Rhawn Joseph, Ph.D.
BrainMind.com
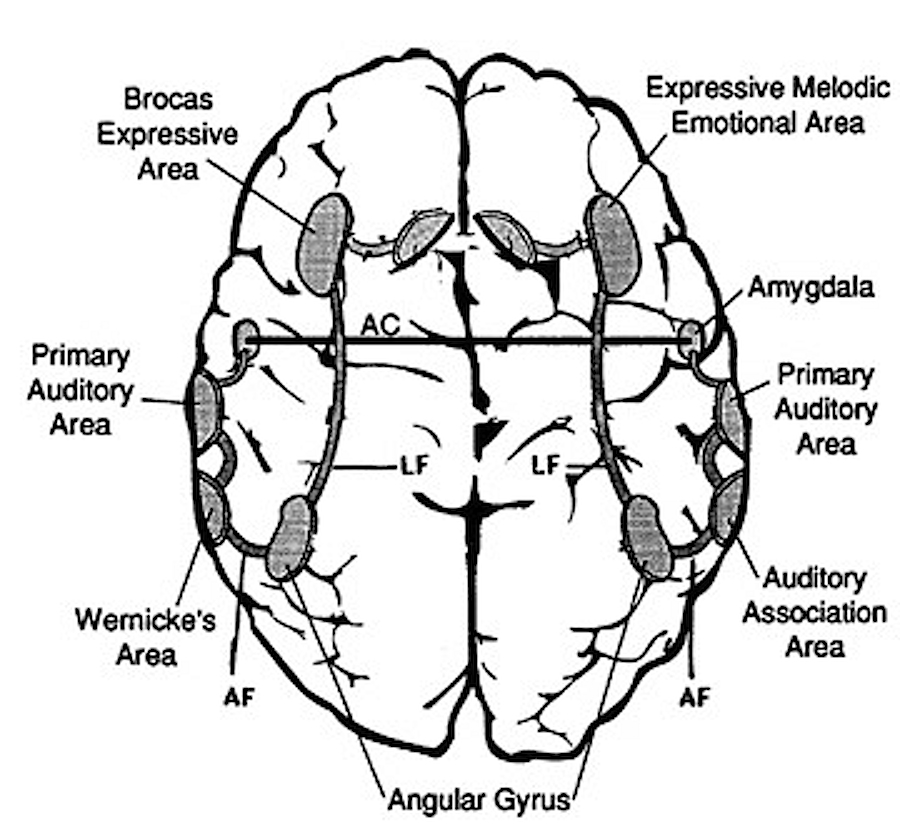
RIGHT HEMISPHERE EMOTIONAL-MELODIC LANGUAGE AXIS
Just as there are areas in the left frontal and temporal-parietal lobes which mediate the expression and comprehension of the denotative, temporal-sequential, grammatical-syntactial aspects of language, there are similar regions within the right hemisphere that mediate emotional speech and comprehension (Gorelick & Ross, 2016; Heilman et al. 1975; Joseph, 1982, 1988a, 2013; Lalande et al. 2012; Ross, 1981; Shapiro & Danly, 1985; Tucker et al., 1977); regions which become highly active when presented with complex nonverbal auditory stimuli (Roland et al. 2011) and when engaged in interpreting the figurative aspects of language (Bottini et al., 2014).
Moreover, it appears that during the early stages of neonatal and infant development, that the role of the right hemisphere in language expression and perception was even more pronounced. As originally proposed by Joseph (1982, 1988a), language in the neonate and infant is dominated by the right hemisphere, which in turn accounts for the initial prosodic, melodic, and emotional qualities of their vocalizations. Of course, the left hemisphere is genetically programmed to gain functional dominance and to acquire the grammatical, temporal sequential, word-rich, and expressive-motor aspects of speech--as is evident neuronatomically by the presence of asymmetries in the fetal and neontal planum temporal (Wada et al., 1975; Witelson & Palli, 1973), and the fact that the left cerebral pyramidal tract descends and establishes synaptic contact with the brainstem and spinal cord in advance of the right (Kertesz & Geschwind 1971; Yakovlev & Rakic 1966).
However, as also based on evoked potential studies, the pattern of neurological activity, during the performance of language tasks, does not begin to resemble the adult pattern until the onset of puberty (Hollcomb et al., 1992). Moreover, although the left hemisphere gradually acquires language, the right hemisphere continues to participate even in non-emotional language processing, including reading, as demonstrated by functional imaging studies (Bottini et al., 1994; Cuenod, et al., 1995; Price et al., 1996).
For example, the right temporal and parietal areas are activated when reading (Bottini et al., 1994; Price et al., 1996), and the right temporal lobe becomes highly active when engaged in interpreting the figurative aspects of language (Bottini et al., 1994). Moreover, bilateral frontal activation is seen when speaking--though this activity is greater on the left (Passingham, 1997; Peterson et al., 1988). In part, however, these latter findings may well reflect those aspects of right hemisphere language processing (temporal-parietal) and expression (frontal-parietal) which are concerned with extracting and vocalizing emotional, motivational, personal, and contextual details.
For example, right frontal damage has been associated with a loss of emotional speech and emotional gesturing and a significantly reduced ability to mimic various nonlinguistic vocal patterns (Joseph 1988a; Ross, 1981, 2003; Shapiro & Danly, 1985). In these instances, speech can becomes flat and monotone or characterized by inflectional distortions.
With lesions that involve the right temporal-parietal area, the ability to comprehend or produce appropriate verbal prosody, emotional speech, or to repeat emotional statements is reduced significantly (Gorelick & Ross, 2016; Heilman et al. 1975; Lalande et al. 1992; Ross, 1981; Starkstein et al. 1994; Tucker et al. 1977). Indeed, when presented with neutral sentences spoken in an emotional manner, right hemisphere damage disrupts perception and discrimination (Heilman et al. 1975; Lalande et al. 1992) and the comprehension of emotional prosody (Heilman et al. 1984; Starkstein et al. 1994) regardless of whether it is positive or negative in content. Moreover, the ability to differentiate between different and even oppositional emotional qualities (e.g., "sarcasm vs irony" or "love" vs "hate") can become distorted (Cicone et al. 1980; Kaplan et al. 2007), and the capacity to appreciate and comprehend humor or mirth may be attenuated (Gardner et al. 1975).
The semantic-contextual ability of the right hemisphere is not limited to prosodic and paralinguistic features, however, but includes the ability to process and recognize familiar, concrete, highly imaginable words (J. Day, 1977; Deloch et al. 2016; Ellis & Shephard, 1975; Hines, 1976; Joseph 1988b; Landis et al., 1982; Mannhaupt, 1983), as well as emotional language in general.
The disconnected right hemisphere also can read printed words (Gazzaniga, 1970; Joseph, 1986b, 1988b; Levy, 1983; Sperry, 1982; Zaidel, 1983), retrieve objects with the left hand in response to direct and indirect verbal commands, e.g. "a container for liquids" (Joseph, 1988b; Sperry, 1982), and spell simple three- and four-letter words with cut-out letters (Sperry, 1982). However, it cannot comprehend complex, non-emotional, written or spoken language.
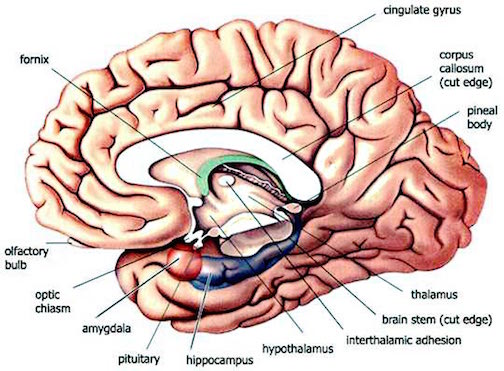
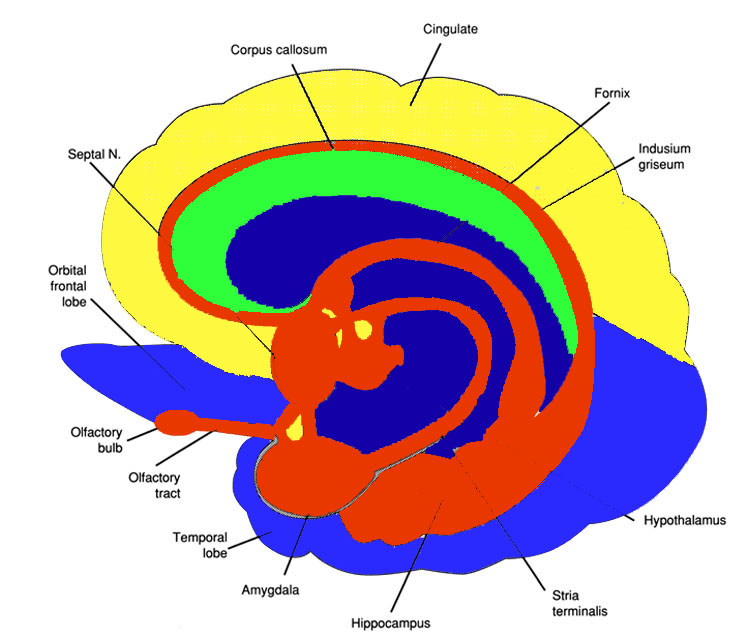
As noted, the right hemisphere dominance for vocal (and non-verbal) emotional expression and comprehension is believed to be secondary to hierarchical neocortical representation of limbic system functions. It may well be dominance by default, however. That is, at one time both hemispheres may well have contributed more or less equally to emotional expression, but with the evolution of language and right handedness, the left hemisphere gradually lost this capacity where it was retained in the right cerebrum (chapter 6). Even so, without the participation of the limbic system, the amygdala and cingulate gyrus in particular, emotional language capabilities would for the most part be nonexistent.
