Rhawn Gabriel Joseph, Ph.D.
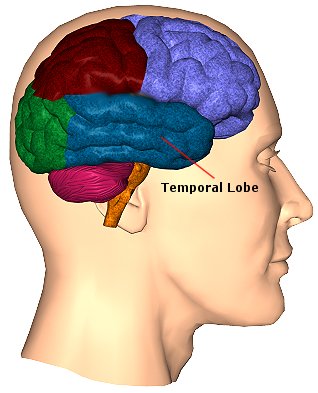
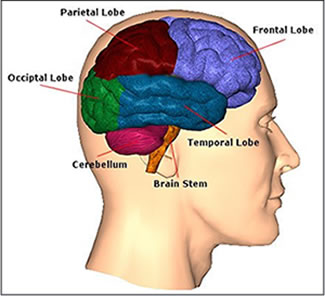
The temporal lobes are unique. These are the only regions of the brain that subserves personalized, subjective emotional and social experience and can store and recall this information from memory. The temporal lobes also contains the core structures of the limbic system involved in emotion and memory, the amygdala and hippocampus.
The temporal lobe/limbic allocortex is responsive to complex auditory and visual stimuli (Binder et al., 2014; Gross & Graziano 1995; Nakamura et al. 2014; Nelken et al., 2008; Nishimura et al., 2008; Price, 2011; Rolls 2002; Tovee et al. 2014), comprehending, in humans, complex speech, and contains the highest density and the greatest diversity of peptides and neurotransmitters as compared to all other brain regions (Niewenhujs, 2015).
Of all brain regions, only stimulation or activation of the temporal lobe or underlying limbic structures, gives rise to personalized, subjective, emotional, and sexual experiences (Gloor, 2011; Halgren, 2002). Although direct electrical stimulation of the frontal motor cortex can induce twitching of the lips, flexion or extension of a single finger joint, protrusion of the tongue or elevation of the palate, patients never claim to have willed these movements. That is, stimulation of the frontal lobes evokes behavior that seems outside the control of the patient (Penfield & Boldrey, 1937; Penfield & Jasper, 1954; Penfield & Rasmussen, 1950; Rothwell et al. 2007).
By contrast, electrode stimulation of the temporal lobes evokes experiences which become part of the subjective stream of consciousness, embedded into the very fabric of the personality, such that the personality, and even sexual orientation may be altered. Moreover, patients may experience profound visual and auditory hallucinations and even feel as if they have left their bodies and are floating in space or soaring across the heavens.

There is no subjective sensations with frontal or parietal or occipital stimulation. In fact, with stimulation to these other brain areas the patient only becomes aware of the stimulation when they attempt to speak or move, or if a finger begins to twitch or if they feel painful tingling or see flashes of light. Although damage to the frontal lobe can produce the "frontal lobe personality" it is the temporal lobe which subserves those aspects of experience which are experienced as personal and subjective, of pertaining to the self and one's personal and even spiritual identity. Indeed, stimulation of the temporal lobe can give rise to profound personal, emotional, sexual, and even religious feelings which are experienced as personally, spiritually and philosophically meaningful, including even sensations of having the "truth" revealed and of receiving knowledge regarding the meaning of life and death.

TEMPORAL TOPOGRAPHY
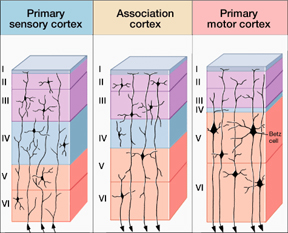
The temporal lobe is the most heterogenous of the four lobes of the human brain, as it consists of six layered neocortex, four to five layered mesocortex, and 3 layered allocortex, with the hippocampus and amygdala forming its limbic core.
