Rhawn Gabriel Joseph, Ph.D.
BrainMind.com
Sexually, the human female is unique. Unlike all other (noncaptive) females, she is continuously sexually receptive and can have sexual intercourse at any time, morning, noon, or night--so long as she doesn't have a headache. Whereas other females of the animal kingdom have sex almost exclusively when in estrus or "heat" the human female can have sexual relations 24 hours a day, 365 days a year.
In other respects, however, her sexual behavior is similar to other primates and mammals. Like other female animals who enter estrus (or "heat") the human female, (depending on cultural restraints) sometimes flaunts and aggressively advertises her sexual availability; young women in particular, via makeup, short-skirts, high heels, lipstick, perfume, pushup bras emphasizing enlarged artificial breasts, and other behaviors designed to sexually arouse and harass males. And like other social primates, she is capable of experiencing multiple orgasms and enjoying multiple sex partners, one after another.




(Left) bonobo in full estrus (Right) Human in full estrus
A female in heat, behaves like a female in heat regardless of species, simply because biologically sexuality serves a single purpose: to attract numerous male sex partners and to breed. Moreover, be it female cat, dog, wolf, or chimpanzee, all possess basically identical brain structures which mediate sexuality, the limbic system.
Moreover, pre-human species continued to have an estrus, and thus, behaved little different from other apes--the chimpanzee in particular, for millions of year after apes and pre-humans diverged, and for almost 2 million years after the first Australopithecus and Homo habilis stood blinking upward into the moonlight. Full time female sexuality, and enlarged breasts and buttocks to signal her available, did not become the norm until 700,000 to 500,000 years ago.


FROM HOMINOID TO HOMINID CHIMPANZEES, AUSTRALOPITHECUS AND H. HABILIS
The human female shares numerous sexual, cognitive, emotional, and behavioral characteristics with other species, the chimpanzee in particular (Bygott,1979; de Waal, 1989; Goodall, 1986, 1990; Itani & Suzuki, 1967; McGrew, 1981; Tanner, 1981, 1987; Stanford, 1998).
For example, although there is considerable variation, the sexual behavior of the female human and the female chimpanzee increases at the time of ovulation; that is, at midcycle; though in humans there is a second peak just before and after menstruation. Moreover, human and group living (multi-male/female) hominoid females are capable of experiencing multiple orgasms and commonly (though there are exceptions) have sex with multiple partners. Multiple sex partners ensures she will become pregnant, and multiple orgasms reward her with increasing pleasure as she sexually dallies with male after male.
It is this ancestral hominoid heritage which explains not only her capacity for multiple orgasms, but the fact that the human female's "monthly rhythm of ovogenesis, ovulation, estrogen and progesterone secretion, uterine stimulation, and menstrual bleeding follows the basic primate pattern" (Beach, 1974, p. 356). Because they share common ancestors and a limbic system organized in a "female" pattern, female chimps and humans are sexually similar.
The human female sometimes behaves like, and biologically, in many respects is similar to a female chimpanzee in heat. This is not only because we share a common ancestor, but because our most distant "human" ancestors were essentially apes.
And, being more ape-like than human, it can also be inferred that these first pre-human females were extremely sexually promiscuous, and probably had sex up to fifty times a day when they entered estrus. Like other social apes, our pre-human female ancestors probably had sex with every desirable and high status male of the troop, and she likely snuck off to an adjacent troop where she would then enjoy a romantic vacation by again having sex up to fifty times a day with every desirable and high status male-- a common behavior of female chimps.
THE HOMINOID TO HOMINID TRANSITION
It was perhaps as recently as 5 million years ago that chimps and humans diverged from a common ancestor (Sibley & Alhquist, 1984; Takahata et al., 1995) with the first pre-humans, Ardipithecus ramidus ("ground ape"), and Australopithecus, emerging soon thereafter. However, these were still basically apes. For example, Ardipithecus ramidus (the remains of which are 4.4 million years old) Australopithecus anamesis (who emerged 4 million years B.P.), A. aferensis (3.6 million B.P.), A. africanus (3 million years B.P.), A. garhi (2.5 million B.P.), and early Homo habilis (2.2 million B.P), possessed forelimbs or hind limbs which were more ape-like than human-like (McHenry & Berger, 1998; White et al., 1994. 1999; Wood, 1994). Australopithecus was especially ape-like in regard to head and brain size (Conroy, 1998). Also ape-like were their small semi-circular canals (inner ear), robust body build, conical chest, and curved feet. Australopithecus, like chimpanzees, climbed and probably nested in trees (Fleagle, 2008; Stern & Susman, 1983; Wood, 1994).
Australopithecus
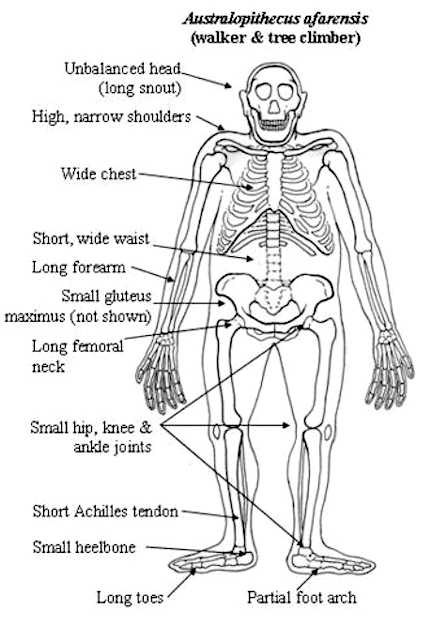

