Rhawn Gabriel Joseph, Ph.D.
BrainMind.com
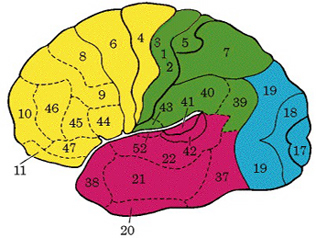
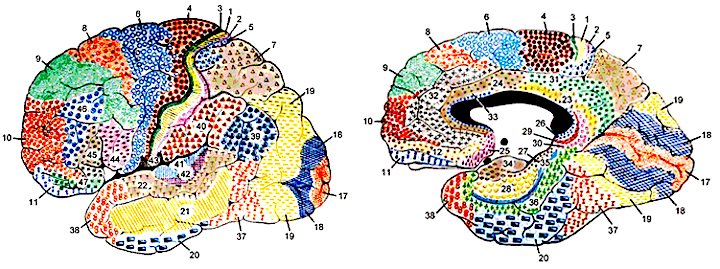
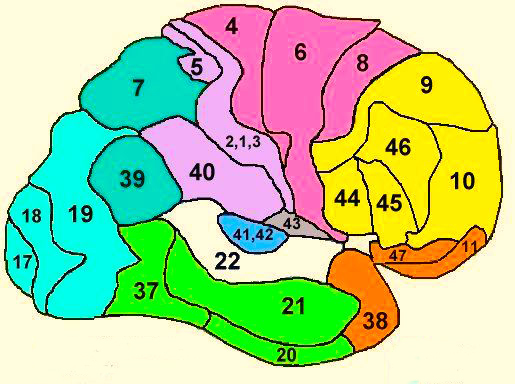
PARIETAL TOPOGRAPHY
There are nine major somesthetic areas within the parietal lobe, such that the primary, association, and assimilation areas actually consist of numerous subareas. Broadly, and most generally, however, the parietal lobe may be subdivided into a primary receiving area (involving Brodmann's areas 3ab,1,2) within the post central gyrus, an immediately adjacent somesthetic association area (Brodmann's area 5ab), a polymodal (visual, motor, somesthetic) receiving area located in the superior-posterior parietal lobule (area 7ab), a granular insular area which is located in the inferior convexity and encompasses part of the marginal gyrus, and a multimodal-assimilation area within the inferior parietal lobule (areas 7, 39, 40) which encompasses the angular and supramarginal gyrus.

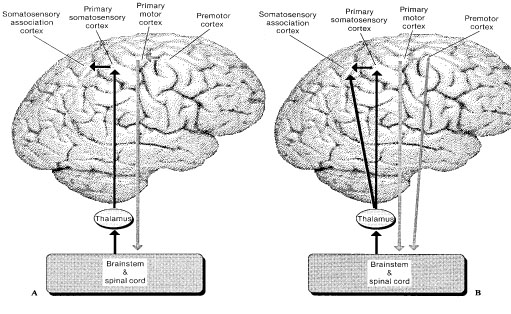
The primary somesthetic as well as portions of the association area contribute almost one third of the fibers which make up the cortical-spinal (pyramidal) tract. Hence, this region is very involved in motor functioning; e.g., the sensory and postural guidance of movement, including hand movements and the direction of gaze (Cohen et al. 2014; Dong et al. 2014; Snyder et al., 1998). Moreover, the primary motor and somesthetic association regions are richly interconnected (Jones & Powell, 1970) with the primary areas transmitting to the association areas which in turn project to the motor cortex. Indeed, in order to make motoric responses with some precision, there must be tremendous sensory feedback concerning proprioception, including data regarding the positions of the various joints and tendons, etc. --information which is provided by the somesthetic cortices (Cohen et al. 2014; Dong et al. 2014; Lebedev et al. 2014; Pred'Homme & Kalaska 2014; Snyder, et al., 1998). Together, the motor and somesthetic areas comprise a single functional unit which some have referred to as the sensorimotor cortex (Luria, 1980).
THE PRIMARY SOMESTHETIC RECEIVING AREAS
The primary somesthetic areas consists of three narrow strips of tissue (areas 3ab, 1, 2) which differ histologically, in architectural composition, and in sensory input. Moreover, each of these areas maintains a complete and independent representation of the body (Kaas, 2014).
Specifically, area 3a receives input from the muscles spindles (group IA muscle afferents) and can also signal muscle length (e.g. flexion or extension) whereas areas 3b receives cutaneous stimuli. Hence, area 3 appears to maintain cutaneous and muscular maps of the body. However, almost all of the cells in area 3ab receive input only from the contralateral half of the body. Hence, only half the body is represented.