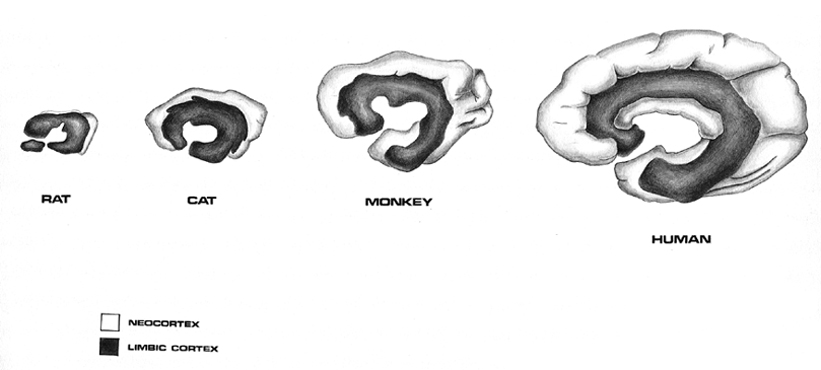
As detailed in chapters, 5, 12, over the course of evolution the dorsally situated hippocampus became displaced and progressively assumed a ventral position, during the course of which it also contributed to the neocortical development of portions of the parietal, occipital and temporal lobe. Similarly, portions of the amygdala became increasingly cortical in structure, and together with the hippocampus, contributed to the evolution of the anterior, medial, superior, and lateral temporal lobes.
Because so much of the temporal lobe evolved from these limbic nuclei, unlike the other lobes, it consists of a mixture of allocortex, mesocortex, and neocortex, with allocortex and mesocortex being especially prominent in and around the medial-anterior inferiorally located uncus, beneath which and which abuts the amygdala and hippocampus.
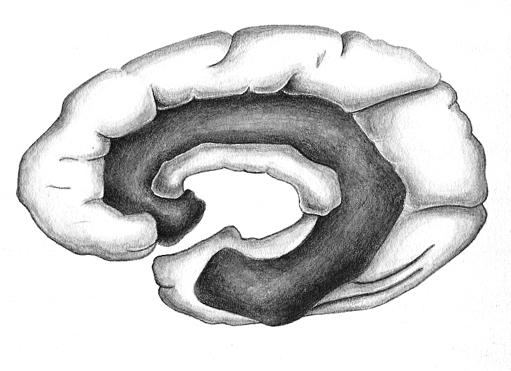
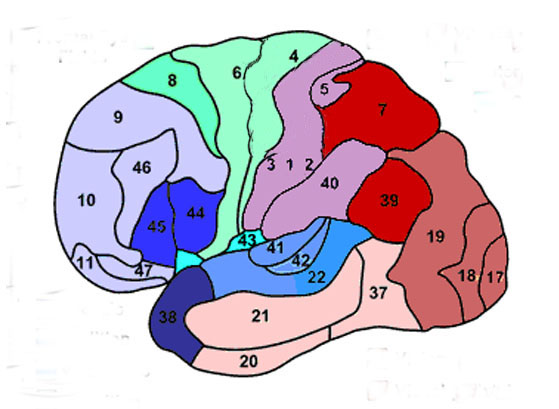
Given the role of the amygdala and hippocampus in memory, emotion, attention, and the processing of complex auditory and visual stimuli, the temporal lobe became similarly organized (Gloor, 2011). Broadly considered, the neocortical surface of the temporal lobes can be subdivided into three main convolutions, the superior, middle, and inferior temporal gyri, which in turn are separated and distinguished by the sylvian fissure and the superior, middle, and inferior temporal sulci. Each of these subdivision performs different (albeit overlapping) functions, i.e. auditory, visual, and auditory-visual-affective perception including memory storage.