Although Australopithecus (aferensis/africanus) had acquired the ability to walk on two legs and was well on the way to becoming human, this pre-human was built like a chimpanzee, and retained chimp-like capabilities and limitations, including an ape-like maturational growth rate (Beynon & Dean, 1988). Similar ape-like traits were characteristic of early H. habilis (McHenry and Berger, 1998).
Sexually, these early hominids probably behaved no different from group living apes, such as chimpanzees. The social life of Australopithecus and Homo habilis was probably more ape-like than human-like.
That our early ancestors, Australopithecus and early H. habilis, behaved and acted in a manner similar to chimpanzees can also be inferred based on present-day human DNA which is almost identical to chimpanzee DNA.
Chimpanzees and modern humans display a 99% homologous sequence identity in nucleotide base pair sequence organization, and 98.4% of activated/coded human and chimpanzee DNA is identical (Goodman et al., 1990). There are very few genes in the chimp genome whose counterparts cannot be found often in the same exact location in the human genome, though it is also apparent that chromosomes 4, 9, and 12 are configured somewhat differently. Those few genes that have been inverted include AF4, which sits on chromosome 4 and which codes for a transcription factor related to leukemia in humans.
By contrast, those which have disappeared from the human genome include a few silent "satellite" introns (non-coded genes) which, in the chimp genome, are adjacent to the telomere. The telomere is a structure which caps the chromosome and regulates gene expression. These chimpanzee introns appear to have shifted to a new position within the human chromosome, which is a common behavior of episoms, transposons, and plasmids. In so doing, they likely became exons and thus activated, and in so doing possibly promoting the transition from hominoid to hominid (Joseph, 2000, 2010).
Given these close genetic similarities, it can thus be inferred that genetically Australopithecus and H. habilis were probably almost DNA- identical to chimps.
Therefore, given these genetic, physical, and other similarities, and the fact that early H. habilis also possessed ape-like characteristics, the modern chimpanzee is thus an excellent evolutionary model for early hominid behavior (Joseph, 2000b, McGrew, 1981; Tanner, 1987), including female sexuality (Symons 1979). In fact, "humans" remained basically ape-like until around 2 million years B.P., and then became increasingly human-like with the emergence of Homo erectus.
AUSTRALOPITHECUS/H. HABILIS SOCIAL-SEXUAL BEHAVIOR
Like modern day chimpanzees,Australopithecus and H. habilis probably lived in troops of up to 50 individuals, and the males may have controlled and patrolled large territories dozens of miles in diameter.
Like chimpanzees, it can also be assumed they employed elaborate vocalizations and facial, hand, arm, and body gestures to communicate, and developed long lasting sibling and mother-infant relationships, and used a variety of strategies for achieving dominance and forming coalitions.



Australopithecines and H. habilis probably greeted their own kind with hugs, pats, and kisses, would hold and shake hands, engage in long periods of mutual grooming, seek reassurance by embracing, and were probably willing to risk their lives to help family members who were in distress or danger.
As is characteristic of chimpanzees (e.g., de Waal, 1989; Goodall, 1986, 1990), female Australopithecus/H. habilis likely spent considerable time socializing with kin and engaged in prolonged child care with mother-son and especially mother-daughter bonds lasting a lifetime. Incessant mutual vocalizing and prolonged daily food gathering activities were probably characteristic, especially between mothers and daughters.
By contrast male Australopithecines and H. habilis were probably more semi-independent, though like chimps, they likely formed coalitions, as well as hunting or raiding parties in which they would kill other animals or hominids from adjacent troops; and, on occasion, each other.