These two maps, however, are also semi-independent, and in some respects they do not directly correspond to the location of body parts along the body surface, but instead are organized in regard to those parts which most frequently interact. That is, certain body parts more greatly represented in accordance with their sensory importance. However, in area 3b, for example, the hands, fingers, and jaw and mouth are juxtaposed--a function of the interaction of the hand and mouth when eating food (Kaas, 2014). In this way, an individual can coordinate their hand movements toward their mouth.
Information received and processed in area 3 is relayed to the immediately adjacent areas 1, and 2; each of which also contain a specialized spatial map of the body (Kaas, 2014; Kaas et al. 1981; Lin et al. 2014; Sur et al. 1982). For example, area 1 appears to maintain an overlapping cutaneous-joint body map (Evarts, 1969; Mountcastle & Powell, 1959; Schwartz et al. 1973). Area 2 maintains a map of the joint receptors and can signal the position and posture of the limbs based on input from the muscle spindles. Hence, the somesthetic cortex maintains four independent maps of the body.
Moreover, within this tiny expanse of tissue there is a sequential hierarchical convergence of input from areas 3, to 1, onto area 2. That is, information is analyzed and then passed from area 3 to 1, and from 3 and 1 onto area 2, as well as from area 3 to area 2. Therefore, a single neuron in area 2 receives multiple input from several cells in area 1 and 3, as well as vestibular input.
This organization is evident anatomically and as based on single cell recording and evoked potentials. For example, evoked potentials appear in area 1 about 5 msec, after they appear in area 3b. However, the degree of activation is also dependent on the attentional state and degree of arousal. With minimal attention to the source of input, there is minimal activation, which is why sensations from the clothing, shoes, or while sitting, lying down, and so on, can rapidly fade from consciousness.
Together these four strips of tissue comprise an interactional functional unit and are responsive to touch, texture, shape, motion, and the direction of stimulus movement, including temporal-sequential patterning, and can directly monitor the position and movement of the extremities (Cohen et al. 2014; Lebedev et al. 2014; Levitt & Levitt, 1968; Lin et al. 2014; Pred'Homme & Kalaska 2014; Mountcastle, 1957; Warren et al. 2006ab; Whitsel et al. 1972). Many cells are also responsive to changes in temperature as well as the presence of noxious stimuli applied to the skin.
Because the majority of these neurons receive input concerning pressure, light touch, vibration, the movement of joints, and muscular activity (Cohen et al. 2014; Lebedev et al. 2014; Levitt & Levitt, 1968; Prud'Homme & Kalaska 2014; Mountcastle, 1957) they can signal and determine whatever posture or position the body is in as well as the amount of force or pressure being exerted by the limbs (Jennings et al. 1983), i.e. if carrying or lifting some object. Conversely, via the reception and analysis of this input an individual can detect an insect crawling up or down their leg, the direction it is moving, as well as determine the position of their arms and legs without looking at them.
Nevertheless, predominantly elementary and simple contralateral somesthetic information is processed in this region (Lin et al. 2014; Prud'Homme & Kalaska 2014). Electrical stimulation of the primary somesthetic area gives rise to simple, albeit well localized sensations on the opposite half of the body (Penfield & Boldrey, 1937; Penfield & Jasper, 1954; Penfield & Rasmuseen, 1950) such as numbness, pressure, tingling, itching, tickling and warmth.
There are nine major somesthetic areas within the parietal lobe, such that the primary, association, and assimilation areas actually consist of numerous subareas. Broadly, and most generally, however, the parietal lobe may be subdivided into a primary receiving area (involving Brodmann's areas 3ab,1,2) within the post central gyrus, an immediately adjacent somesthetic association area (Brodmann's area 5ab), a polymodal (visual, motor, somesthetic) receiving area located in the superior-posterior parietal lobule (area 7ab), a granular insular area which is located in the inferior convexity and encompasses part of the marginal gyrus, and a multimodal-assimilation area within the inferior parietal lobule (areas 7, 39, 40) which encompasses the angular and supramarginal gyrus.
The primary somesthetic as well as portions of the association area contribute almost one third of the fibers which make up the cortical-spinal (pyramidal) tract. Hence, this region is very involved in motor functioning; e.g., the sensory and postural guidance of movement, including hand movements and the direction of gaze (Cohen et al. 2014; Dong et al. 2014; Snyder et al., 208). Moreover, the primary motor and somesthetic association regions are richly interconnected (Jones & Powell, 1970) with the primary areas transmitting to the association areas which in turn project to the motor cortex. Indeed, in order to make motoric responses with some precision, there must be tremendous sensory feedback concerning proprioception, including data regarding the positions of the various joints and tendons, etc. --information which is provided by the somesthetic cortices (Cohen et al. 2014; Dong et al. 2014; Lebedev et al. 2014; Pred'Homme & Kalaska 2014; Snyder, et al., 208). Together, the motor and somesthetic areas comprise a single functional unit which some have referred to as the sensorimotor cortex (Luria, 1980).