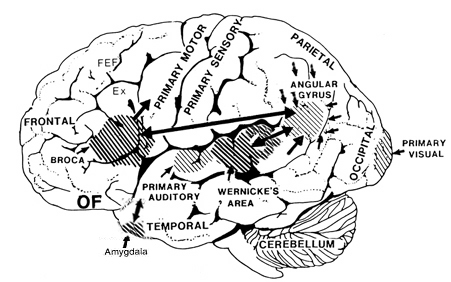
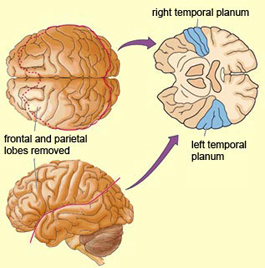
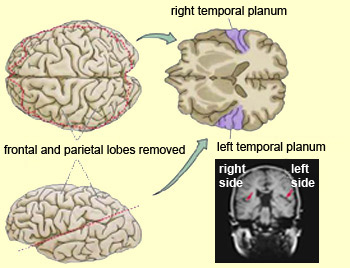
Specifically, the anterior and superior temporal lobes are concerned with complex auditory and linguistic functioning and the comprehension of language (Binder et al., 2014; Edeline et al. 1990; Geschwind 1965; Goodglass & Kaplan, 2008; Keser et al. 2011; Nelken et al., 2008; Nishimura et al., 2008; Price, 2011). The importance of the superior temporal lobe in auditory functioning, and (in the left hemisphere) language, is indicated by the fact that the superior temporal, including the primary auditory area, is larger in humans versus other primates and mammals. In addition, Heschl's gyri, a region of the brain directly implicated in auditory processing, are larger on the left, and the left planum temporal, also an auditory area, is 10 times larger (among the majority of the population) than its counterpart on the right (Geschwind & Levinsky, 1968).
The auditory areas, however, extend along the axis of the superior temporal lobe, extending in a anterior-inferior and posterior-superior arc, such that the connections of the primary auditory area extends outward in all directions throughout the brain, innervating, in a temporal sequences, the second and third order auditory association areas (Pandya & Yeterian, 2015). Thus, each area of the brain involved in auditory processing, from simple to complex analysis, are interlinked and these association areas in turn project back to the primary auditory area.

Vision and the Temporal Lobes
The temporal lobes perform complex analysis of visual stimuli, including the recognition of faces, animals, tools, houses, and other complex geometric forms.
The inferior and medial temporal lobe harbors the amygdala and hippocampus, performs complex visual integrative activities including visual closure, and contains neurons which respond selectively to faces and to the sight of complex objects (Gross & Graziano 1995; Nakamura et al. 2014; Rolls 2002; Tovee et al. 2014).
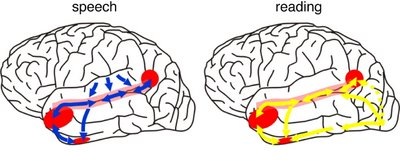

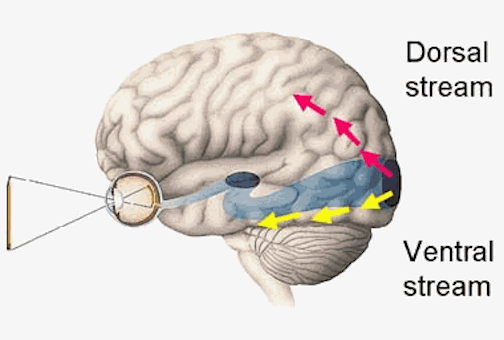
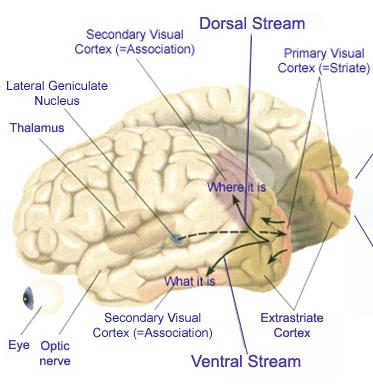
The inferior and middle temporal lobes, are the recipients of one two diverging (dorsal and ventral) streams of visual input arising from within the occipital lobe and thalamus (Ungerlieder & Mishkin, 1982); i.e. the pulvinar and dorsal medial nucleus of the thalamus. The dorsal stream is more concerned with the detection of motion and movement, orientation, binocular disparity, whereas the ventral stream is concerned with the discrimination of shapes, textures, objects and faces, including individual faces (Baylis et al., 2015; Perrett et al., 2004, 2002). This information flows from the primary visual to visual association areas and is received and processed in the temporal lobes and is then shunted to parietal lobe, and to the amygdala and entorhinal cortex (the gateway to the hippocampus) where it may then be learned and stored in memory.