According to Dart (1949), Australopithecines "were confirmed killers; carnivorous creatures that seized others by violence, battered them to death, tore apart their broken bodies, dismembered them limb from limb, and slaking their ravenous thirst with the hot blood of the pitiful victims and greedily devouring their writhing flesh."
Dart's conclusions have since been well established. Male chimps not uncommonly engage in violent, murderous and even cannibalistic interactions with neighboring as well as other troop member. Like modern humans, gangs of male chimpanzees engage in surprise attacks on neighboring colonies, beating and killing the old, infirm, and infants alike, including former friends; even drinking their blood. That Australopithecus behaved in an identical manner, thus, should not be surprising.
Australopithecus as well as H. erectus were sexually dimorphic, with the female weighing half as much as the male. All larger sized primates are sexually dimorphic and tend to live in multi-male, multi-female groups (Fedigan, 1992), with males competing for access to estrus females who may mate with numerous males. By contrast, smaller and similar sized primates are more likely to be monogamous.
Hence, we can assume that monogamous sexual relations had not yet been established with the emergence of these pre-humans. Rather, the females were probably exceedingly promiscuous and enjoyed sex with multiple partners--conduct similar to human females in non-Muslim cultures.
Hence, as with chimps, Australopithecus/H. habilis females were probably highly promiscuous, and males may have only occasionally formed temporary consort relations so long as the female remained in estrus.
AUSTRALOPITHECUS/HOMO HABILIS FEMALE SEXUALITY
As is common among chimps, it can be assumed that early hominids may have been ruled by dominant males or male coalitions. Females and subordinate males probably would bow and grunt submissively or turn and offer their backside for mounting when confronted by dominate males.
Like other social primates, dominant Australopithecus/H. habilis males probably mounted their subordinates and engaged in simulated sexual intercourse. Similar sexual mountings to indicate dominance or submission are employed by gorillas, monkeys, dogs, and humans (Beach, 1965; Eibl-Eisbesfeldt, 1995; Ford & Beach, 1951; Wickler, 1973).

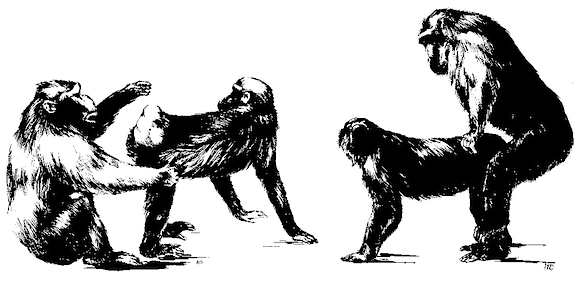
SEXUAL SURRENDER: BREASTS AND BUTTOCKS
Like her chimpanzee cousins, the first female hominids probably frequently employed sexual postures as a form of appeasement, so as to reduce male tension, anger, and aggression. When confronted by a high status, dominant male, she would "turn him on" and make him behave more friendly toward her by submitting and bending over coyly, offering her sexual favors in order to get him into a more pleasant and agreeable mood. To submit and to display mild fear and to be trembling with emotion was probably a common Paleolithic turn on.
And, not just her posterior oriented genitals but the hairless double ovoid pattern of her rump likely served as a social-sexual signal of submission. The double hairless region of the chimp buttocks has a breast- like pattern that is similar to her own breasts which become swollen when pregnant and lactating. The swollen breasts of a lactating female chimpanzee are little different from those of a modern human female.



