regions are injured, selective attention and memory functioning may become impaired such that individuals may experience sensory-perceptual overload, become distractible, disinhibited, confused, and have difficulty keeping their mind on a certain tasks and/or recalling and acting upon events planned for the future. Similar disturbances can result when the dorsal medial nucleus or the bi-directional pathways linking the thalamus and frontal lobe are severed (Graff-Radford et al.1990; Skinner and Yingling 1977; Victor et al. 2005). However, the nature of these disturbances depends on if the right vs left frontal lobe has been negatively impacted.
THE RIGHT FRONTAL LOBE AND THE REGULATION OF AROUSAL
The right cerebral hemisphere is clearly dominant in regard to the mediation and control over most aspects of social-emotional functioning (chapter 10). There is also a variety of findings which strongly suggest that the so called alerting/arousal vigilance network is localized to the right frontal lobe (Posner & Raichle, 2011), and that the right frontal lobe exerts bilateral influences on arousal (DeRenzi & Faglioni, 1965; Heilman & Van Den Abell, 1979, 1980; Joseph, 1982, 1986a, 1988a, 2015a; Konishi, et al., 2015; Tucker, 2011).
The right hemisphere also becomes desynchronized (aroused) following left or right sided stimulation (indicating it is bilaterally responsive), whereas the left brain is activated only with unilateral (right side) stimulation (Heilman & Van Dell Abell, 1980). In fact, the right frontal lobe and hemisphere responds more quickly even to stimuli appearing on the right side (Dimond 1976 1979; Heilman and Van Den Abell 1979, 1980; Jeeves and Dixon 1970). In consequence, if the right frontal lobe (or hemisphere) is injured, the left frontal lobe is unable to attend to events occurring on the left side of the body, which results in unilateral left-side neglect.
The right frontal lobe is also larger than the left suggesting a greater degree of interconnections with other brain tissue, and it appears to exert bilateral inhibitory influences on attention and arousal (Cabeza and Nyberg 2013; DeRenzi and Faglioni 1965; Dimond 1976 1979; Heilman and Van Den Abell 1979, 1980; Jeeves and Dixon 1970; Joseph 1986a, 1988ab; Konishi et al., 2015; Pardo et al. 1991; Tucker 1981). For example, as based on functional imaging, it has been found that when performing a "go/no-go task" and the Wisconsin Card Sort--tasks requiring the inhibition of irrelevant or erroneous responses, activity significantly increases in the right inferior frontal lobe, and that this was the case irrespective of if the human subjects used the right or left hand (Konishi et al., 2015). By contrast, the left frontal region appears to exert unilateral excitatory influences which promotes right sided motor control and speech expression.
However, because the right frontal lobe appears to exert bilateral inhibitory influences, whereas the influences of the left are unilateral and excitatory, when the left frontal region is damaged, the right may act unopposed and there may be excessive left cerebral inhibition or reduced activity (e.g., Bench et al., 1995); for example, as manifested by speech arrest, depression, and/or apathy.
Nevertheless, because left cerebral excitatory influences are predominantly unilateral, with massive right cerebral damage, although the left hemisphere is aroused, the left is unable to activate the right half of the brain. This may result in unilateral inattention and neglect of the left half of the body and space (Heilman & Valenstein, 1972; Joseph, 1986a, 1988a; Na et al., 2015). That is, the patient's (undamaged) left hemisphere may ignore his of her left arm or leg, and if their neglected extremities are shown to them, may claim they belong to the doctor or a person in the next room. That such disturbances occur only rarely with left frontal or left hemisphere damage further suggests that the right hemisphere is able to continue to monitor events occurring on either side of the body. Thus although the damaged left hemisphere is hypoaroused (or inhibited by the right), there is little or no neglect.