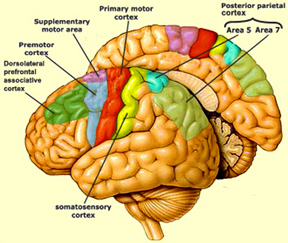
BODY IMAGE REPRESENTATION
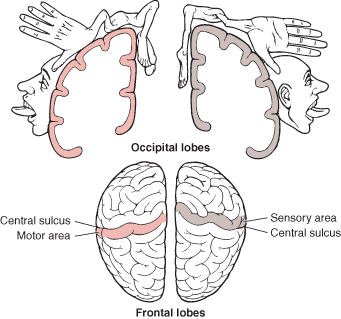
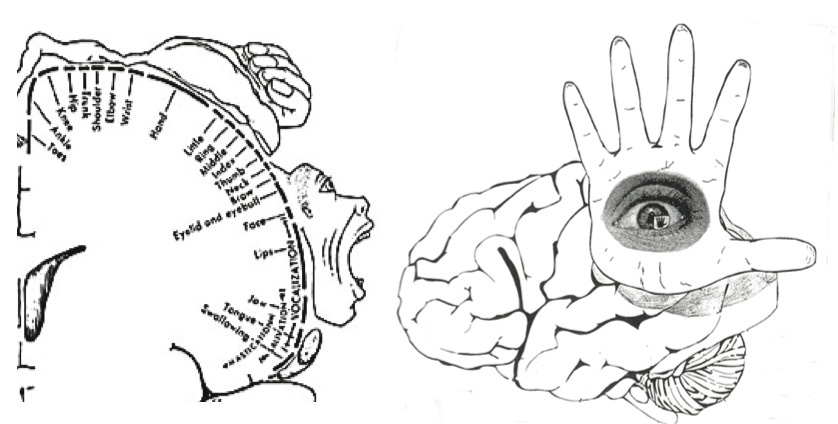
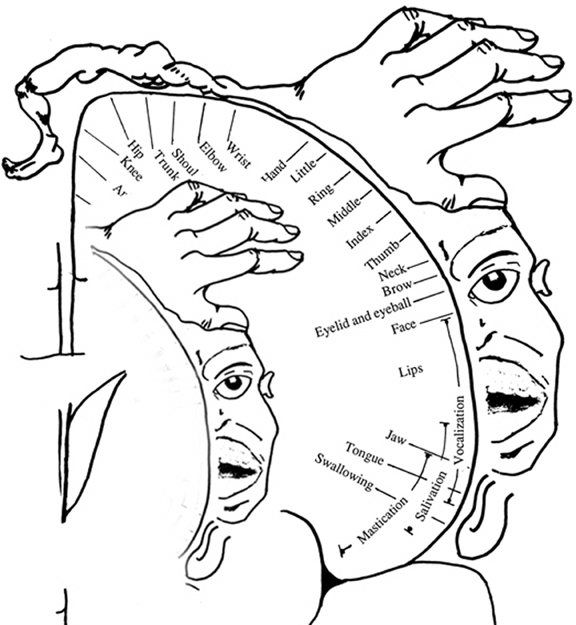
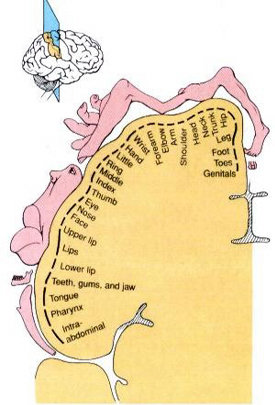
The primary receiving areas for somesthesis continues up and over the top of the hemisphere and along the medial wall where the lower half of the body is represented. Specifically the rectum, genitals, foot and calf are located along the medial wall, the leg along the superior surface of the hemisphere, and the shoulder, arm, hand and then face along the lateral convexity (Penfield & Boldrey, 1937; Penfield & Jasper, 1954; Penfield & Rasmussen, 1950).