Moreover, just as the breasts of other female primates are employed
not just for nourishing, but for soothing and comforting the young, and
sometimes older males, the female Australopithecus/H. habilis likely employed her breasts in an identical
fashion. Over two million years ago our female ancestors were relying on
the sex appeal of their swollen breasts to arouse and manipulate the males
of their community.
In fact, as these hominids were also capable of standing and walking upright, the breasts of the female Australopithecus/H. habilis may have already become increasingly hairless, thus duplicating, in a sense, the hairless and double ovoid shape of her buttocks. Coupled with the submissive and sexual significance of the buttocks (Joseph 2010), once these initial physical changes in the breast area became established this would have enabled the female to display sexually submissive and appeasement appendages which could be easily viewed regardless of her stance or posture. When bending he could see her rump, and when standing, if facing him, he could see her breasts, which soothed the savage beast.
Like the female chimp, female Australopithecus and H. habilis probably had evolved an estrus-like menstrual cycle, becoming sexually receptive for only a few weeks in between successive pregnancies. In general female chimps first enter estrus at about age 10 at which point they give off a strong sex smell and their genitals puff out and turn pink (Goodall, 1986; Wallis, 1992). Hence, like her primate counterparts, once the Australopithecus/H. habilis female entered estrus, her vaginal region probably turned pink or a bright strawberry red, and puffed out in a posterior direction, while simultaneously secreting sex-related odors.
THE MULTI-ORGASMIC SEXUALLY INSATIABLE FEMALE
As with chimpanzees, if the Australopithecus/H. habilis female was high status and sexually experienced, she probably purposefully attracted a whole retinue of suitors, some of whom probably offered her meat or prolonged grooming in exchange for sex. Modern human females commonly exchange sex in return for an expensive date or the reception of gifts.
Like other estrus primates, the Australopithecus/H. habilis female was probably capable of exercising a very limited degree of sexual choice, while simultaneously behaving in a promiscuous manner; even if guarded by a male who was supremely dominant. That is, although promiscuous, she probably avoided low status and undesirable males and instead repeatedly had sex with the more popular and dominant males.
Females, when in heat, are sexually insatiable, but not entirely indiscriminate. Due to the high sex drive of the estrus female, the females of many primate and mammalian species behave in a sexually promiscuous, though not completely indiscriminate fashion (Carpenter, 1942, 1964; Fedigan, 1992; Ford & Beach, 1951).
For example, a female chimp may mate with up to 8 different males in just a few minutes, and she may copulate up to 50 times a day for two weeks or more; though she may also solicit many of the same males (Goodall, 1986).
The estrus female of many species commonly advertise and aggressively solicit male sexual attention. As noted, among primates, the genitals may puff out, and turn pink or a bright strawberry red. Moreover, female primates typically bend over and sway their derriere enticingly to draw male sexual attention, even rubbing her genitals in his face, and she may approach and repeatedly crouch in front of male after male, frantically soliciting sex.
Hence, the Australopithecus/H. habilis female probably did likewise, and like modern (Western/Eastern) females expended considerable effort in advertising her sexuality and to attract male sexual attention. That is, when Australopithecus/H. habilis females became receptive, they probably flaunted their swollen genitals and their sexuality.
Moreover, as with chimpanzees (and modern women), these hominid females were probably biologically predisposed to seek sex with multiple partners, even risking a severe beating to do so. Females behaved promiscuously because a single Australopithecus/H. habilis male (like their chimp counterparts) was probably incapable of satisfying her sexually. Multiple male sex partners ensures she will not only become pregnant and all males will be solicitous of her young, but it provides her with multiple orgasms.
A male chimp may make 12 to 20 pelvic thrusts, and take no more than just 10 to 15 seconds to ejaculate. And, like modern human males (Masters & Johnson, 1966), he then goes into a refractory period in which he becomes drowsy and disinterested in sex (Goodall, 1986; Yerkes, 1939). Obviously, a single male cannot sexually satisfy a multi-orgasmic female who may well require several minutes of thrusting before experiencing her first of several orgasms. She is motivated to have sex repeatedly, and in the case of the female chimpanzee, she may do so 50 times a day.
MULTIPLE ORGASMS & FEMALE PROMISCUITY
The female chimpanzee, like modern human females and other primates, is capable of having sex with multiple partners, one after the other. She also appears capable of experiencing increasingly pleasurable multiple orgasms (e.g. Burton, 1971; Goldfoot et al., 1980; Michael et al., 1974); each successive orgasm rewarding her for her promiscuity. The ability to experience multiple orgasms promotes promiscuity.
Female primates which experience orgasm are not monogamous (Allen & Lemmon, 1981; Burton, 1971; Chevalier-Skolnikoff, 1974), and few males are biologically capable of providing the stimulation necessary for multiple, or even single orgasms. Males quickly become sexually unresponsive. Females, therefore, are sexually motivated to search for that next orgasm and require the sexual services of additional males.
Primate males such as the rhesus, howler, gorilla, and chimpanzee, typically become unresponsive to the aggressive sexual solicitation of the female after three or four ejaculations in a single day, and cease to respond after three or four days of sexual activity (Carpenter, 1942, 1964; Nadler, 1976; Schaller, 1964; Yerkes & Elder, 1936; Zuckerman, 1932). In these and other species, the sexual hunger of the female, and her capacity for copulation completely exceeds that of any single male.
"A single estrus female may satiate, entirely... several sexually vigorous males" (Carpenter, 1942, p. 141). Female chimps, baboons, gorillas, monkeys, and humans are capable of exhausting male and after male without showing any lessening of sexual desire (Carpenter, 1942, 1964; Goodall, 1986; Ford & Beach, 1951).
FULL TIME SEXUAL AVAILABILITY: EVOLUTION OF FEMALE BREASTS AND BUTTOCKS
The Home Base and the Domestication of the "Human" Males. The species known as Homo Erectus appeared around 1.9 million years ago, and was much taller and had a larger brain that H. habilis. However, both species (and multiple other variations of "Homo" coexisted for hundreds of thousands of years.
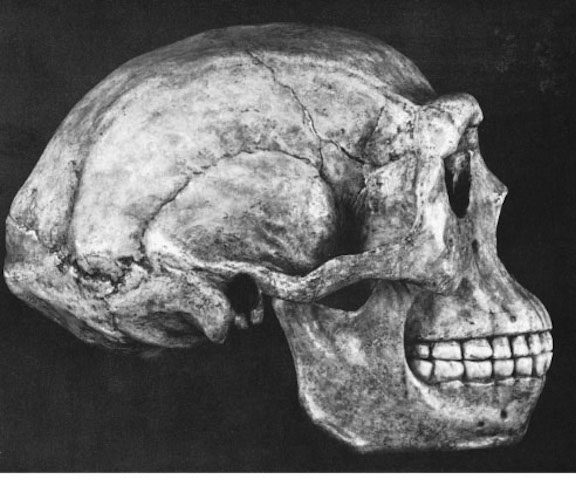