COMPREHENSION AND EXPRESSION OF EMOTIONAL SPEECH
Although language is often discussed in terms of grammar and vocabulary, there is a third major aspect to linguistic expression and comprehension by which a speaker may convey and a listener discern intent, attitude, feeling, mood, context, and meaning. Language is both emotional and grammatically descriptive. A listener comprehends not only the content and grammar of what is said, but the emotion and melody of how it is said -what a speaker feels.
Feeling, be it anger, happiness, sadness, sarcasm, empathy, etc., often is communicated by varying the rate, amplitude, pitch, inflection, timbre, melody and stress contours of the voice. When devoid of intonational contours, language becomes monotone and bland and a listener experiences difficulty discerning attitude, context, intent, and feeling. Conditions such as these arise after damage to select areas of the right hemisphere or when the entire right half of the brain is anesthetized (e.g., during sodium amytal procedures).
It is now well established (based on studies of normal and brain-damaged subjects) that the right hemisphere is superior to the left in distinguishing, interpreting, and processing vocal inflectional nuances, including intensity, stress and melodic pitch contours, timbre, cadence, emotional tone, frequency, amplitude, melody, duration, and intonation (Blumstein & Cooper, 1974; Bowers et al. 2016; Carmon & Nachshon, 1973; Heilman et al. 1975; Ley & Bryden, 1979; Mahoney & Sainsbury, 2016; Ross, 1981; Safer & Leventhal, 1977; Samson & Zatorre, 1988, 1992; Shapiro & Danly, 1985; Tucker et al. 1977). The right hemisphere, therefore, is fully capable of determining and deducing not only what a persons feels about what he or she is saying, but why and in what context he is saying it --even in the absence of vocabulary and other denotative linguistic features (Blumstein & Cooper, 1974; DeUrso et al. 1986; Dwyer & Rinn, 1981). This occurs through the analysis of tone and melody.
Hence, if I were to say, "Do you want to go outside?" although both hemispheres are able to determine whether a question vs. a statements has been made (Heilman et al. 1984; Weintraub et al. 1981), it is the right cerebrum which analyzes the paralinguistic emotional features of the voice so as to determine whether "going outside" will be fun or whether I am going to punch you in the nose. In fact, even without the aid of the actual words, based merely on melody and tone the right cerebrum can determine context and the feelings of the speaker (Blumstein & Cooper, 1974; DeUrso et al. 1986; Dwyer & Rinn, 1981). This may well explain why even preverbal infants are able to make these same determinations even when spoken to in a foreign language (Fernald, 2013; Haviland & Lelwica, 2016). The left hemisphere has great difficulty with such tasks.
For example, in experiments in which verbal information was filtered and the individual was to determine the context in which a person was speaking (e.g. talking about the death of a friend, speaking to a lost child), the right hemisphere was found to be dominant (Dwyer & Rinn, 1981). It is for these and other reasons that the right half of the brain sometimes is thought to be the more intuitive half of the cerebrum.
. Correspondingly when the right hemisphere is damaged, the ability to process, recall, or even recognize these nonverbal nuances is greatly attenuated. For example, although able to comprehend individual sentences and paragraphs, such patients have difficulty understanding context and emotional connotation, drawing inferences, relating what is heard to its proper context, determining the overall gist or theme, and recognizing discrepancies such that they are likely to miss the point, respond to inappropriate details, and fail to appreciate fully when they are being presented with information that is sarcastic, incongruent or even implausible (Beeman 2013; Brownell et al. 1986; Foldi et al. 1983; Gardner et al. 2013; Kaplan et al. 2014; Rehak et al. 2012; Wapner et al. 1981).
Such patients frequently tend to be very concrete and literal. For example, when presented with the statement, "He had a heavy heart" and requested to choose several interpretations, right-brain damaged (vs. aphasic) patients are more likely to choose a picture of an individual staggering under a large heart vs. a crying person. They also have difficulty describing morals, motives, emotions, or overall main points (e.g. they lose the gestalt), although the ability to recall isolated facts and details is preserved (Delis et al. 1986; Hough 2010; Wapner et al. 1981) -details being the province of the left hemisphere.
Although they are not aphasic, individuals with right hemisphere damage sometimes have difficulty comprehending complex verbal and written statements, particularly when there are features which involve spatial transformations or incongruencies. For example, when presented with the question "Bob is taller than George. Who is shorter? ", those with right-brain damage have difficulties due, presumably, to a deficit in nonlingusitic imaginal processing or an inability to search a spatial representation of what they hear (Carmazza et al. 1976).
In contrast, when presented with "Bob is taller than George. Who is taller?" patients with right-hemisphere damage perform similar to normals, which indicates that the left cerebrum is responsible for providing the solution (Carmazza et al. 1976) given that the right hemisphere is injured and the question does not require any type of spatial transformation. That is, because the question "Who is shorter?" does not necessarily follow the first part of the statements (i.e., incongruent), whereas "Who is taller?" does, these differential findings further suggest that the right hemisphere is more involved than the left in the analysis of incongruencies.
MUSIC AND NON-VERBAL ENVIRONMENTAL AND ANIMAL SOUNDS
Individuals with extensive left-hemisphere damage and/or severe forms of expressive aphasia, although unable to discourse fluently, may be capable of swearing, singing, praying or making statements of self-pity (Gardner, 1975; Goldstein, 1942; Smith, 1966; Smith & Burklund, 1966; Yamadori et al., 1977). Even when the entire left hemisphere has been removed completely, the ability to sing familiar songs or even learn new ones may be preserved (Smith, 1966; Smith & Burklund, 1966) --although in the absence of music the patient would be unable to say the very words that he or she had just sung (Goldstein, 1942). The preservation of the ability to sing has, in fact, been utilized to promote linguistic recovery in aphasic patients, i.e., melodic-intonation therapy (Albert et al. 1973; Helm-Estabrooks, 1983).
Similarly, there have been reports that some musicians and composers who were suffering from aphasia and/or significant left hemisphere impairment were able to continue their work (Alajounine, 1948; Critchley, 1953; Luria, 1973). In some cases, despite severe receptive aphasia and/or although the ability to read written language (alexia) was disrupted, the ability to read music or to continue composing was preserved (Gates & Bradshaw, 1977; Luria, 1973).
One famous example is that of Maurice Ravel, who suffered an injury to the left half of his brain in an auto accident. This resulted in ideomotor apraxia, dysgraphia, and moderate disturbances in comprehending speech (i.e., Wernicke's Aphasia). Nevertheless, he had no difficulty recognizing various musical compositions, was able to detect even minor errors when compositions were played, and was able to correct those errors by playing them correctly on the piano (Alajounine, 1948).
Conversely, it has been reported that musicians who are suffering from right hemisphere damage (e.g., right temporal-parietal stroke) have major difficulties recognizing familiar melodies and suffer from expressive instrumental amusia (Luria, 1973; McFarland & Fortin, 1982). Even among nonmusicians, right hemisphere damage (e.g. right temporal lobectomy) disrupts time sense, rhythm, and the ability to perceive, recognize or recall tones, loudness, timbre, and melody (Chase, 1967; Gates & Bradshaw, 1977; Milner, 1962; Samsom & Zattore, 1988; Yamadori et al., 1977). In fact, right temporal injuries can disrupt the ability to remember musical tunes or to create musical imagery (Zatorre & Halpen, 2013).
Right hemisphere damage also can disrupt the ability to sing or carry a tune and can cause toneless, monotonous speech, as well as abolish the capacity to obtain pleasure while listening to music (Reese. 1948; Ross, 1981; Shapiro & Danly, 1985), i.e., a condition also referred to as amusia. For example, Freeman and Williams (1953) report that removal of the right amygdala in one patient resulted in a great change in the pitch and timbre of speech and that the ability to sing also was severely affected. Similarly, when the right hemisphere is anesthetized the melodic aspects of speech and singing become significantly impaired (Gordon & Bogen, 1974).