As detailed below, mania, confabulation, hypersexuality, tangentiality, and impulsive, labile, disinhibited and inappropriate social and emotional behaviors are predominately associated with right frontal dysfunction (Bogousslavsky et al. 2018; Clark and Davison 2003; Cohen and Niska 1980; Cummings and Mendez 2004; Fischer et al. 1995; Forrest 1982; Girgis 1971; Jack et al. 1983; Jamieson and Wells 1979; Joseph 1986a, 1988a, 2015a; Kapur and Coughlan 1980; Lishman 1973; Miller et al. 1986; Oppler 1950; Rosenbaum and Berry 1975; Shapiro et al.1981; Starkstein et al. 2003; Stern and Dancy 1942; Stuss and Benson 1986); a function of loss of inhibitory control as well as its role in all aspects of emotion. In fact, and as originally pointed out by Lisman (1968, 1973), injuries to the right frontal lobe are clearly associated with what has been described as the "frontal lobe personality," including, in the extreme, the development of a full blown manic psychosis coupled with disinhibited sexuality.
DISINHIBITED SEXUALITY
In some cases following frontal lobe damage patients may engage in inappropriate sexual activity (Benson and Geschwind 1971; Brutkowski 2005; Freeman and Watts 1942, 1943; Girgis 1971; Leutmezer et al., 2015; Lishman 1973; Miller et al. 1986; Strom-Olsen 1946; Stuss and Benson 1986). One patient, after a right frontal injury began patronizing up to 4 prostitutes a day, whereas his premorbid sexual activity had been limited to Tuesday evenings with his wife of 20 years (Joseph 1988a). Another patient with a right frontal stroke propositioned nurses and would spontaneously reach out and fondle large breasted women (Joseph 1988a).
It is not unusual for a hypersexual, disinhibited frontal lobe injured individual to employ force. One individual who was described as quite gentle and sensitive prior to his injury, subsequently raped and brutalized several women. Similar behavior has been described following lobotomy. As stated by Freeman and Watts (1943, p. 805): "Sometimes the wife has to put up with some exaggerated attention on the part of her husband, even at inconvenient times and under circumstances which she may find embarrassing. Refusal, however, has led to one savage beating that we know of, and to an additional separation or two" (p. 805).
Curiously, in these situations Freeman and Watts (1943, p. 805) have suggested that "spirited physical self-defense is probably the best strategy of the woman. Her husband may have regressed to the cave-man level, and she owes it to him to be responsive at the cave-women level. It may not be agreeable at first, but she will soon find it exhilarating if unconventional."
By contrast, a young woman I examined with right frontal-temporal seizures would spread her legs and engage in pelvic thrusting, coupled with grunting, lip licking and tongue protrusion. Currier et al., (1971), have also reported pelvic thrusting and moaning, and sex appropriate vocalizations, with temporal lobe seizures.
However, according to Leutmezer et al., (2015) and as based on prolonged scalp-EEG monitoring, sexual automatisms, such as "sexual hypermotoric pelvic or truncal movements are common in frontal lobe seizures," whereas "discrete genital automatisms, like fondling and grabbing the genitals are more common in seizures involving the temporal lobe." Presumably, the results of Leutmezer et al., (2015) differ from that of Currier, et al., (1971), Joseph (1988a, 2015a), Spencer et al., (1983), and Williams et al., (2001), due to their use of scalp rather depth electrodes which are more sensitive and exacting. On the other hand, temporal lobe/hippocampal sclerosis and atrophy were also documented in the Leutmezer et al. (2015) study.
Nevertheless, given the close functional association between the frontal and temporal lobes, and the fact that even a frontal seizure can propagate to the amygdala and thus involve the temporal lobe, perhaps the dysfunctional differences in abnormal sexual behavior are due to seizure origin and the subsequent spread of seizure activity.