Perhaps between 700,000 to 500,000 years ago, the H. erectus female became sexually receptive at all times. Sexually, the human female is unique. Unlike all other (noncaptive)
females, she is continuously sexually receptive and can have sexual intercourse
at any time, morning, noon, or night. Whereas other females of the
animal kingdom have sex almost exclusively when in estrus or "heat" the
human female can have sexual relations 24 hours a day, 365 days a year.
The human female is the sexiest female on the planet, and she continually signals this fact as she has evolved an enlarged derriere and prominent breasts which remain swollen even when she is not pregnant, lactating, or sexually aroused. Those swollen, luscious breasts, and that perfect posterior protuberance, her buttocks, have been driving males sexually wild with sexual desire for over half a million years. Likewise, it was probably around 500,000 years ago, that the H. erectus female not only became continuously sexually receptive, but evolved swollen breasts and an enlarged buttocks, to signal her sexual availability--a characteristic of modern women today. Indeed, the larger H. erectus buttocks was not only directly related to sexual signaling, but an expansion of the pelvis and birth canal which would enable the birth of big brained babies... and evolution marches on.
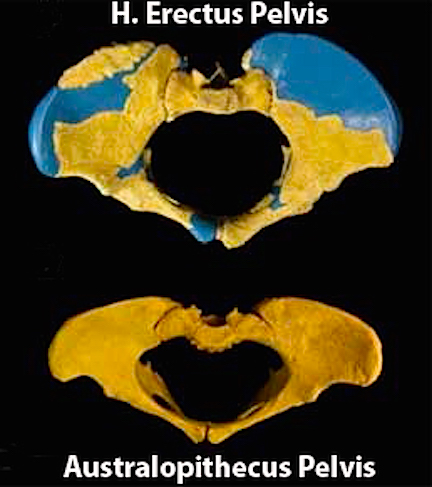


Other species of female may develop swollen breasts or genitals only when they are maximally sexually receptive, whereas for much of the year these sex organs are flat and hidden. Among other social primates, these swollen sexual allurements serve to attract sex partners, which is why the human female, being continually sexually receptive, also continually advertises her sexual condition.




In other respects, however, her sexual behavior is similar to other
primates and mammals. Like other female animals who enter estrus (or
"heat") the human female, (depending on cultural restraints) sometimes
flaunts and aggressively advertises her sexual availability; young women
in particular. And like other social primates, she is capable of experiencing
multiple orgasms and enjoying multiple sex partners, one after another.
It was probably not until around 700,000 to 500,000 years B.P., during the latter stages of Homo erectus evolution, that she evolved those characteristics which made her sexually unique, i.e. full time sexual receptivity, as well as an enlarged pelvis and permanently swollen buttocks and breasts (Joseph, 2000a, 2010).



It may have been during this time, which corresponds to the establishment of the first home bases and significant increases in brain size, that the human female developed the cognitive and creative capacity to artificially
emphasize and exaggerate her sexuality. The H. erectus female may
have begun applying natural pigments such as rouge obtained from red
ocher (as sharpened sticks of this pigment were found at one site), and later
(around 40,000 years B.P.), creating and wearing personal ornaments.
Why rouge and cosmetics? As is common among present day females, they were employed to mimic the stereotypical colorful signs of estrus in other species, and thus served to sexually signal and attract potential mates (Joseph, 2000a, 2010).