It also has been demonstrated consistently in normals (such as in dichotic listening studies) and with brain-injured individuals that the right hemisphere predominates in the perception (and/or expression) of timbre, chords, tone, pitch, loudness, melody, meter, tempo, and intensity (Breitling et al .2016; Curry, 1967; R. Day et al. 2011, Gates & Bradshaw, 1977; Gordon, 1970; Gordon & Bogen, 1974; Kester et al. 1991; Kimura, 1964; Knox & Kimura, 1970; McFarland & Fortin, 1982; Milner, 1962; Molfese et al. 1975; Piazza,2010; Reese, 1948; Segalowitz & Plantery, 2015; Spellacy, 1970; Swisher et al. 1969; Tsunoda, 2015; Zurif, 1974)--the major components (in conjuction with harmony) of a musical stimulus.
For example, in a functional imaging study, it was found that when professional pianists played Bach (Bach's Italian concerto, third movement) that there was increased activity in the right but not left temporal lobe, whereas when they played scales, activity increased in the left but not right temporal lobe (Parsons & Fox, 1997). Likewise, Evers and colleagues (1999) in evaluating cerebral blood velocity, found that a right hemisphere increase in blood flow when listening to harmony (but not rhythm), among non-musicians in general, and especially among females.
In addition, Penfield and Perot (1963) report that musical hallucinations most frequently result from electrical stimulation of the right superior and lateral surface of the temporal lobe. Berrios (2016) also concluded from a review of lesions studies that musical hallucinations were far more likely following right cerebral dysfunction; whereas conversely destruction of this tissue disrupt the ability to conjure up musical imagery (Zatorre & Halpen, 2013). Findings such as these have added greatly to the conviction that the right cerebral hemisphere is dominant in regard to the non-temporal sequential aspects of musical perception and expression.
However, this does not appear to be the case with professional musicians who in some respects tend to treat music as a mathematical language that is subject to rhythmic analysis. As noted when professional pianists played scaled, activity increased in the left but not right temporal lobe (Parsons & Fox 1997). Moroever, Evers and colleagues (1999) found that musicians displayed an increase in left hemisphere blood flow when listening to both harmony and rhythm.
ENVIRONMENTAL AND HUMAN/ANIMAL SOUNDS
In addition to music the right hemisphere has been shown to be superior to the left in discerning and recognizing nonverbal and environmental sounds (Curry, 1967; Joseph, 1988b; Kimura, 1963; King & Kimura, 1972; Knox & Kimura, 1970; Nielsen, 1946; Piazza, 1980; Roland et al. 1981; Schnider et al. 1994; Spreen et al. 1965; Tsunoda, 1975). Similarly, damage that involves select areas within the right hemisphere not only disturb the capacity to discern musical and social-emotional vocal nuances, but may disrupt the ability to perceive, recognize, or disciminate between a diverse number of sounds which occur naturally within the environment (Fujii et al., 2010; Joseph, 2013; Nielsen, 1946; Schnider et al. 1994; Spreen et al. 1965), such as water splashing, a door banging, applause, or even a typewriter; this is a condition which also plagues the disconnected left hemisphere (Joseph, 1988b).
A 47-year old woman I examined who was subsequently found to have a calcium cyst growing from the skull into the right superior temporal lobe, was able to name pictures of animals, tools and household objects. However, she was almost completely unable to recognize and correctly name animal and humans sounds (e.g. a baby crying, a crowd cheering, a lion roaring) which had been briefly presented, but was better able to recognize non-living sounds such as a creaking door, or a hammer hammering--though these abilities were also compromised. However, coupled with other findings to be reviewed below, there is some possibility that the right temporal lobe is better able to recognize living and true environmental sounds, whereas the left may be better able to recognize and name non-living sounds.
The possibility has been raised that music, verbal emotion, and nonverbal environmental-living sounds are, in some manner, phylogenetically linked (Joseph, 1982, 1988a, 2013). For example, it is possible that right hemisphere dominance for music may be a limbic outgrowth and/or strongly related to its capacity to discern and recognize environmental acoustics as well as its ability to mimic these and other nonverbal and emotional nuances. That is, music may have been invented as a form of mimicry, and/or as a natural modification of what has been described as "limbic language" -(a term coined by Joseph 1982).
For example, it is somewhat probable that primitive man and woman's first exposure to the sounds of music was environmentally embedded, for obviously musical sounds are heard frequently throughout nature (e.g. birds singing, the whistling of the wind, the humming of bees or insects). For example, bird songs can encompass sounds that are "flute-like, truly chime- or bell-like, violin-or guitar-like" and "some are almost as tender as a boy soprano" (Hartshorne, 1973, p. 36).
Hence, perhaps our musical nature is related to our original relationship with nature and resulted from the tendency of humans to mimic sounds that arise from the environment --such as those which conveyed certain feeling states and emotions. Perhaps this is also why certain acoustical naunces, such as those employed in classical music, can affect us emotionally and make us visualize scenes from nature (e.g., an early spring morning, a raging storm, bees in flight).
MUSIC AND EMOTION
Music is related strongly to emotion and, in fact, may not only be "pleasing to the ear," but invested with emotional significance. For example, when played in a major key, music sounds happy or joyful. When played in a minor key, music often is perceived as sad or melancholic. We are all familiar with the "blues" and perhaps at one time or another have felt like "singing for joy," or have told someone, "You make my heart sing!"
Interestingly, it has been reported that music can act to accelerate pulse rate (Reese, 1948), raise or lower blood pressure, and, thus, alter the rhythm of the heart's beat. Rhythm, of course, is a major component of music.
Music and vocal emotional nuances also share certain features, such as melody, intonation., etc., all of which are predominantly processed and mediated by the right cerebrum. Thus, the right hemisphere has been found to be superior to the left in identifying the emotional tone of musical passages and, in fact, judges music to be more emotional as compared to the left cerebrum (Bryden et al. 1982).
AUDITORY AGNOSIA
An individual with cortical deafness, due to bilateral lesions suffers from a generalized auditory agnosia involving words and non-linguistic sounds. However, in many instances an auditory agnosia, with preserved perception of language may occur with lesions restricted to the right temporal lobe (Fujii et al., 2010). In these instances, an indvidual loses the capability to correctly discern environmental (e.g. birds singing, doors closing, keys jangling) and acoustically related sounds, emotional-prosodic speech, as well as music (Nielsen, 1946; Ross, 2013; Samson & Zattore, 1988; Schnider et al. 2014; Spreen et al. 1965; Zatorre & Hapern, 2013).
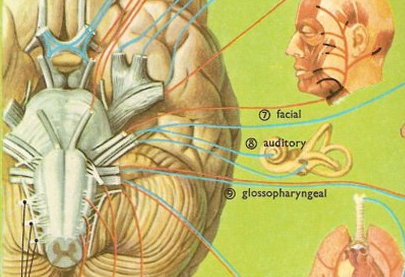
These problems are less likely to come to the attention of a physician unless accompanied by secondary emotional difficulties. That is, most individuals with this disorder, being agnosic, would not know that they have a problem and thus would not complain. If they are their families notice (for example, if a patient does not respond to a knock on the door) the likelihood is that the problem will be attributed to faulty hearing or even forgetfulness.
However, because such individuals may also have difficulty discerning emotional- melodic nuances, it is likely that they will misperceive and fail to comprehend a variety of paralinguistic social-emotional messages; a condition referred to as social-emotional agnosia (Joseph, 1988a) and phonagnosia (van Lancker, et al., 2015). This includes difficulty correctly identifying the voices of loved ones or friends, or discerning what others may be implying, or in appreciating emotional and contextual cues, including variables such as sincerity or mirthful intonation. Hence, a host of behavioral difficulties may arise (see chapter 10).
For example, a patient may complain that his wife no longer loves him, and that he knows this from the sound of her voice. In fact, a patient may notice that the voices of friends and family sound in some manner different, which, when coupled with difficulty discerning nuances such as humor and friendliness may lead to the development of paranoia and what appears to be delusional thinking. Indeed, unless appropriately diagnoses it is likely that the patients problem will feed upon and reinforce itself and grow more severe.
It is important to note that rather than completely agnosic or word deaf, patients may suffer from only partial deficits. In these isntances they may seem to be hard of hearing, frequently misinterpret what is said to them, and/or slowly develop related emotional difficulties.