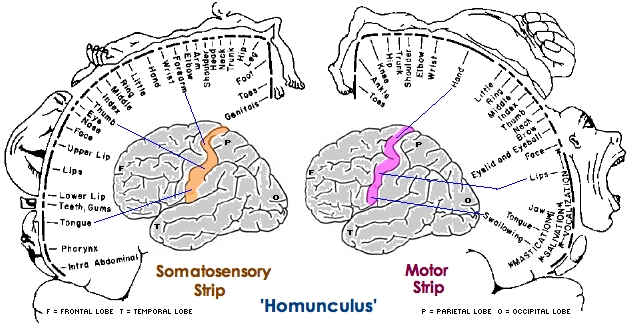
Body parts are also represented in terms of their sensory importance, i.e. how richly the skin is innervated. For example, more cortical space is devoted to the representation of the mouth, fingers and the hand than to the elbow or trunk (Warren et al. 2006). In fact, the area devoted to representation of the fingers is 100 times larger than the area devoted to the trunk. Because of this the cortical body map is very distorted. However, as noted, some areas are also juxtaposed, such as the hand and mouth area.
In summary, the primary receiving area receives very precise information regarding events occurring anywhere along the internal/external body and responds to converging inputs from muscle spindles, cutaneous and joint receptors, as well as proprioceptive and vestibular stimuli. In this manner, not only the body but the global properties of objects held in the hand can be determined (Iwamura & Tanaka, 1978); i.e. stereognosis.
THE BODY IN SPACE
Signals from joint and cutaneous receptors are transmitted to association neurons (Skata & Iwanura 1978). Many associaton cells also receive converging input from primary neurons concerned with different body parts (Dong et al. 2014; Sakata, et al. 2013), and can combine these signals with visual information (Snyder et al., 208) and are thus able to determine positional interrelationships.
For example, a single association neuron may receive information regarding the elbow and the shoulder, and become activated only when these two body parts are simultaneously stimulated or in motion. A considerable number of cells are especially sensitive to the posture and position of the trunk and extremities during movement (Hyvarienen, 1982). By associating this convergent input these cells are thus able to monitor, coordinate and guide limb movement (Cohen et al. 2014) as well as determine the position of the body and objects in space.
Through the integrative and associative activities of the cell assemblies within area five, an interactional image of the body is maintained. In this manner, an individual is able to ascertain the position of the body and the limbs at rest and in motion (Gross, et al. 1974). In part, this may be accomplished through comparisons with a more stable image of the body which is possibly maintained via the combined interactions of neurons in areas 3,1,2. That is a stable body image (or body image memories) are stored in these tissues. Hence, when the body has moved, this new information (received and processed in area 5) can be compared to the more stable trace (or memory maintained in the primary regions) so that the new position of the limbs and body can be ascertained. In this regard it could be argued that body-related memories are stored in the parietal lobe.

Nevertheless, to determine position, sensation per se is not sufficient. Rather, sensation must be combined with input regarding movement or positional change (Gandevia & Burke 1992). It is for this reason that in the absence of movement (and in the absence of visual cues, such as when one wakes up in the middle of the night) one usually cannot tell where or in what position their arms or legs may be in. However, with a slight movement we can immediately determine position.
FUNCTIONAL LATERALITY
As detailed in Chapter 10, there is clear evidence that the right parietal area is dominant in regard to many aspects of somesthetic information processing. Hence, neurons in this half of the brain appear to be more sensitive and more responsive and to more greatly monitor events occurring on either half of the body, but particularly the left. In fact, this relationship was noted over 150 years ago by Weber.
According to Weber (1834/1977), the left half of the body exceeds the right in regard to most forms of tactual sensitivity. The left hand and the soles of the left foot, as well as the left shoulder are more accurate in judging weight, have a more delicate sense of touch and temperature, such that "a greater sense of cold or of heat is aroused in the left hand" (p. 322). That is, the left hand judges warm substances to be hotter, and cold material to be colder as compared to the right hand, even when both hands are simultaneously stimulated.
The right half of the brain, in fact, appears to maintain multiple images of the body. Therefore, the parietal lobe of the right hemisphere appears to have more neocortical space devoted to maintaining images of the body.
PAIN: AREAS 5, 7, & THE SUPRAMARGINAL GYRUS
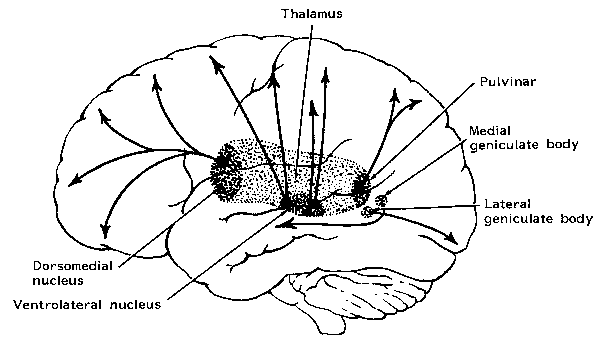
Neurons in area 5, as well as those located in the insula, receive direct thalamic input from the ventral and posterior portion of VPL. The ventral portion in particular, however, in addition to somesthetic information, may also convey pain sensation to the parietal lobe. In fact, Penfield and Boldrey (1937) reported that electrical stimulation of the parietal lobe resulted in the sensation of pain, albeit about 1% of the time.