EVOLUTION OF THE BIG BRAIN - A WOMAN'S HOME IS HER CASTLE
It was around 500,000 B.P. the first hearth equipped home bases were established in China, France, Hungary and elsewhere (Clark & Harris 1985; Rightmire, 1990; Zhang, 1985). These latter developments implies that at least some groups of H. erectus may have established a rudimentary home life. For example, a home base equipped with a hearth indicates that these hunters and gatherers were returning home with food that was to be cooked and prepared as a group meal.
This great change in diet and food preparation is also evident from an examination of those evolutionary changes which occurred in the teeth, and facial and jaw muscles (Howell, 1997; Potts, 1996; Rightmire, 1990). Food that is cooked is easily chewed and digested. In consequence, large teeth, massive canines, and huge jaw muscles were soon discarded. In this regard, full time female sexual receptivity may have promoted not only the development of the nuclear family, but physical and neurological evolution.
For example, when the need for massive jaw muscles was no longer adaptive and became reduced in size, the inner skull was able to expand as there was reduced pressure for the outer skull to accommodate
bulging jaw muscles. As the size of the inner skull expanded, and due
also to the increased capacity to digest protein secondary to cooking (as
well as other factors related to gathering and tool making), the brain also
expanded. The expansion of the brain coupled with the reduction in the
jaw and improved protein digestion, in turn coincided with and promoted
the emergence of increasingly complex hominids, including the first archaic
H. sapiens, some 500,000 years ago.
Because he now had access to a continuously sexually receptive female who was not only providing him with sex, but a home cooked meal, males were also becoming increasingly "domesticated." And as males became more domesticated, and as the brain increased in size, social life and intellectual functioning became more complex as well.
FEMALE SEXUAL CHOICE
These physical, neurological, intellectual and ensuing social changes may have also been a function of female choice and her tendency to selectively mate with certain types of males. That is, as she was probably more likely to form a long-term mating relationship with a male who could be more easily domesticated, and who also demonstrated intelligence and good social skills, she would have produced male children with similar qualities.
Although promiscuous, female primates are not necessarily indiscriminate. They also display certain preferences in sex partners (Fedigan, 1992; Small, 1989; Smuts, 1987; Tutin, 1975; Yerkes, 1933). They will in fact refuse to mate with certain males by lowering their hindquarters or attaching themselves to a more dominant male (Lancaster, 1978; Smuts, 1987; Taub, 1980; Tutin, 1975). As noted by Lancaster (1978, p. 68) "fieldwork publications are filled with reports of females... refusing copulation attempts by keeping their hindquarters lowered."
Female primates prefer high status males and those who offer them meat or prolonged grooming in exchange of sex (Carpenter, 1942; Stanford, et al., 1994; Yerkes, 1933). Likewise, human females prefer high status males who offer them resources. Therefore, as is the case with human and non-human primates, the H. erectus female as well as the archaic H. sapiens female, at least those of high status, may have been able to exercise some degree of choice in mating partners (e.g. Reynolds, 1991; Small, 1989; Smuts, 1987; Taub, 1980). By exercising sexual choice, female hominids would have also significantly affected the course of human evolution.
FEMALE SEXUAL CHOICE, EVOLUTION, PENIS SIZE, MALE DOMESTICATION
Male Australopithecus and H. habilis were almost twice as large as the females. However, the size differential steadily decreased over the course of H. erectus evolution. Males became smaller and their brains became larger, which made for a more intelligent but less physically imposing sex partner.
It is likely that the decrease in physical size may have been due to sexual selection on the part of continuously receptive females who mated with males who they found more attractive in appearance and more pleasing behaviorally. As female primates tend to avoid dangerous, assaultive, belligerent, domineering, and frightening males (Fedigan, 1992; Herbert, 1968; Michael et al., 1978), and as they also demonstrate sexual preferences, they may have exerted a degree of sexual selection by selectively mating with males who were not as huge or aggressive, and which were less frightening. That is, these females may have avoided the most frightening and assaultive males, and instead formed long term relationships with and allowed themselves to be impregnated by males who were more gentle in appearance and demeanor.
For example, when provided a choice of mating partners (who are caged), primate females avoid releasing large, aggressive and domineering males, but instead select those which are less frightening, and who are more likely to groom, socialize, as well as mate with them (Herbert, 1968; Michael et al., 1978). In fact, among modern human females, there is a definite trend to prefer males whose facial characteristics are more feminine than masculine and which are thus less frightening (Perrett et al., 1998). Moreover, among Westernized human females, there is a growing propensity among females to feminize normal male behavior.
However, although the modern woman may prefer a more sensitive male as a long term companion, when they ovulate human females also tend to prefer males who are obviously more male and masculine than female in appearance. It is also when they ovulate, that human females are most likely to "cheat" on their husbands and boyfriends and to seek out sex partners including anonymous sex with handsome strangers they meet online.
Females may have also selectively mated with those males best equipped to please them and provide orgasms; such as men with a big penis. Just as males may have selectively mated with large breasted females, thus exercising not just sexual choice but sexual selection, such that swollen breasted females became the norm, females may have done likewise in regard to male penis size. Hence, female breasts got bigger and so did the male penis.


As noted, although promiscuous, female primates are not necessarily
indiscriminate as they also display certain preferences in sex partners including those who offer them resources. It has also been reported that
the majority of female chimpanzees seek to have sex most often with
those males with the longest penis, and those who make the greatest number
of thrusts per copulation, and who copulate for the longest time (Yerkes,
1933). In this regard, since the human male penis has become huge in
size; almost three times that of a chimpanzee, male penis size may also
have been "selected for" by choosy females. Indeed, like the female chimp,
the human female is aroused by the sight of the male penis (Bancroft,
1980; Friday, 1991) and the bigger the penis, the more aroused she becomes.
Therefore, since female primates can exercise sexual choice smaller less frightening males with big sex organs became the norm. In fact, given her continual sexual receptivity, and her ability to accentuate her sexuality through cosmetics (Joseph, 2000a) the H. erectus/archaic H. sapiens female was probable able to attract a whole retinue of suitors and could repeatedly chose among the many males who were offering her food, grooming, and resources, while simultaneously denying sex to those who were frightening, who sported a small penis, and who she did not find attractive (e.g. Herbert, 1968; Lancaster 1978; Michael et al., 1978; Perrett, et al., 1998; Tutin, 1975). Indeed, as is common among chimpanzees (de Wall, 1989; Goodall, 1986) she may have been able to motivate the males of her choice and those vying for her affections, to protect her from those huge, brutal, violent males who frightened her.
Therefore, given female choice and her tendency to avoid mating with those who were huge and frightening while selectively mating with those who would groom, feed, protect and provision her, and especially those males who were high status and good hunters, over the course of evolution males became smaller, more sociable, more intelligent, and less likely to dominate large groups of females through violence, terror, and aggression.
Moreover, even if forced to mate with males who were not of her liking, these females may have been able to reject the sperm and avoid becoming pregnant. That is, through sperm selection made possible by control of the vaginal and uterine muscles, the female could determine which male would have the honor of actually impregnating her.
Although this capacity has almost been lost to "modern" women due to the fact that they spend so much of their lives sitting which weakens these muscles, females nevertheless retain the potential to manipulate and reject the sperm that has been sprayed into their body (Baker & Bellis, 1995). This is accomplished through control over uterine contractions which can induce "flow back" and thus sperm ejection, vs "upsuck" which literally causes the sperm to be drawn into the uterus. Thus, although mating with numerous males, the female can exert choice at the level of sperm.
Through selective mating with high status males who were more sociable and more to her liking, those males who were twice her size were gradually replaced by more sociable males with big penises (and bigger brains), including those seeking to form (what they believed to be) exclusive sexual relations. Males were becoming domesticated.