SPATIAL LOCALIZATION, ATTENTION & ENVIRONMENTAL SOUNDS
In conjunction with the inferior colliculus and the frontal lobe (Graziano et al., 1999), and due to bilateral auditory input, the primary auditory area plays a significant role in orienting to and localizing the source of various sounds (Sanchez-Longo & Forster, 1958); for example, by comparing time and intensity differences in the neural input from each ear. A sound arising from one's right will reach and sound louder to the right ear as compared to the left.
Indeed, among mammals, a consideral number of auditory neurons respond or become highly excited only in response to sounds from a particular location (Evans & Whitfield, 1968). Moreover, some of these neurons become excited only when the subject looks at the source of the sound (Hubel et al., 1959). Hence, these neurons act so that location may be identified and fixated upon. In addition to the frontal lobe (Graziano et al., 1999) these complex interactions probably involve the parietal area (7), as well as the midbrain colliculi and limbic system. As based on lesions studies in humans, the right temporal lobe is more involved than the left in discerning location (Nunn et al., 1999; Penfield & Evans, 1934; Ploner et al., 199; Shankweiler, 1961).
There is also some indication that certain cells in the auditory area are highly specialized and will respond only to certain meaningful vocalizations (Wollberg & Newman, 1972). In this regard they seemed to be tuned to respond only to specific auditory parameters so as to identify and extract certain meaningful features, i.e. feature detector cells. For example, some cells will respond to cries of alarm and sounds suggestive of fear or indicating danger, whereas others will react only to specific sounds and vocalizations.
Nevertheless, although the left temporal lobe appears to be more involved in extracting certain linguistic features and differentiating between semantically related sounds (Schnider et al. 1994), the right temporal region is more adept at identifying and recognizing acoustically related sounds and non-verbal environmental acoustics (e.g. wind, rain, animal noises), prosodic-melodic nuances, sounds which convey emotional meaning, as well as most aspects of music including temp and meter (Heilman et al. 1975, 1984; Joseph, 1988a; Kester et al. 1991; Schnider et al. 1994; Spinnler & Vignolo 1966).
Indeed, the right temporal lobes spatial-localization sensitivity coupled with its ability to perceive and recognize environmental sounds no doubt provided great survival value to early primitive man and woman. That is, in response to a specific sound (e.g. a creeping predator), one is able to immediately identify, localize, locate, and fixate upon the source and thus take appropriate action. Of course, even modern humans relie upon the same mechanisms to avoid cars when walking across streets or riding bicycles, or to ascertain and identify approaching individuals, etc.
HALLUCINATIONS
Electrical stimulation of Heschyl's gyrus produces elementary hallucinations (Penfield & Jasper, 1954; Penfield & Perot, 1963). These include buzzing, clicking, ticking, humming, whispering, and ringing, most of which are localized as coming from opposite side of the room. Tumors involving this area also give rise to similar, albeit transient hallucinations, including tinnitus (Brodal, 1981). Patients may complain that sounds seem louder and/or softer than normal, closer and/or more distant, strange or even upleasant (Hecaen & Albert, 1978). There is often a repetitive quality which makes the experience even more disagreeable.
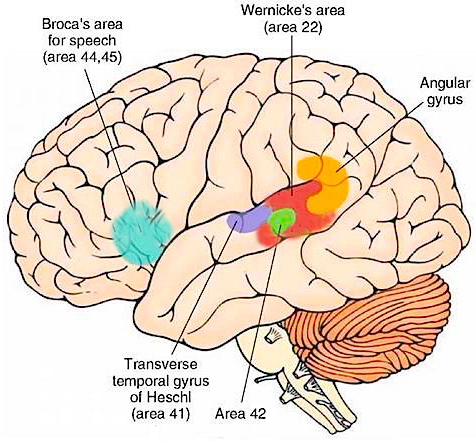
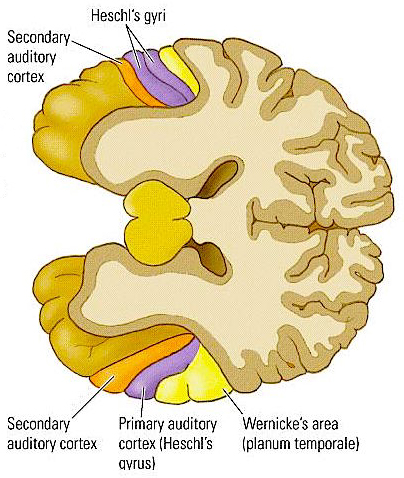
In some instances the hallucination may become meaningful. These include the sound of footsteps, clapping hands, or music, most of which seem (to the patient) to have an actual external source.
Penfield and Perot (1963) report that electrical stimulation of the superior temporal gyrus, the right temporal lobe in particular results in musical hallucinations. Patients with tumors and seizure disorders, particularly those involving the right temporal region, may also experience musical halluciantions. Frequenty the same melody is heard over and over. In some instances patients have reported the sound of singing voices and individual instruments may be heard (Hecaen & Albert, 1978).
Conversely, it has been frequently reported that lesions or complete surgical destruction of the right temporal lobe significantly impaires the ability to name or recognize melodies and musical passages. It also disrupts time sense, the perception of timbre, loudness, and meter (Chase, 1967; Joseph, 1988a; Kester et al. 1991; Milner, 1962; Shankweiler, 1966).
Auditory verbal hallucinations seem to occur with right or left temporal destruction or stimulation (Hecaen & Albert, 1978; Penfield & Perot, 1963; Tarachow, 1941) --although left temporal involvement is predominant. The hallucination may involve single words, sentences, commands, advice, or distant conversations which can't quite be made out. According to Hecaen and Albert (1978), verbal hallucinations may precede the onset of an aphasic disorder, such as due to a developing tumor or other destructive process. Patients may complain of hearing "distorted sentences", "incromprehensible words" etc.
CONSTRUCTIONAL AND SPATIAL PERCEPTUAL SKILLS
Based on studies of brain injured, neurosurgical (e.g., temporal lobectomy, split-brain), and normal populations, the right cerebral hemisphere has been found to be dominant over the left in the analysis of geometric and visual-space, the perception of depth, distance, direction, shape, orientation, position, perspective, and figure-ground, the detection of complex and hidden figures, the performance of visual closure, and the ability to infer the total stimulus configuration from incomplete information, route finding and maze learning, localizing targets in space, the performance of reversible operations, stereopsis, and the determination of the directional orientation of the body as well as body-part positional relationships. Hence, if the right hemisphere is injured, visual-spatial perceptual functioning is negatively impacted.
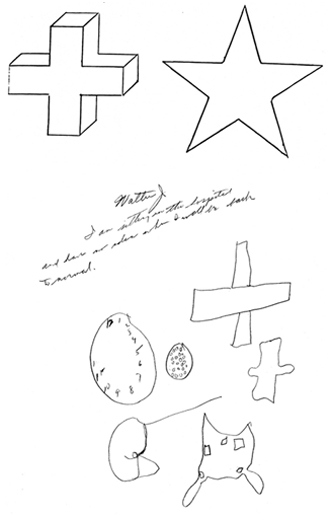
For example, Kimura (1963) found that patients with right vs left temporal lobe injury were impaired when presented with overlapping nonsense shapes and then immediately tested for recognition. Likewise, Meier and French (1965), found that those with right vs left temporal lobe injuries were impaired when asked to make visual discriminations when presented with fragmented concentric circle patterns--skills which are also related to visual closure and gestalt formation.
In addition, the isolated right hemisphere has been found to be superior in "fitting designs into larger matrices, judging whole circle size from a small arc, discriminating and recalling nondescript shapes, making mental spatial transformations, sorting block sizes and shapes into categories, perceiving wholes from a collection of parts, and the intuitive perception and apprehension of geometric principles" (Sperry, 1982, p.1225).
Thus, it is the right hemisphere which enables us to find our way in space without getting lost, to walk and run without tripping and falling, to throw and catch a football with accuracy, to drive a car without bumping into things, to draw conclusions based on partial information, and to see the forest when looking at the trees. The right is also superior to the left in analyzing manipulo-spatial problems, drawing and copying simple and complex geometric-like figures and performing constructional tasks, block designs and puzzles (Benson & Barton, 1970; Black & Strub 1976; Critchley, 1953; DeRenzi, 1982; Gardner, 1975; Hecaen & Albert, 1978; Hier et al. 1983; Kertesz, 1983b; Levy, 1974; Luria, 1973, 1980; Piercy et al. 1960). It is for these and other reasons that the right brain is often viewed as the artistic half of the cerebrum.
The right hemisphere is also dominant over the left in regard to localizing and thus referencing the position of an object in space (Cook et al. 1994; Nunn et al., 1999; Ploner et al., 1999), as well as in aiming and closed loop throwing accuracy (Guiard et al. 1983; Haaland & Harrington, 2017). Of course, most individuals use the right hand to throw (as well as draw). Presumably the right hemisphere is able to guide right limb and related axial movements (Rapcsak et al. 2013) via bilateral SMA and parietal lobe innervation of the basal ganglia and lower motor neurons (see chapters 16, 19).