Indeed, insofar as the behavior involves fondling and grabbing, the hands are being employed, and the hands are generally represented along the medial walls, within the SMA, cingulate, and along the lateral surface of the frontal lobe (see below), whereas truncal movements are more the province of the striatum with which the amygdala is intimately interconnected. Indeed, even with complete destruction of the anterior temporal lobe human and non-human primates may fondle their genitals and masturbate--a common components of the Kluver-Bucy syndrome (see chapter 13).
However, as the amygdala has been removed bilaterally, then this part of the brain cannot be directing hand-movements toward the genitals, which thus implicates the frontal lobes. Although this issue cannot be resolved here, it is nevertheless rather obvious than frontal-temporal seizures can produce abnormal and/or inappropriate sexual behavior.
There is also some evidence for functional laterality in regard to sexual automatisms and abnormal sexual behavior. In most instances, "sexual" seizures are associated with right frontal seizure foci (Joseph 1988a; Spencer et al. 1983). However, patients may also become hyposexual (Greenblatt 1950; Miller et al. 1986), especially with left frontal injuries, and/or experience genital pain with left temporal seizures (Leutmezer et al., 2015).
MANIA
When the right orbital and/or right lateral convexity are damaged, behavior often becomes inappropriate, labile, and disinhibited. Individuals may become hyperactive, distractible, hypersexual, tangential, delusional, and confabulatory (Bogousslavsky et al. 1988; Clark and Davison 2003; Cohen and Niska 1980; Cummings and Mendez 2004; Joseph,1986a, 1988a, 2015a; Lishman 1973; Robinson and Downhill 1995; Starkstein et al. 2003). Although laughing and joking one moment, these same patients can quickly become irritated, angered, enraged, destructive, or conversely tearful and depressed with slight provocation. That is, the patient may present with manic-depressive symptoms, with mania predominating. Hence, manic-depression (bipolar affective disturbances) may be due to waxing and waning abnormalities involving the right and left frontal lobes.
Mania and manic-like features have been reported in many patients with injuries, tumors, and even seizures involving predominantly the frontal lobe and/or the right hemisphere (Bogousslavsky et al. 1988; Clark and Davison 2003; Cohen and Niska 1980; Cummings and Mendez 2004; Forrest 1982; Girgis 1971; Jack et al. 1983; Jamieson and Wells 1979; Joseph 1986a, 1988a, 2015a; Lishman 1973; Miller et al. 1986; Oppler 1950; Robinson and Downhill 1995; Rosenbaum and Berry 1975; Starkstein et al. 2003; Stern and Dancy 1942).
One frontal patient described as formerly very stable, and a happily married family man, became excessively talkative, restless, grossly disinhibited, sexually preoccupied, extravagantly spent money and recklessly purchased a business which soon went bankrupt (Lishman 1973).
In another case, a 46-year old woman was admitted to the hospital and observed to be careless about her person and room, and incontinent of urine and feces. She slept very little and acted in a hypersexual manner. Her symptoms had developed several months earlier when she began accusing a neighbor of taking things she had misplaced. She also would confront him and strip off her clothes. She began going about in just a slip and bra, and informed people she was descended from queens, was fabulously wealthy, and that many men wanted to divorce their wives and marry her. During her hospitalization she was frequently quite loud, disoriented to time and place, and extremely tangential, jumping from subject to subject. After several years she died and a meningioma involving the orbital surface of the right frontal lobes was discovered (Girgis 2011).
One formerly very conservative engineer with over 20 patents to his name suffered a right frontal injury when he fell from a ladder. He became sexually indiscriminate and reportedly patronized up to 3 prostitutes a day, whereas before his injury his sexual activity was limited to once weekly with his wife. He also spent money lavishly, suffered delusions of grandeur, camped out at Disney Land and attempted to convince personnel to fund his ideas for a theme park on top of a mountain, and at night had dreams where the Kennedy's would appear and offer him advice --and he was a republican!