FEMALE PROMISCUITY AND GROUP COHESION
As is characteristic of modern humans (and chimpanzees), H. erectus, or archaic H. sapiens males would have banded together to protect and guard their villages, their homes, and in particular, their sexually receptive women. And these males would have tried to prevent their females from leaving the troop or village so as to mate with the males of neighboring tribes or communities.
However, no matter how well guarded, many of their women likely continued to engage in clandestine sexual affairs (e.g. Baker et al., 2009) as is common among other female primates and modern humans. Female chimps and other species of primates go to great lengths to circumvent male mate-guarding strategies in order to have sex with the males of other communities (Gagneux et al., 2017). In fact, even if prevented from mating with foreign males, these females would have continued to have had numerous opportunities to have sex with the males of her own troop, even if in a supposed monogamous relationship.
Over the course of human social evolution, and as the number of H. erectus or archaic H. sapiens males in the tribe increased, so too would her opportunities for having sex with multiple (high status) partners. As in other primates, as the number of females and males in a group increases, the ability to prevent these females from mating with other males declines (Stacey, 1982). In consequence, as bands became tribes, this would have also provided the H. erectus/Archaic H. sapiens female with increased opportunities to mate with different, albeit high status, males, which in turn would have resulted in the birth of males with similar qualities.
FEMALE SEXUAL CHOICE, FEMALE KINSHIP & SOCIAL EVOLUTION
When a female enters estrus, males and females are stimulated to band together, the males so as to prevent foreign males from mating with their estrus females, and the females banding together so as to prevent these estrus females from mating with their males. Female sexuality arouses males and females, and promotes group cohesion.

Continuous sexual availability, coupled with female sexual choice, thus contributes to group cohesion as well as the development of group stability. That is, not only might males form permanent fighting groups to patrol and guard their estrus female containing land, but males who were not as social or friendly and who were not acceptable to the females, may have become isolated and then expelled from the troop.
As noted, sometime a young female chimp may leave her troop in order to have sex with the males of a neighboring troop. Generally she returns home to her mother once her estrus is over. Mothers, daughters, and younger siblings generally form permanent associations, whereas it is a pronounced male tendency to wonder, often completely alone. These mother-daughter associations and those of the extended female-family typically wield tremendous influence over the males of their community, and assist in determining which males become dominant over the others. Male chimps rise in power when supported by the females, whereas some males may in fact be brutalized and expelled from the troop due to the collective actions of certain females.
Over the course of evolution, and again due to female sexual choice, unacceptable and brutal males would have been increasingly removed from the gene pool as these females were selectively breeding with those sociable males who were more to their liking. Moreover, because females selectively mate with males who are high status, and thus successful and (presumably more intelligent), increasingly intelligent offspring were produced, thus leading to increasingly intelligent hominids.
MATRIARCHAL SOCIAL-SEXUAL EVOLUTION: HOME IS WHERE THE HEART IS - DOMESTICATION OF MAN, CULTURE, SOCIETY
Yet another major factor in the development of group cohesion, increased male sociability, and the elimination of unacceptable males from the breeding pool, is the mother-offspring bond and the development of extended maternal/matriarchal kinship groups involving grandmothers, mothers, daughters, and their young, including sons and male siblings. A group of like-minded females can wield enormous influence and control over the males of the troop, such as by refusing sex or by avoiding males who even dare pal around with unacceptable males. To be accepted and to retain access to these females, males would be forced to modify their behavior or suffer permanent banishment and sexual deprivation.