VISUAL-PERCEPTUAL ABNORMALITIES
When the male or female right hemisphere is damaged, most aspects of visual-spatial and perceptual functioning can become altered, including nonlingusitic memory. For example, right temporal lobe damage impairs memory for abstract designs, tonal melodies, objects, positions, and visual mazes (Kimura, 1963; Milner, 1968; Nunn et al., 1999). Deficits in left sided attention, the ability to make judgments which involve visual-figural relationships, in detecting hidden, embedded, and overlapping nonsense figures, recognizing or recalling recurring shapes, and disturbances in the capacity to perceive spatial wholes and achieving visual closure can result (Bartolomeo et al. 2014; Benton, 2013; Binder et al. 1992; DeRenzi, 1982; DeRenzi et al., 1969; Ettlinger, 2000; Gardner, 1975; Kimura, 1963, 1966, 1969; Landis et al., 1986; Lansdell, 1968; 1970; Levy, 1974).
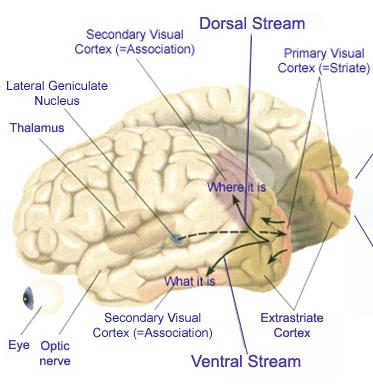
In some instances, the deficit can be quite subtle and circumscribed. For example, one patients only complaint (3 months after he suffered a circumscribed blunt head injury that resulted in a subdural hemotoma over the right posterior temporal-parietal area) was that his golf game had deterioarted significantly and he was no longer as accurate when throwing wads of paper into the trash can in his office. Formal testing also indicated mild constructional and manipulo-spatial disturbances, with most other capacities in the high average to superior range.
FACIAL-EMOTIONAL RECOGNITION AND PROSOPAGNOSIA
Possibly due in part to the visual-spatial complexity as well as the social-emotional significance of the human face, the right hemisphere has been shown to be dominant in the perception and recognition of familiar and unfamiliar faces and to become more greatly activated when viewing faces as measured by positron emission tomography and regional cerebral blow flow (Sergent, et al. 1992).
There is some indication, however, that the left hemisphere is involved in the recognition of famous faces (Marzi & Berlucchi, 1977; Rizzolatti, et al. 2011) and the differentiation of highly similar faces (presented in outline form) when analysis of fine detail is necessitated (Patterson & Bradshaw, 1975).
Be it the face of a friend or that of a stranger, the right hemisphere superiority for facial recognition is augmented by the additional display of facial emotion (Ley & Bryden, 1979; Suberi & McKeever, 1977). Indeed, not only is it predominant in perceiving facial emotion, regardless of the emotion conveyed (Buchtel et al. 1978; Dekosky et al. 1990; Landis et al. 1979; Strauss & Moscovitch, 1981; Suberi & McKeever, 1977) faces also are judged to be more intensely emotional when viewed exclusively by the right hemisphere (Heller & Levy, 1981).
Conversely, with right (but not left) cerebral injuries, patients tend to demonstrate an overall impairment in recalling, imaging, identifying and visualizing facial emotional expressions (Bowers et al. 1991; Young et al. 1995), and with right temporal atrophy, patients may suffer a progressive prosopagnosia (Evans et al. 1995).
In addition the left side of the face has been found to be more emotionally expressive (Campbell 1978; Chaurasis & Goswami 1975; Moreno et al. 2016; Sackheim et al. 1978) and to be perceived as more intensely emotional as well (Borod & Caron 1980; Sackheim & Gur 1978). In response to emotional stimuli, the left half of the face becomes more activated and a significant majority of individuals respond with conjugate lateral eye movements to the left (Schwartz et al. 1975; Tucker 1981); the left half of the body being under the control of the right hemisphere.
Conversely, damage to the right (but not left) hemisphere significantly reduces facial emotional expressiveness (Blonder et al. 2013).
In fact, the right hemisphere superiority for facial recognition is augmented by the additional display of facial emotion (Ley & Bryden 1979; Moreno, et al. 2010; Suberi & McKeever 1977), regardless of the emotion conveyed (Buchtel et al. 1978; Dekosky et al. 1980; Landis et al. 1979; Strauss & Moscovitch 1981; Suberi & McKeever, 1977). Faces also are judged to be more intensely emotional when viewed exclusively by the right hemisphere (Heller & Levy 1981) and the right hemisphere is dominant in regard to memory for facial expression as well (Weddell, 1989; reviewed in Bradshaw & Mattingly, 1997). Hence, the right half of the cerebrum is dominate for visual, facial, and auditory modes of emotional expression and perception including memory for faces and facial emotion.
Conversely, when the right hemisphere is damaged, particularly the occipital-temporal region, there can result a severe disturbance not just in the capacity to perceive facial emotion, but in the ability to recognize the faces of friends, loved ones, or pets (DeRenzi, 1986; DeRenzi et al., 1968; DeRenzi & Spinnler, 1966; Evans et al. 1995; Hecaen & Angelergues, 1962; Landis et al., 1986; Levine, 1978; Whiteley & Warrington, 1977; Young et al. 1995); i.e. prosopagnosia. Some patients may be unable to recognize their own face in the mirror.
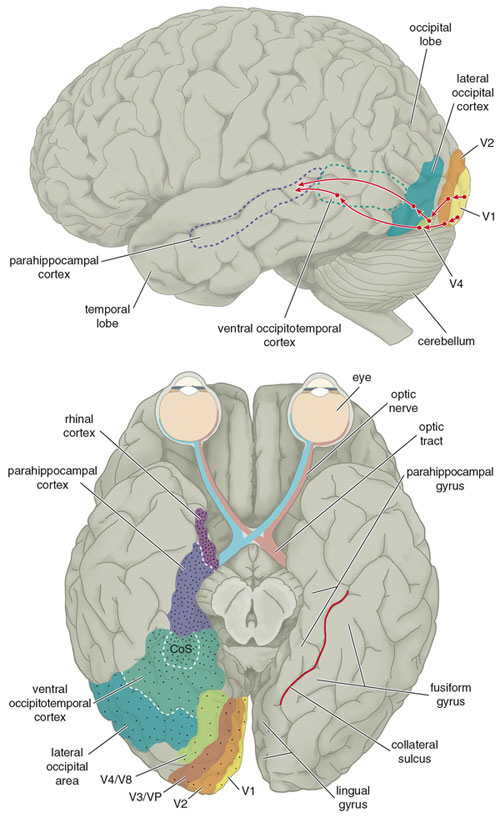
DELUSIONS & FACIAL RECOGNITION
As noted above, lesions to the right cerebrum may result in difficulty recognizing, distinguishing or differentiating facial emotion (Bowers et al. 1991; DeKosky et al. 1980; Evans et al. 1995). That is, patients are unable to recognize or determine what others are feeling via facial expression.
Electrical stimulation of the posterior portion of the right middle temporal gyrus also results in an inability to correctly label the emotion shown in faces, whereas posterior right temporal stimulation disrupts visual-spatial memory for faces in general (Fried et al., 1982). Hence, the right hemisphere is clearly dominant for perceiving, recognizing, differentiating, expressing, and even recalling facial emotion.
In some instances, depending on the extent of damage, rather than a frank failure to recognize, patients may notice that friends, lovers, or their children, look different, strange, or unfamiliar --perceptions which may give rise to a host of abnormal emotional reactions and upheavals including frank paranoia, for example, fear that one's wife may have been replaced by an imposter.
Delusional misperception of familiar and unfamiliar individuals, as well as disturbances such as Capgras syndrome (delusional doubles; reduplication) or false identification, also can result from right hemisphere and/or (bilateral) frontal damage (Alexander et al. 1979; Benson et al. 1976; Hecaen, 1964; Jacocic & Staton 2013). For example, one patient who was looking into a dark tachistoscope suddenly said in an emotional voice, "I see my daughter --oh, she 's gone" and was unable to recognize ward personel or relatives when present (Levine, 1978).