Some neurons located in area 5 and 7 of the parietal lobe also demonstrate pain sensitivity, with some are 7 neurons responding exclusively to thermal and nociceptive stimuli (Dong et al. 2014) and with area 5 presumably acting to localize the source of pain. Hence, in some instances, such as when the more inferior portion of area 5, 7 or the supramarginal gyrus (Broadmann's area 40) has been destroyed, patients may demonstrate a lack of emotional responsiveness to painful stimuli, become indifferent, develop an increased pain threshold, tolerate pain for an unusually lengthy time period and fail to respond even to painful threat (Berkley & Parmer, 1974; Biemond, 1956; Geschwind, 1965; Greenspan & Winfield 1992; Hyvarinen, 1982; Schilder, 1935) --particularly with right parietal destruction (Cubelli et al 2012). However, disturbance or lack of pain sensation has been noted to occur when lesions to either hemisphere (Hecaen & Albert, 1978).
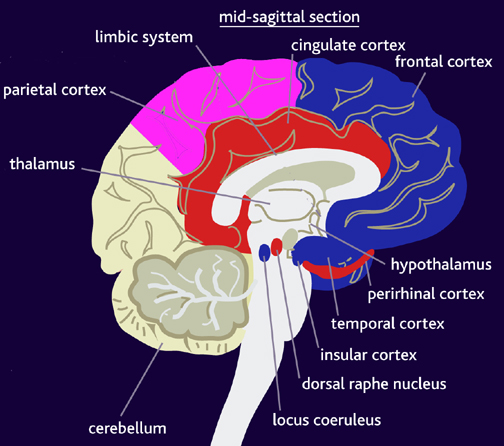
Moreover, loss of sensation or an inability to react to pain may also occur from subcortical lesions, especially within the thalamus, and less often, with surgical destruction of the anterior cingulate--the so called center of "pain and misery." In this regard, there appears to be two major cerebral pain pathways, a subcortical medial pathway involving the thalamus and cingulate, and a neocortical pathway involving the parietal lobe.
At the neocortical level, although pain responsiveness may be diminished or absent following damage to these tissues, elementary sensation remains intact and the ability to differentiate, for example, between dull and sharp is retained. The deficit is usually bilateral.
Some researchers have claimed that in order to lose pain sensitivity the lesion sometimes involves the frontal-parietal cortex (Hecaen & Albert, 1978). However, the supramarginal gyrus of the inferior parietal lobule (Geschwind, 1965; Hyvarinen, 1982; Schilder, 1935) and area 7 of the superior parietal lobule (Dong et al. 2014; Greenspan & Winfield 1992) are the most likely candidates for this condition --particularly in that a second somesthetic area is located here as well as yet another image of the human body (Penfield & Rasmussen, 1950).
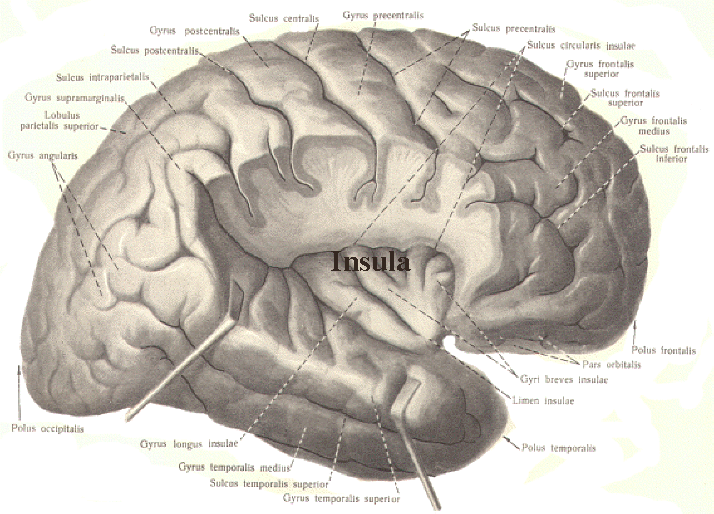
In this regard, Schilder (1935), has argued that the loss of reaction to pain is due to disturbances in the image of the body. That is, the experience or threat of pain is no longer related to the body image. Geschwind, (1965), however, raises the possibility that this condition is due to disconnection from the limbic system (see Cavada & Goldman-Rakic 1989). If this were the case, somesthetic (painful) sensation would no longer be assigned emotional significance and would thus implicate the insular region of the parietal lobe, which also receives visceral as well as somesthetic information and funnels this data to the limbic system.