Many if not most primate societies are structured around female kinship groups. The mother-offspring bond serves as a foundation for social life (Fedigan, 1992; Goodall, 1990; Lancaster, 1976). And, among ancient humans, social life would have centered not only upon the females, but the huts and "homes" that they established. That is, in general, in "primitive" and presumably among ancient humans, the home, hut, tent, or shelter is the possession and the dwelling of the women and children, whereas males tend to sleep wherever they happen to grow tired and are only allowed into these dwellings if they are family or have brought presents.
Females are not only the mistress of the home, but in many ancient cultures and societies she also constructed the home. She not only owns the home, but would rule over her children and her children's children, thus giving rise to a matriarchal dominated society. Indeed, these tribal female-dominated clans would typically be headed by a clan-mother and would be comprised of a hierarchy of daughters and granddaughters, collectively incarnating that purity of the blood which is the tribe (Briffault, 1931). Thus, the women, including all sexually receptive women, so long as they were kin, would form associations that would last a life time; associations that gave rise to the "tribe" and to village life.
Hence, in order to gain sexual access to women, and upon establishing a relationship with one of these maidens, and given the close ties between mother and daughter, in primitive and ancient cultures, the male would essentially move in with his lover/wife, and her family. He would also have to modify his behavior accordingly.
As summarized by Briffault (1931) regarding 18th and 19th century societies, "among the Eskimo of Labrador, the young man goes to the home of the maiden where as man and wife they dwell together, the son-in-law helping to support the family. Of the Algonkin tribes of Canada, the man joins the woman and her family and she is the mistress and heiress of the home. Among the Crees when a young man marries he resides with his wife's parents. Among the Pawnees the husband took up residence with his wife. In Florida among the Seminole Indians, it is the man and not the woman who leaves father and mother and cleaves to a mate. Among the Ainu of Japan, the women remain in their own home and their husbands join them there. Likewise, in Samoa the husband takes up is abode with his wife."
Known for their utter ferocity, the males of the confederated tribes of the Iraquois were also dominated by matriarchs, and the people would dwell in long-houses which might be sixty to one hundred feet long, being divided into compartments. The interior economy of those clan- dwellings was under the authority of a matron. Sometimes up to 20 families lived together, the women taking husbands from other clans.
"However, no matter how many children, or what goods he might have in the house, he might at any time be ordered to pick up his blanket and move out; and after such orders it would not be healthful for him disobey; the house would be too hot for him; and unless saved by the intercession of some aunt of grandmother, he must retreat to his own clan" (Briffault, 1931).
In fact, among those ancient cultures and primitive cultures where the woman would be removed from her home in order to live with the man, she would often pack up and run back to her mother and family if he displeased or upset her in some fashion.
Since many or perhaps all ancient human cultures may have also been matriarchal (Briffault, 1931), including those of the Cro-Magnon peoples of the Upper Paleolithic (Joseph, 2000a,c), it is likely that over the course of evolution, similar mother-daughter matriarchal bonds also formed the social glue which held together and thus regulated hominid troop and tribal behavior.
Over the course of social and physical evolution, the further development of multiple extended female kinship associations would have provided its increasing population of females with considerable influence over other troop members including even dominant males who could be ostracized and expelled from the group. Grandmothers, mothers, daughters, sisters, aunts and cousins, and thus the development of multiple female-dominated kinship groups and their female allies, would have also been able to directly as well as indirectly exert profound influences on which males were accepted into the troop, as well as acting to collectively modify male behavior toward females in general.
Female dominated kinship groups, coupled with the evolution of full time female sexuality and female "choice," would have promoted not only social-physical evolution, but the development of tribal village life. That is, as with chimps, as the number of sexually receptive females increased, additional males would have vied for acceptance into the group in order to mate with and establish long term sexual relations with one (or several) females.
Once accepted, these males would have been motivated to behave in a manner acceptable to these continuously sexually receptive females, and would have therefore contributed not only to population growth but the stability and cohesion of the group. The alternative was social banishment and sex deprivation. Indeed, it has been argued that permanent social groupings of monkeys, apes, and humans remain permanent because one or more females within the group are always sexually available (Zuckerman, 1932). Female sexuality promotes and maintains group cohesion (Rowell, 1972, Saayman, 1975) and coupled with matriarchal dominated kinship groups, gave rise to the development of society and the " civilization" and domestication of man.
According to Briffault (1931), "the primitive male is a homeless potentate sojourning like the rest of his fellows in the huts erected by their wives. He is welcome in the home only so long as he pleases his wife and the women. If she thinks her husband is not a good worker, or depending on her whims, she can dismiss him and take another husband."
Therefore, it can be assumed that the evolution of full time female sexual receptivity would have exerted a cohesive, and socializing influence on males who were seeking to join or who were already members of the troop. Hence, as ever larger groups of men and sexually receptive women began congregating together, bands of H. erectus soon became tribes of up to 100 or more individuals (Clark, 1977) which consisted, presumably of multiple female kinship groups, their consorts, and males seeking mates. In fact, near the end of their rein, 400,000 to 300,000 years ago, H. erectus (or perhaps archaic H. sapiens) were building crude shelters and huts of branches and stone, some of which were large enough to house large extended families consisting of twenty or more people.
"Long houses," that is, those which are designed to accommodate numerous individuals are typically associated with matriarchal societies. It can thus be concluded that not only was Homo erectus and archaic Homo sapien village life dominated by females, but that evolution of the continuously sexually receptive female is the foundation stone which gave rise to the domestication of the male and the development of civilized society.