The temporal lobes, however, also receive extensive projections from the somesthetic and visual association areas, and processes gustatory, visceral, and olfactory sensations (Jones & Powell, 1970; Previc 1990; Seltzer & Pandya, 1978) including the feeling of hunger (Fisher 2014). Hence, the temporal lobes perform an exceedingly complex array of divergent and interrelated functions.
FUNCTIONAL OVERVIEW
The right and left temporal lobe are functionally lateralized, with the left being more concerned with non-emotional language functions, including, via the inferior-medial and basal temporal lobes reading and verbal (as verbal-visual) memory. For example, as determined based on functional imaging, when reading and speaking the left posterior temporal lobe becomes highly active, due, presumably to its involvement in lexical processing (Binder et al., 2014; Howard et al., 199). The superior temporal lobe (and supramarginal gyrus) also becomes more active when reading aloud than when reading silently (Bookheimer, et al., 1995), and becomes active during semantic processing as does the left angular gyrus (Price, 2011).
These same temporal areas are activated during word generation (Shaywitz, et al., 1995; Warburton, et al., 1996), and sentence comprehension tasks (Bottini, et al., 2014; Fletcher et al., 1995). and (in conjunction with the angular gyrus) becomes highly active when retrieving the meaning of words during semantic processing and semantic decision tasks (Price, 2011). Likewise, single cell recordings from the auditory areas in the temporal lobe demonstrate that neurons become activated in response to speech, including the sound of the patient's own voice (Creutzfeldt, et al., 2008).

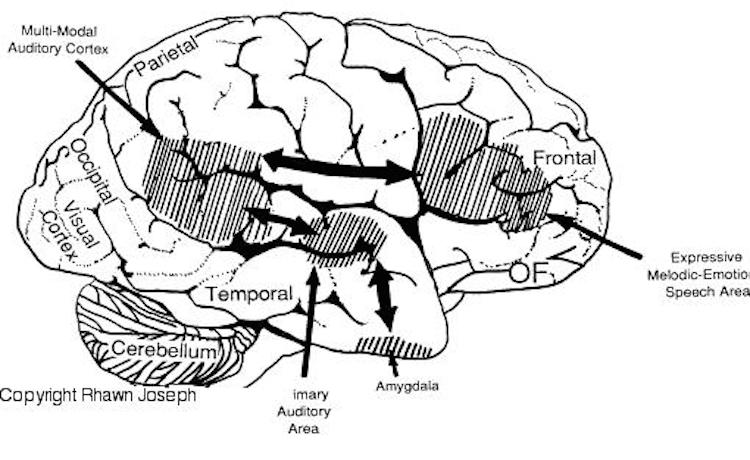
By contrast, the right is more concerned with perceiving emotional and melodic auditory signals (Evers et al., 2008; Parsons & Fox, 2011; Ross, 1993), and is dominant for storing and recalling emotional and visual memories (Abrams & Taylor, 1979; Cimino, et al. 2011; Cohen, Penick & Tarter, 1974; Deglin & Nikolaenko, 1975; Kimura, 1963; Shagass et al.,1979; Wexler, 1973). The right temporal lobe becomes highly active when engage in interpreting the figurative aspects of language (Bottini et al., 2014).
However, there is considerable functional overlap and these structures often become simultaneously activated when performing various tasks. For example, as based on functional imaging, when reading, the right posterior temporal cortex also becomes highly active (Bookheimer, et al., 1995; Bottini et al., 2014; Price, et al., 1996), and when making semantic decisions (involving reading words with similar meanings), there is increased activity bilaterally (Shaywitz, et al., 1995).
Presumably, in part, both temporal lobes become activated when speaking and reading, due to the left temporal lobe's specialization for extracting the semantic, temporal, sequential, and the syntactic elements of speech, thereby making language comprehension possible. By contrast, the right temporal lobe becomes active as it attempts to perceive, extract and comprehend the emotional (as well as the semantic) and gestalt aspects of speech and written language.