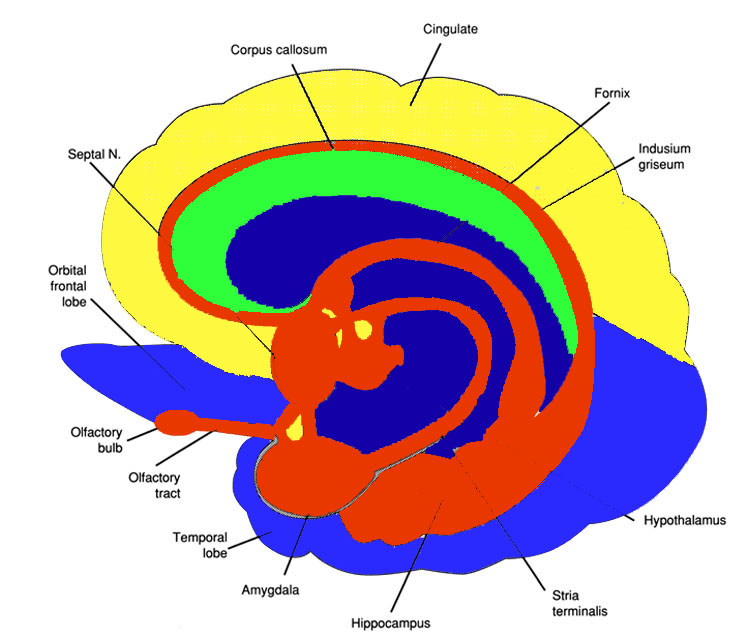
In fact, this same insular-limbic pathway may serve to promote tactile memory; that is, via the funneling of complex somesthetic information to the hippocampus and amygdala. Conversely, it may be this same pathway which when abnormally activated or injured, may give rise to abnormal emotional significance being attributed to bodily sensations.
PAIN AND HYSTERIA
Whereas destruction of the inferior portions of areas 7, 5, and 40 may result in loss of pain sensation, when the injury is secondary to tumor or seizure activity patients may instead report experiencing pain (Davidson & Schick, 1935; Hernandez-Peon et al. 1963; Ruff, 1980; Wilkinson, 2013; York et al. 1979). In addition patients may experience sensory distortions that concern various body parts due to abnormal activation of the parietal neocortex.
For example, one 48 year old housewife complained of diffuse, poorly localized (albeit intense) pain in her left leg, which occurred in spasms that lasted minutes. She subsequently was found to have a large tumor in the right parietal area, which, when removed, alleviated all further attacks. Head and Holmes (1911) reported a patient who suffered brief attacks of "electric shock"-like pain that radiated from his foot to the trunk; a glioma in the right parietal area subsequently was discovered.
Sometimes the pain may be related to abnormal sexual or genital sensations. For example, one 9-year-old boy seizure activity in the right parietal area experienced spontaneous attacks of intense scrotal and testicular pain (York et al.1979). Ruff (1980) reports two cases who experienced paroxsymal episodes of spontaneous and painful orgasm, which was secondary to right parietal seizure activity. In one patient the episodes began with the sensation of clitoral warmth, engorgement of the breasts, tachycardia, etc., all of which rapidly escalated to a painful climax.
It is important to note, however, that although the predominant focus for paroxysmal pain is the right hemisphere, pain also has been reported to occur with tumors or seizures activity that involves the left parietal region (Bhaskar, 1987; McFie & Zangwill, 1960).
Unfortunately, when the patient's symptoms are not considered from a neurological perspective, their complaints with regard to pain may be viewed as psychogenic in origin. This is because the sensation of pain, stiffness, engorgement, is, indeed, entirely "in their head" and based on distorted neurological perceptual functioning. Physical exam may reveal nothing wrong with the seemingly affected limb or organ. Thus such patients may be viewed as hysterical or hypochondriacle, particularly in that right hemisphere damage also disrupts emotional functioning.
THE RIGHT & LEFT PARIETAL LOBE: LESIONS & LATERALITY
CONSTRUCTIONAL AND SPATIAL PERCEPTUAL SKILLS
Based on studies of brain injured, neurosurgical (e.g., temporal lobectomy, split-brain), and normal populations, the right cerebral hemisphere has been found to be dominant over the left in the analysis of geometric and visual-space, the perception of depth, distance, direction, shape, orientation, position, perspective, and figure-ground, the detection of complex and hidden figures, the performance of visual closure, and the ability to infer the total stimulus configuration from incomplete information, route finding and maze learning, localizing targets in space, the performance of reversible operations, stereopsis, and the determination of the directional orientation of the body as well as body-part positional relationships (Benton 1993; Butters & Barton, 1970; Carmon & Bechtoldt, 1969; DeRenzi & Scotti, 1969; DeRenzi et al. 1969; Ettlinger, 2010; Fontenot, 2013; Franco & Sperry, 1977; Fried et al. 2012; Hannay et al., 2017; Kimura, 1966; 1969, 1993; Landis et al. 20166; Lansdell, 1968, 1970; Levy, 1974; Milner, 1968; Nebes, 2011; Sperry, 1982). Hence, if the right hemisphere is injured, visual-spatial perceptual functioning is negatively impacted.