AGNOSIA AND TEMPORAL LOBE FUNCTIONAL LATERALITY
Abnormalities affecting the middle temporal lobe (area 37) can result in agnosic disturbances (Giannokapoulos et al., 1999). Patients have difficulty correctly naming and identifying visual percepts. However, depending on the laterality and location of the injury, e.g., right vs left inferior/medial vs superior temporal lobe, patients may display category specific agnosias. For example, a 27-year old man I examined who had sustained a massive right inferior-posterior temporal lobe injury that also involved surgical removal, was able to recognize and name pictures of tools--or at least demonstrate their use (he had been a carpenter) but could not recognize or correctly name pictures of animals and he could not correctly remember facial stimuli that he had been shown five minutes earlier and could not differentiate them from faces he had not seen. By contrast, a 43 year old woman who had been a waitress, and had developed a left inferior temporal lobe glioma that required surgical removal (coupled with chemotherapy) was able to recognize and name pictures of animals and could remember different pictures of faces, but had considerable difficulty recognizing and naming common household objects.
As noted, a 47-year old woman who was suffering from a right superior temporal lobe, was able to name pictures of animals, tools and household objects but was almost completely unable to recognize and correctly name animal and humans sounds. However, she was better able to recognize non-living sounds such as a creaking door, or a hammer hammering--though these abilities were also compromised.
These findings, which require independent confirmation, raises the possibility that the right temporal lobe is specialized for perceiving faces and living creatures (and the sounds they make) whereas the left is somewhat more adept at perceiving and naming non-living things, such as tools and household objects.
"POSITIVE" EMOTIONS & THE LEFT HALF OF THE BRAIN
Almost all other studies demonstrate increased right frontal activity in response to positive (Teasdale, et al., 1999) and negative stimuli or mental imagery (Rauch et al., 1996; Shin et al., 1997, 1999; Teasdale et al., 1999; however, see Mayberg et al., 1999 for contrary results). For example, in a recent functional MRI study (Teasdale et al., 1999), increases in right frontal (and right cingulate) activity was demonstrated when subjects were shown pairs of pictures and captions evoking negative feelings or positive feelings; a finding which is consistent with most all other reports which indicate that the right hemisphere is dominant for almost all aspects of emotion whereas the left hemisphere is less well endowed in this regard.
There is some (albeit, controversial) evidence that the left hemisphere processes positive emotions, and is likely to view negative and neutral emotions as positive (Davidson, 1984; Davidson et al. 1985, 2016; Dimond & Farrington, 1977; Dimond, et al, 1976; Gainotti, 1972; Lee, et al. 2009, 2013; Ostrove et al. 2010; Otto et al. 2016; Rossi & Rosadini, 1967; Sackeim, et al. 1982; Schiff & Lamon, 1989; Silberman & Weingartner 1986; Terzian,1964). However, this appears to be a bias rather than a perceptual capability and in fact appears to be based on an inability to correctly perceive emotion. For example, when right cerebral influences are eliminated, such as due to right hemisphere damage or anesthetization, many patients are likely to view and report neutral and even negative events in a positive manner and to exhibit a positive mood including laughter (Gainotti, 1972; Lee et al. 2010; Rossi & Rosadini, 1967; Sackeim et al. 1982; Terzian 1964).
Rossi and Rosadini (1967), for example, injected sodium amytal into the left vs right hemisphere and found that in 68% of those with left hemisphere inactivation reacted with depression (expressed presumably by the awake right hemisphere). In contrast, 84% of those with right sided inactivation responded with euphoria (expressed presumably by the completely awake left hemisphere) and 16% responded in a depressed fashion (see also Gainotti, 1972; Lee et al. 2010; Rossi & Rosadini, 1967; Terzian 1964). These differences are not always observed, however. Indeed, I have observed approximately a dozen sodium amytal tests and never witnessed these changes in affect.
Nevertheless, when the left anterior region of the brain has been damaged or is dysfunctional, individuals are likely to respond with severe depression, or anger, irritability, and paranoia (Gainotti, 1972; Gruzelier & Manchanda, 1982; Hillbom, 1960; Joseph, 1999a; Lebrun, 2016; Robinson & Benson, 1981; Robinson & Szetela, 1981; Sherwin et al. 1982; Sinyour et al., 1986).
This suggests that when left cerebral ("positive") influences are negated, positive emotions are replaced by "negative" feeling states which in turn are a consequence of right cerebral emotional dominance; i.e. the right half of the brain is accurately perceiving the consequences of the injury and is understandably upset and depressed. In fact, with right prefrontal transcranial stimulation, patients report a significant reduction in depression (Kelin et al., 1999). However, in some cases those with massive left posterior and temporal lobe damage may respond with euphoria which in turn may be due to subsequent emotional disorganization in response to loss of comprehension. That is, they become euphoric as they no longer comprehend.
Therefore, although the left plays a minor role in regard to emotions, there is evidence to suggest that it is inclined to view or express emotional material in either a neutral or positive light, regardless of its actual affective value. This may also explain why following massive right frontal injuries (at least in males), "positive" emotions are sometimes expressed indiscriminately and inappropriately . Indeed, affected patients may appear to be in a manic state (see below).
DISTURBANCES OF EMOTION AND PERSONALITY
OVERVIEW
The right cerebral hemisphere appears to be dominant in regard to most aspects of somesthesis, including the maintanance of the body-image, visual-spatial-geometric analysis, facial expression and perception, and musical and paralinguistic, melodic-intonational processing. The right hemisphere also predominates in regard to almost all aspects of emotional functioning and exerts bilateral influences on autonomic nervous system functioning. Moreover, norepinephrine (NE) concentrations are higher in the right thalamus (Oke et al. 1978), whereas conversely, damage to the right hemisphere disrupts NE levels on both sides of the brain, whereas similar damage to the left hemisphere only effects local NE levels (Robinson 1979). This is highly significant given the role and importance of NE in emotional and arousal.
Hence, when the right cerebrum is damaged there can result a myriad of peculiar disturbances that involve a number of modalities. Patients with body-image disturbances may seem emotionally abnormal and possibly hysterical rather than neurologically impaired. Those with facial agnosia may become paranoid and convinced that friends or lovers have been replaced by imposters. Individuals with intonational-melodic and emotional-linguistic deficiences may be unable to adequately vocally express their feelings, fail to recognize or misinterpret the feelings conveyed by others, as well as "miss the point" or fail to recognize discrepancies in speech, such as when presented with implausible information. Conversely, their own speech patterns and behavior may become abnormal, tangential, disinhibited, and contaminated by implausible, confabulatory, and delusional ideation.
Hence, in all instances, regardless of where within the right hemisphere damage occurs, social-emotional abnormalities may result. Indeed, emotional disturbances may be the dominant or only manifestation of a patient's illness. Unfortunately, if not accompanied by gross neurological signs, the possiblity of right hemisphere damage may be overlooked.
MANIA AND EMOTIONAL INCONTINENCE
In December of 1974, associate Supreme Court Justice William O. Douglas suffered a massive infarct in the right cerebral hemisphere that left him paralyzed and in pain for many years. As reviewed by Gardner et al., (1983): "For all public purposes, Douglas acted as if he were fine, as if he could soon assume full work on the Court. He insisted on checking himself out of the hospital where he was receiving rehabilitation and then refused to return. He responded to seriously phrased queries about his condition with off handed quips: Walking has very little to do with the work of the Court; If George Blanda can play, why not me? He insisted in a press release that his left arm had been injured in a fall, thereby baldly denying the neurological cause of his paralysis. Occasionally, he acted in a paranoid fashion, claiming, for example, that the Chief Justice's quarters were his and that he was the Chief Justice. During sessions of the Court, he asked irrelevant questions, and sometimes rambled on. Finally, after considerable pressure, Douglas did resign. But the Justice refused to accept that he was no longer a member of the Court. He came back, buzzed for his clerks and tried to inject himself into the flow of business. He took aggressive steps to assign cases to himself, asked to participate in, author, and even publish separately his own opinions, and he requested that a tenth seat be placed at the Justices' bench" (p. 170).
In short this formerly highly impressive and dignified man acted for a long time period after his stroke in a highly unusual and bizzare manner.
Since the right hemisphere is dominant in the perception and expression of facial, somesthetic and auditory emotionality, damage to this half of the brain can result in a variety of affective and social-emotional abnormalities including indifference, lability, hysteria, florid manic excitement, pressured speech, ideas of reference, bizzare confabulatory responding, childishness, irritability, euphoria, impulsivity, promiscuity and abnormal sexual behavior . For example, seven of 10 patients with sexual seizures described by Remillard, et al. (1983) had right hemisphere foci. Similar findings were reported by Freemon and Nevis (1969), Penfield and Rasmussen (1950), and Spencer et al. (1983).
For example, M. Cohen and Niska (1980) report an individual with a subarachnoid hemorrhage and right temporal hematoma who developed an irritable mood; shortened sleep time; loud, grandiose, tangential speech; flight of ideas; and lability and who engaged in the buying of expensive commodities. Similarly, Oppler (1950) documented an individual with a good premorbid history who began to deteriorate over many years' time. Eventually, the patient developed flight of ideas, emotional elation, increased activity, hypomanic behavior, lability, extreme fearfulness, distractability, jocularity, and argumentativeness. The patient was also overly talkative and produced a great deal of tangential-circumstantial ideation with fears of persecution and delusions. Eventually a tumor was discovered (which weighed over 74 grams) and removed from the right frontal-parietal area.