EMOTIONAL & PROSODIC SPEECH
Right frontal injuries are thus associated with disinhibited states, including manic neuropsychosis coupled with disturbances of thought and speech. Although language per se, is not aphasic, speech may be rushed, contaminated with unusual, tangential, and delusional ideas, and in some cases, melodic control over speech appears to be lost, such that the patient's melody of voice may not correspond with or parallel what is being said; e.g. they may be saying one thing, but by their labile tone of voice, seem to be meaning something else altogether.
Although language is usually discussed in regard to grammar and vocabulary, it is also emotional, melodic and prosodic --features which enable a speaker to convey and a listener determine, intent, attitude, feeling and meaning (chapters 10, 15). A listener comprehends not only what is said, but how it is said--what a speaker feels.
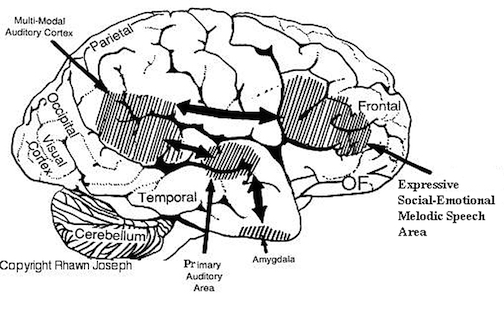
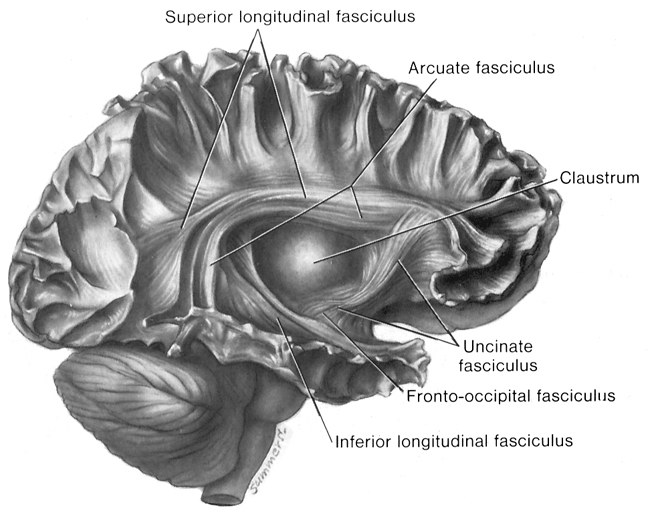
Patients with severe forms of Broca's expressive aphasia are unable to discourse fluently. However, they may be capable of swearing, making statements of self-pity, praying, singing, and even learning new songs (Gardner 1975; Goldstein 1942; Gorelick and Ross 2003; Joseph 1988a; Ross 1981; Smith 1966; Smith and Burklund 1966; Yamadori, Osumi, Mashuara, and Okuto, 1977)--although in the absence of music they would be unable to say the very words they had just sung. This is because the ability to produce non-linguistic and musical/emotional sounds is mediated by the undamaged right frontal lobe and limbic nuclei (Gardner 1975; Gorelick and Ross 2003; Joseph 1988a; Ross 1993; Shapiro and Danly 2001). Indeed, just as the left frontal convexity (i.e. Broca's area) subserves the syntactical, temporal-sequential, motoric, and grammatical aspects of linguistic expression (Foerster 1936; Fox 1995; Goodglass & Kaplan, 2000; LeBlanc 2012; Petersen et al. 2008, 2014; Sarno, 2015), there is a homologous region within the right frontal area which mediates the expression of emotional and melodic speech (Gorelick and Ross 2003; Joseph 1982, 1988a, 2015a; Ross 1981, 1993; Shapiro and Danly 2001).
With massive damage involving the right frontal melodic-emotional speech area, speech may become flat and monotonous, or conversely, the ability to alter or convey melodic and prosodic elements may become exceedingly abnormal and distorted. With extensive injuries to the right frontal lobe, patients may lose control over their voice and at times may sound as if they are crying, wailing, or screeching. Such patients may loose the ability to engage in vocal mimicry or to accurately repeat various statements in an emotional manner (Gorelick and Ross 2003; Joseph 1988a; Ross 1981, 1993).