In addition, the isolated right hemisphere has been found to be superior in "fitting designs into larger matrices, judging whole circle size from a small arc, discriminating and recalling nondescript shapes, making mental spatial transformations, sorting block sizes and shapes into categories, perceiving wholes from a collection of parts, and the intuitive perception and apprehension of geometric principles" (Sperry, 1982, p.1225).
Thus, it is the right hemisphere which enables us to find our way in space without getting lost, to walk and run without tripping and falling, to throw and catch a football with accuracy, to drive a car without bumping into things, to draw conclusions based on partial information, and to see the forest when looking at the trees. The right is also superior to the left in analyzing manipulo-spatial problems, drawing and copying simple and complex geometric-like figures and performing constructional tasks, block designs and puzzles (Benson & Barton, 1970; Black & Strub 1976; Critchley, 1953; DeRenzi, 1982; Gardner, 1975; Hecaen & Albert, 1978; Hier et al. 2013; Kertesz, 1983b; Levy, 1974; Luria, 2013, 1980; Piercy et al. 1960). It is for these and other reasons that the right brain is often viewed as the artistic half of the cerebrum.
The right hemisphere is also dominant over the left in regard to localizing and thus referencing the position of an object in space (Cook et al. 2014; Nunn et al., 1999; Ploner et al., 1999), as well as in aiming and closed loop throwing accuracy (Guiard et al. 1983; Haaland & Harrington, 2010). Of course, most individuals use the right hand to throw (as well as draw). Presumably the right hemisphere is able to guide right limb and related axial movements (Rapcsak et al.2013) via bilateral SMA and parietal lobe innervation of the basal ganglia and lower motor neurons (see chapters 16, 19).
CONSTRUCTIONAL APRAXIA
Constructional apraxia is by no means a unitary disorder (Benton, 1969; Benson & Barton, 1970) and can may be expressed in a number of ways. On a drawing or copying task this may include the addition of unncessary/non-existant details or parts, misalignment or inattention to details, disruptions of the horizontal and verticle axis with reversals or slight rotations in reproduction, and scattering of parts. For example, in performing the Block Design subtest from the WAIS-R, the patient may correctly reproduce the model but angle it incorrectly. In drawing or copying figures, the patient may neglect the left half, draw over the model, and misalign details.
Moreover, although constructional deficits are more severe after right hemisphere damage (Arrigoni & DeRenzi, 1964; Black & Strub, 1976; Benson & Barton, 1970; Critchley, 1953; Hier et al., 1983; Joseph, 1988a; Kimura 1993; Piercy et al. 1960), disturbances involving constructional and manipulo-spatial functioning can occur with lesions to either half of the brain (Arrigoni & DeRenzi, 1964; Mehta et al., 1987; Piercy et al., 1960). Hence, depending on the laterality, as well as the extent and site of the lesion, the deficit may also take different forms. For example, following posterior reight cerebral lesions, rather than apraxic, the patient is spatially-agnosic, i.e. suffering from constructional agnosia and a failure to perceive and recognize visual-spatial and object interrelationships. In other cases, such as following left cerebral injury, the disturbance may be secondary to a loss of control over motor programming (Kimura 1993; Warrington et al., 1966; Warrington, 1969).
Although visual motor deficits can result from lesions in either hemisphere (Arrigoni & DeRenzi, 1964; Piercy et al., 1960; Kimura 1993), visual-perceptual disturbances are more likely to result from right hemisphere damage. In contrast, lesions to the left half of the brain may leave the perceptual aspects undisturbed whereas visual motor functioning and selective organization may be compromised (Kim et al. 2012; Mehta et al., 1987; Poeck et al. 2013). As such the patient is likely to recognize that errors have been made.
In general, the size and sometimes the location of the lesion within the right hemisphere has little or no correlation with the extent of the visual-spatial or constructional deficits demonstrated, although right parietal lesions tend to be worst of all. With right parietal involvement patients tend to have trouble with the general shape and overall organization, the correct alignment and closure of details, and there may be a variable tendency to ignore the left half of the figure or to not fully attend to all details. Moreover the ability to perceive (or care) that errors have been made is usually compromised.