Similarly, Spreen et al., (1965) describe a 65 year old man who following a right sided stroke (with left hemiparesis), developed extremely unpredictable behavior and lability. Regardless of external circumstances he would begin crying at one moment and at the next demonstrate irritability, happiness, or extreme depression. Secondary mania also has been reported with right frontal encephalopathy accompanied by biolectric epileptiform activity (Jack et al. 1983).
Over the course of the last 20 years, I have examined two dozen patients who who developed manic like symptoms after suffering a right frontal stroke or trauma to the right hemisphere (some of whom are described in Joseph, 1986a, 1988a); the majority of whom were males. All but three of the males had good premorbid histories and had worked steadily at the same job for over 3-5 years. Upon recovering from their injuries, all developed delusions of grandeur, pressured speech, flight of ideas, decreased need for sleep, indiscriminant financial activity, extreme emotional lability, and increased libido.
One patient, formerly very reserved, quiet, conservative, and dignified with more than 20 patents to his name, and who had been married to the same woman for over 25 years, began patronizing up to 4 different prostitutes a day and continued this activity for months. He left his job, began thinking up and attempting to act upon extravagant, grandiose schemes, and camped out at Disneyland and attempted to convince personnel there to finance his ideas for developing an amusement park on top of a mountain. At night he frequently had dreams in which either John F. or Robert Kennedy would appear and offer him advice --and he was a Republican!
LATERALIZED MEMORY FUNCTIONING
Although a variety of neurochemical and neuroanatomical regions are involved in the formulation of memory (Brewer et al., 1998; Gloor, 1997; Graff-Radford et al.2010; Halgren, 1992; Murray, 1992; Rolls, 1992; Sarter & Markovitch, 1985; Squire, 1992; Wagner et al., 1998; Victor et al 1989), functional specialization greatly determines what type of material can be memorized or even recognized by each half of the cerebrum. This is because the code or form in which a stimulus is represented in the brain and memory is largely determined by the manner in which it is processed and the transformations that take place. Because the right and left cerebral hemispheres differentially process information, the manner in which this information is represented also will be lateralized (Bradshaw & Mattingly, 1997). Hence, some types of information only can be processed or stored by the right vs. the left cerebrum.
For example, it is well known that the left hemisphere is responsible for the encoding and recall of verbal memories, whereas the right cerebrum is dominant in regard to visual-spatial, non-verbal, and emotional memory functioning (Barr, et al. 2017; Brewer et al., 1998; Fried et al. 1982; Frisk & Milner 1970; Hecaen & Albert, 1978; Kimura, 1963. Levy, 1983; Milner, 1962, 1968; Nunn et al., 1999; Sperry et al., 1979; Squire, 1992; Suberi & McKever, 1977; Wechsler, 1973; Whitehouse, 1981). If the left temporal lobe were destroyed, verbal memory functioning would become impaired since the right cerebrum does not readily store this type of information. Conversely, the left has great difficulty storing or remembering nonlinguistic, visual, spatial, and emotional information.
Specifically, left temporal lobectomy, seizures or lesions involving the inferior temporal areas can moderately disrupt immediate and severely impair delayed memory for verbal passages, and the recall of verbal paired-associates, consonant trigrams, word lists, number sequences, and conversations (Barr et al. 2010; Delaney et al. 1980; Kapur et al. 1992; Meyer & Yates,1955; Milner 1968; Milner & Teuber 1968; Samson & Zatorre, 1992; Weingartner 1968). Similarly, severe anterograde and retrograde memory loss for verbal material has been noted when the left anterior and posterior temporal regions (respectively) are electrically stimulated (Ojemann et al. 1968, 1971), lobectomized or injured (Barr et al. 2010; Kapur et al. 1992).
In contrast, right temporal lesions or lobectomy significantly impairs recognition memory for tactile and recurring visual stimuli such as faces and meaningless designs, memory for object position and orientation, and visual-pictorial stimuli, and short-term memory for melodies (Corkin 1965; Delaney et al. 1980; Kimura 1963; Milner 1968; Nunn et al., 1999; Ploner et al., 1999; Samson & Zatorre, 1988, 1992; Taylor 1979). Similarly, memory for emotional material is also significantly impaired with right vs left cerebral lesions (Cimino et al. 1991; Wechsler 1973) including the ability to recall or recognize emotional faces (DeKosky, et al. 1980; Fried et al. 1982; Weddell, 1989). Individuals with right hemisphere damage also have more difficulty recalling personal emotional memories (Cimino et al. 1991).
Hence, it is the left hemisphere which is responsible for the encoding and recall of verbal, temporal-sequential, and language related memories, whereas the right cerebrum is dominant in regard to visual-spatial, non-verbal, and social emotional memory functioning. Each hemisphere stores the type of material that it is best at recognizing, processing, and expressing.

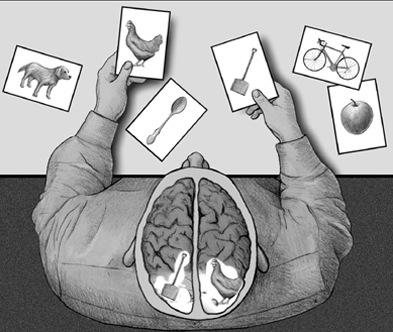
UNILATERAL MEMORY STORAGE
In the intact, normal brain, even non-emotional memory traces appear to be stored unilaterally rather than laid down in both hemispheres (Bures & Buresova 1960; Doty & Overman 1977; Hasegawa et al., 1998; Kucharski et al. 2010; Levy, 1974; Risse & Gazzaniga, 1979). Moreover when one hemisphere learns, has certain experiences, and/or stores information in memory, this information is not always available to the opposing hemisphere; one hemisphere cannot always gain access to memories stored in the other half of the brain (Bures & Buresova 1960; Doty & Overman 1977; Hasegawa et al., 1998; Joseph, 1986b, 1988ab, 1992b; Kucharski et al. 2010; Levy, 1974; Risse & Gazzaniga 1979).
To gain access to these lateralized memories, one hemisphere has to activate the memory banks of the other brain half via the corpus callosum (Doty & Overman, 1977; Hasegawa et al., 1998) or anterior commissure (Kucharski et al. 2010). This has been demonstrated experimentally in primates. For example, after one hemisphere had been trained to perform a certain task, although either hemisphere could respond correctly once it was learned, when the commissures were subsequently cut, only the hemisphere that originally was trained was able to perform--i.e., could recall it. The untrained hemisphere acted as though it never had been exposed to the task; its ability to retrieve the original memories was now abolished (Doty & Overman, 1977; see also Hasegawa et al., 1998).
In a conceptually similar study, Risse and Gazzaniga (1979) injected sodium amytal into the left carotid arteries of intact patients so as to anesthetize the left cerebral hemisphere. After the left cerebrum was inactivated, the awake right hemisphere, although unable to speak, was still able to follow and behaviorally respond to commands, e.g., palpating an object with the left hand.
Once the left hemisphere had recovered from the drug, as determined by the return of speech and motor functioning, none of the eight patients studied was able to verbally recall what objects had been palpated with the left hand, "even after considerable probing." Although encouraged to guess most patients refused to try and insisted that they did not remember anything. However, when offered multiple choices in full field, most patients immediately raised the left hand and pointed to the correct object!
According to Risse and Gazzaniga (1979), although the memory of touching and palpating the object was not accessible to the verbal (left hemisphere) memory system, it was encoded in a nonverbal form within the right hemisphere and was unavailable to the left hemisphere when normal function returned. The left (speaking) hemisphere was unable to gain access to information and memories stored within the right half of the brain. Nevertheless, the right brain not only remembered, but was able to act on its memories.
This indicates that when exchange and transfer is not possible, is in some manner inhibited, or if for any reason the two halves of the brain become functionally disconnected and are unable to share information, the possibility of information transfer at a later time is precluded (Bures & Buresova, 1960; Hasegawa et al., 1998; Kucharski et al. 2010; Risse & Gazzaniga, 1979) -even when the ability to transfer is acquired or restored. The information is lost to the opposite half of the cerebrum.
Moreover, because some types of information are processed by the right and left hemisphere in a wholly different fashion, they are unable to completely share or gain access to the data or even the conclusions reached by the other -as they are unable to process or recognize it -which in turn precludes complete interhemispheric transfer (Berlucchi & Rizzolatti, 1968; Hicks, 1974; Joseph, 1982, 1988a; Marzi, 1986; Merriam & Gardner, 2016; Miller, 1991; Myers, 1959, 1962; Rizzolatti et al. 1971; Taylor & Heilman 1980); information is lost during the transfer process.