With mild damage, rather than severe distortions or a loss of melody, the intonational qualities of the voice can become mildly abnormal and patients may seem to be speaking with an odd midwestern-like accent--particularly with deep lesions of the right frontal area, perhaps involving the cingulate or basal ganglia. Prosodic distortion in the form of an unusual accent is sometimes seen in seizure disorders involving deep right frontal or frontal-temporal areas.
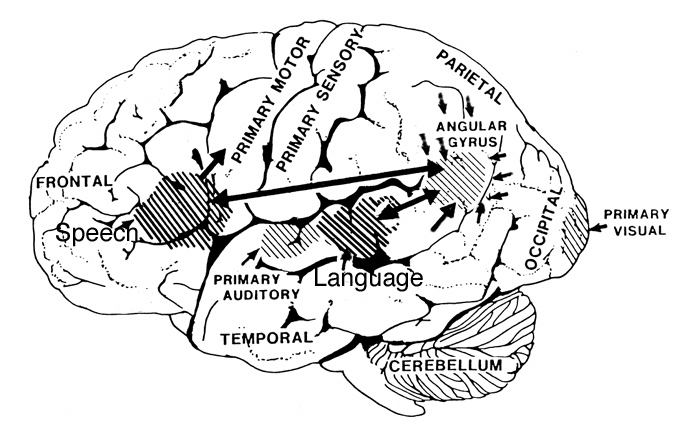
When damage is limited to this right frontal emotional-motor speech area, the ability to comprehend and understand prosodic-emotional nuances appears to be somewhat intact (Gorelick & Ross, 2003; Joseph, 1988a; Ross, 1993). However, with right temporal injuries, the ability to comprehend these nuances may be lost (Ross, 1993).
It is interesting to note, however, that despite the clinical, neuropsychological, and neuroanatomical evidence indicating that the right frontal and right temporal areas contribute to the production of emotional prosodic melodic speech, that functional imaging and blood flow studies have failed to display significant activity in these regions during language-related activities (Frost, et al., 2015; Pujol, et al., 2015)--a function perhaps, of the insensitivity of the tasks and/or measures employed in this regard.
TANGENTIAL, PRESSURED SPEECH & CIRCUMLOCUTORY SPEECH
Patients suffering from mania often display pressure, tangential and delusional speech. Likewise, with bilateral or right frontal lobe damage, speech may become pressured and tangential such that the patient rapidly diverges to other and unrelated topics (Joseph, 1986a, 1988a, 2015a). For example, when a patient with severe right orbital damage was asked if his injury affected his thinking, he replied, "yeah--it's affected the way I think--It's affected my senses--the only thing I can taste are sugar and salt--I can't detect a pungent odor--ha ha--to tell you the truth it's a blessing this way" (Blumer & Benson, 1975, p.197).
One frontal patient when asked what he received for Christmas replied, "I got a record player and a sweater." (Looking down at his boots) "I also like boots, westerns, popcorn, peanuts and pretzels." Another right frontal patient when asked in what manner an orange and a banana were alike replied, "fruit. Fruitcakes--ha ha--tooty fruity." When asked how a lion and a dog were alike he responded, "They both like fruit--ha ha. No. That's not right. They like trees--fruit trees. Lions climb trees and dogs chase cats up trees, and they both have a bark."
Tangentiality is in some manner related to impulsiveness as well as circumlocution. In contrast, patients with circumlocutous speech often have disturbances involving the left cerebral hemisphere and frequently suffer from word finding difficulty and sometimes receptive or expressive dysphasia. They experience difficulty expressing a particular idea or describing some need as they have trouble finding the correct words. Thus talk around the central point and only through successive approximations are able to convey what they mean to say.