Conversely, constructional disturbances associated with left hemisphere damage are positively correlated with lesion size, and left anterior lesions are worse than left posterior (Benson & Barton, 1970; Black & Bernard, 2012; Black & Strub, 1976; Kimura 1993; Lansdell, 1970). This is because the capacity to control and program the motor system has been compromised. The larger the lesion, the more extensive the deficit.
Moreover, because the left hemisphere is concerned with the analysis of parts or details and engages in temporal- sequential motor manipulations, lesions result in oversimplification and a lack of detail although the general outline or shape may be retained (Gardner, 1975, Levy, 1974). However, in some cases, when drawing, there may be a tendency to more greatly distort the right half of the figure with some preservation of left sided details.
DRAWING AND CONSTRUCTIONAL DEFICITS
The left hemisphere also contributes to visual-spatial processing and expression such that when damaged drawing ability can be affected (Kimura, 1993), albeit in a manner different from that of the right (Mehta et al. 1987). For example, because the left is concerned with the analysis of parts or details lesions result in sequencing errors, oversimplification and a lack of detail in drawings such that details may be ignored, although the general outline or shape may be retained (Bradshaw & Mattingly, 1995; Gardner, 1975; Joseph, 1988a; Kimura, 1993; Levy, 1974).
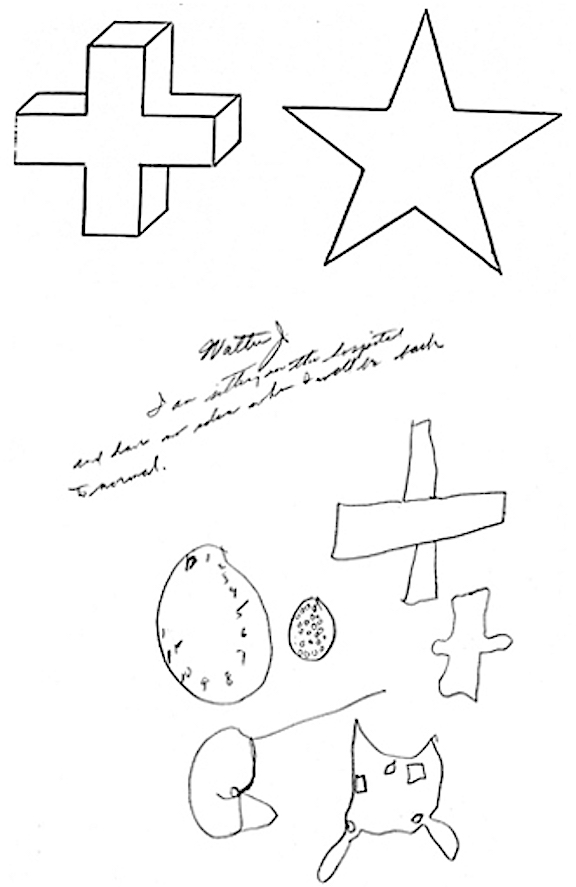
Constructional/Drawing Apraxia: Difficulty copying geometric patterns
In contrast, the right cerebrum is more involved in the overall perceptual analysis of visual and object interrelations including visual closure and gestalt formation. Thus, patients with right cerebral injuries have trouble with general shape and organization, although certain details may be drawn correctly. Drawings may also be grossly distorted and/or characterized by left sided neglect.
Right sided damage also can affect writing. When patients are asked to write cursively, writing samples may display problems with visual closure, as well as excessive segmentation due to left hemisphere release (Joseph, 1988a). That is, cursively the word "recognition" may be written "re cog n i tion," or letters such as "o" may be only partly formed.
Constructional deficits are more severe after right hemisphere damage (Arrigoni & DeRenzi, 1964; Black & Strub, 1976; Benson & Barton, 1970; Bradshaw & Mattingly, 1995; Critchley, 1953; Hier et al., 1983; Piercy et al., 1960). However, lesions to either hemisphere can create disturbances in constructional and manipulo-spatial functioning -including performance on the WAIS Block Design and Object Assembly subtests (Arrigoni & DeRenzi, 1964; Cubelli et al. 2011; Kimura, 1993; Mehta et al., 1987; Piercy et al., 1960).
If the left hemisphere is damaged, performance may be impaired due to motor programming errors and/or an inability to transform the percept into a motor action with preservation of good spatial-perceptual functioning (Warrington et al., 1966); in which case errors may be recognized by the patient. With right cerebral injuries, visual-spatial perceptual functioning becomes distorted (although motor activities per se are preserved) and the patient may not realize they have made an error (Hecaen & Assal, 1970).