Nevertheless, although inaccessible or lost, these memories, details, and attached feelings can continue to influence whole brain functioning in subtle as well as in profound ways. That is, one hemisphere may experience and store certain information in memory and at a later time in response to certain situations act on those memories, much to the surprise, perplexity, or chagrin of the other half of the brain; one hemisphere cannot always gain access to memories stored in the other half of the brain.
Dreaming.
Of course, complete functional deactivation is probably quite rare in the normal brain. However, there is some evidence to suggest that interhemispheric communication is reduced, for example, during sleep and possibly during dreaming (Banquet, 1983; Joseph, 1988a).
Most dreaming occurs during REM, which possibly is associated with right hemisphere activation and low-level left hemisphere arousal (Goldstein et al. 1972; Hodoba, 1986; Meyer et al. 2016). It also becomes progressively more difficult to recall one's dreams as one spends time in or awakens during NonREM (Wolpert & Trosman, 1958), which is associated with high left hemisphere and low right brain activation (Goldstein et al. 1972). Thus are dreams really forgotten, or are they locked away in a code which is not accessible to the speaking left hemisphere?
DREAMING AND HEMISPHERIC OSCILLATION
Although up to five stages of sleep have been identified in humans, for our purposes we will be concerned only with two distinct sleep states. These are the REM (rapid eye movement) and non-REM (N-REM) periods. N-REM occurs during a stage referred to as "slow-wave" or synchronized sleep. In contrast, REM occurs during a sleep stage referred to as "paradoxical sleep." It is called paradoxical, for electrophysiologically the brain seems quite active and alert, similar to its condition during waking. However, the body musculature is paralyzed, and the ability to perceive outside sensory events is greatly attenuated (reviewed in Hobson et al. 1986; Steriade & McCarley 2010; Vertes 2010).
Most individuals awakened during REM report dream activity approximately 80% of the time. When awakened during the N-REM period, dreams are reported approximately 20% of the time (Foulkes, 1962; Goodenough et al. 1959; Monroe et al. 1965) However, the type of dreaming that occurs during REM vs. N-REM is quite different. For example, N-REM dreams (when they occur) are often quite similar to thinking and speech (i.e. lingusitic thought), such that a kind-of rambling verbal monologue is experienced in the absence of imagery (Foulkes 1962; Monroe et al. 1965) It is also during N-REM in which an individual is most likely to talk in his or her sleep (Kamiya, 1961). In contrast, REM dreams involve a considerable degree of visual imagery, emotion, and tend to be distorted and implausible to various degrees (Foulkes, 1962; Monroe et al. 1965).
REM is characterized by high levels of activity within the brainstem, occipital lobe, and other nuclei (Hobson, et al. 1986; Steriade & McCarley 2010; Vertes 2010) It also has been reported that electrophysiologically the right hemisphere becomes highly active during REM, whereas, conversely, the left brain becomes more active during N-REM (Goldstein et al. 1972; Hodoba, 1986). Similarly, measurements of cerebral blood flow have shown an increase in the right temporal and parietal regions during REM sleep and in subjects who upon wakening report visual, hypnagogic, hallucinatory and auditory dreaming (Meyer et al., 2016).
Interestingly, abnormal and enhanced activity in the right temporal and temporal-occipital area acts to increase dreaming and REM sleep for an atypically long time period. Similarly, REM sleep increases activity in this same region much more than in the left hemisphere (Hodoba, 1986), which indicates that there is a specific complementary relationship between REM sleep and right temporal-occipital electrophysiological activity.
At least one group of investigators, however, have failed to find significant hemispheric EEG differences between REM and NREM (Ehrlichman et al. 1985).
DAY DREAMS, NIGHT DREAMS, AND HEMISPHERIC OSCILLATION
There is some evidence to suggest that during the course of the day and night the two cerebral hemispheres oscillate in activity every 90 to 100 minutes and are 180 degrees out of phase --a cycle that corresponds to changes in cognitive efficiency, the appearance of day dreams, REM (dream sleep), and, conversely, N-REM sleep (Bertini et al. 1983; Broughton, 1982; Gordon et al. 1982; cited by Hodoba, 1986; Klein & Armitage, 1979; Kripke & Sonnenschein, 1973; Levie et al. 1983, cited by Hodoba, 1986). That is, like two pistons sliding up and down, it appears that when the right cerebrum is functionally at its peak of activity, the left hemisphere is correspondingly at its nadir.
Similarly, shifts in cognitive abilities associated with the right and left hemisphere have been found during these cyclic changes during the day and after awakenings from REM and N-REM sleep. That is, performance across a number of tasks associated with left hemisphere cognitive efficiency is maximal during N-REM, whereas, conversely, right hemisphere performance (e.g., point localization, shape identification, orientation in space) is maximal after REM awakenings (Bertini et al.,1983; Gordon et al., 1982; Levie et al., 1983; cited by Hodoba, 1986). Moreover, Bertini et al., (1983) found that left hand motor dexterity (in right handed subjects) was superior to the right when awakened during REM and that the opposite relationship was found during NREM, i.e. right hand superiority (see Hodoba, 1986, for review.)
Conversely, there have been reports of patients with right cerebral damage who have ceased dreaming altogether or to dream only in words (Humphrey & Zangwill, 1951; Kerr & Foulkes, 1978, 1981). For example, defective dreaming, deficits that involve visual imagery, and loss of hypnagogic imagery have been found in patients with focal lesions or hypoplasia of the posterior right hemisphere and abnormalities in the corpus callosum (Botez et al. 1985; Kerr & Foulkes, 1981; Murri et al. 1984).
An absence or diminished amount of dreaming during sleep also has been reported after split-brain surgery; i.e., as reported by the disconnected left hemispehere (Bogen & Bogen, 1969; Hoppe & Bogen, 1977). Similarly, a paucity of REM episodes have been noted in other callosotomy patients, although these particular individuals continued to report some dream activity (Greenwood, Wilson, & Gazzaniga, 1977).
On the other hand it has been reported that when the left hemisphere has been damaged, particularly the posterior portions (i.e. aphasic patients), the ability to verbally report and recall dreams also is greatly attenuated (Murri et al., 1984; Pena-Casanova & Roig-Rovira, 1985; Schanfald et al. 1985). Of course, aphasics have difficulty describing much of anything, let alone their dreams. Moreover, Language Axis disconnection from the right hemisphere would also account for this failure to verbally report dreams and related imagery.
In some respects, however, a parallel between these latter findings and those of Risse and Gazzaniga (1979) in their amytal studies may be explanatory regarding the failure to report dreams with left hemisphere lesions. That is, with left hemisphere damage or when it is at a low level of arousal, the ability to verbally recall or report events experienced or generated by the right hemisphere appears to be reduced; i.e., the left (speaking) half of the brain cannot remember because it cannot gain access to right cerebral memory centers.
Thus, it appears that the right hemisphere provides the physiological foundation from which dreams in part derive their source and origin (Goldstein, et al. 1972; Hodoba, 1986; Joseph, 1988a; Meyer et al.,2016). However, in some instances in which these dream centers are disconnected from the language dominant left hemisphere, due to posterior right or left hemisphere lesions or after callosotomy, the ability to recall, report, and/or to produce vivid visual and hypnogogic dream imagery is attenuated (Joseph, 1988a).
However, also important in the capacity to engage in memory search and retrieval, or to dream and fantasize, are the frontal lobes (chapter 19). Likewise, frontal lobe damage and lobotomy also have been reported to abolish dreaming (Freeman & Watts, 1942, 1943).
HALLUCINATIONS
In addition to dream production, the right hemisphere also appears to be the dominant source for complex non-linguistic hallucinations. Specifically, tumors or electrical stimulation of the right hemisphere or temporal lobe are much more likely to result in complex visual as well as musical and singing hallucinations, whereas left cerebral tumors or activation gives rise to hallucinations of words or sentences (Berrios, 2010; Halgren, et al. 1978; Hecaen & Albert, 1978; Jackson, 1880; Mullan & Penfield, 1959; Penfield & Perot, 1963; Teuber et al. 1960). Conversely, LSD induced hallucinations are significantly reduced following right but not left temporal lobe surgical removal (Serafetinides, 1965), and dreaming is sometimes abolished with right but not left temporal lobe removals (Kerr & Foulkes, 1981). In one study, however, it was reported that an alcoholic patient with a right subcortical injury and left sided neglect, experienced hallucinations only in the right (non-neglected) half of visual-space (Chamorro et al. 2010).
REFERENCES
