Patients with tangential speech lose the point altogether. Instead, words or statements trigger other words or statements which are related only in regard to sound (e.g. like a clang association) or some obscure and ever shifting semantic category. Speech may be rushed or pressured and the patient may seem to be free associating as they jump from topic to topic.
Hence, in contrast to the aphasia, or speech arrest associated with left frontal injuries, right frontal lesions may result in speech release ("motor mouth"). Speech becomes disinhibited, pressure, and contaminated with tangential associations, and the patient may seem to be free associating as they jump from topic to topic. In the extreme speech becomes filled with confabulatory ideas.
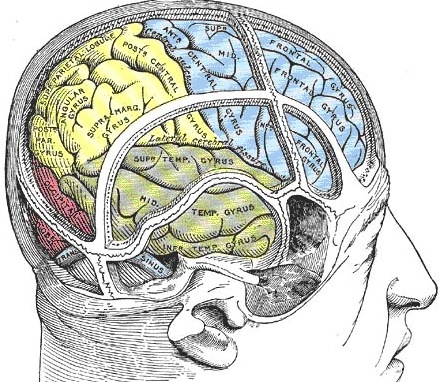
When secondary to right (or bilateral) frontal damage, speech may become exceedingly bizarre, delusional, and fantastical, as loosely associated ideas become organized and anchored around fragments of current experience.
For example, one patient, when asked as to why he had been hospitalized, denied that there was anything wrong, but instead claimed he was there to do some work. When asked what kind of work, he pointed to the air conditioning unit, and stated: "I'm a repair man. I'm here to fix the air conditioner. Now, if you'd please excuse me. I've got work to do."
A 24 year old store clerk who received a gunshot wound (during the course of a robbery) which resulted in destruction of the right inferior convexity and orbital areas, attributed his hospitalization to a plot by the government to steal his inventions (Joseph 1986a). He claimed he was a famous inventor, had earned millions of dollars and had even been on TV. When it was pointed out that he had undergone surgery for removal of bone fragments and the bullet, he pointed to his head and replied, "that's how they are stealing my ideas."
Another patient, formerly a janitor, who suffered a large right frontal subdural hematoma (which required evacuation) soon began claiming to be the owner of the business where he formerly worked (Joseph 1988a). He also alternatively claimed to be a congressman and fabulously wealthy. When asked about his work as a janitor he reported that as a congressman he had been working under cover for the C.I.A. Interestingly, this patient, also stated he realized what he was saying was probably not true. "And yet I feel it and believe it though I know it's not right."
Frontal lobe confabulation seems to be due to disinhibition, difficulties monitoring responses, withholding answers, utilizing external or internal cues to make corrections, accessing appropriate memories, maintaining a coherent line of reasoning, or suppressing the flow of tangential and circumstantial ideas (Fischer et al. 1995; Joseph 1986a,1988a, 2015a; Johnson, O'Connor and Cantor 2013; Kapur and Coughlan 1980; Shapiro et al.1981; Stuss et al. 1978; Stuss and Benson 1986). That is, since the right frontal lobe can no longer regulate information processing and the flow of perceptual and ideational activity, information that is normally filtered out and suppressed is instead expressed. In consequence, the Language Axis of the left hemisphere becomes overwhelmed and flooded by irrelevant, bizarre associations, leading sometimes to the expression of false memories, which the patient (that is, Broca's area) repeats (Joseph, 1982, 1986ab, 1988ab).
As noted, in some respects injuries involving the orbital frontal lobes can result in symptoms similar to those with right frontal injuries, including the production of confabulatory ideation. However, in contrast to right (or bilateral) frontal injuries which may result in the production of fantastical spontaneous confabulations where contradictory facts are ignored or simply incorporated, confabulatory responses associated with orbital injuries tend to be more restricted, transitory, and in some cases must be provoked (Fischer et al. 2005).