AUDITORY NEOCORTEX
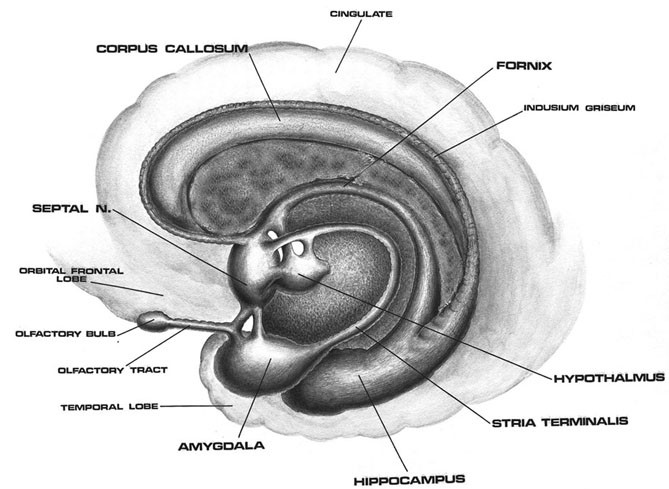
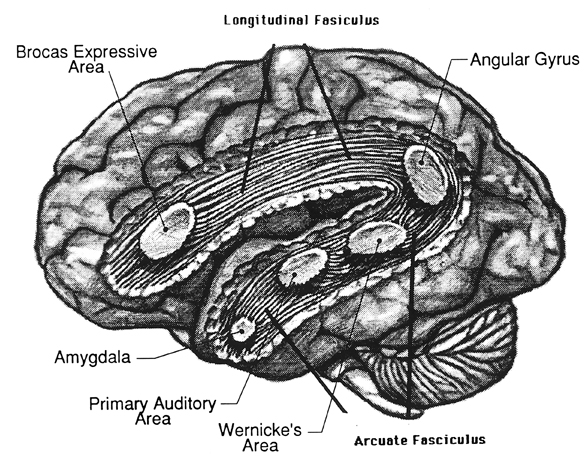
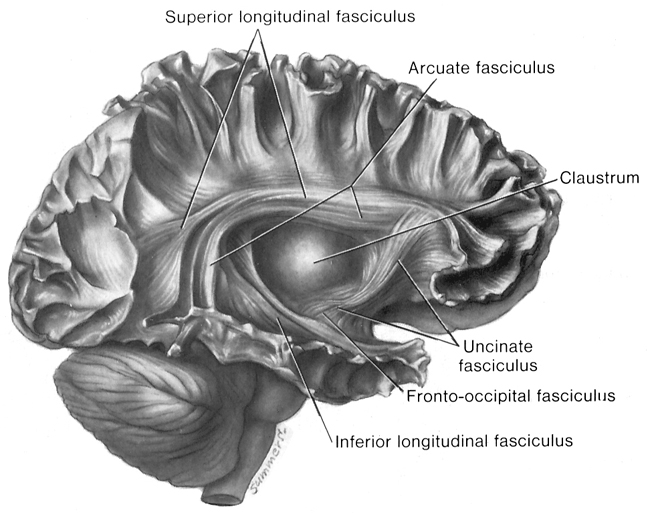
Although the various cytoarchitectural functional regions are not well demarcated via the use of Brodmann's maps, it is possible to very loosely define the superior-temporal and anterior-inferior and anterior middle temporal lobes as auditory cortex (Pandya & Yeterian, 2015). These regions are linked together by local circuit (inter-) neurons and via a rich belt of projection fibers which include the arcuate and inferior fasciculus. The arcuate and inferior fasciculus project in a anterior-inferior and posterior-superior arc innervating (inferiorally) the amygdala and entorhinal cortex (Amaral et al., 1983) and posteriorally the inferior parietal lobule--as is evident based on brain dissection. That is, this rope of fibers links the auditory and language areas of the brain, from the amygdala, to the auditory neocortex, to the inferior parietal lobule and to Broca's speech area in the frontal lobes, and then back again.

It is also via the arcuate and inferior fasciculus that the inferior temporal lobe, entorhinal cortex (the "gateway to the hippocampus") as well as the amygdala (receive from) and transfer complex auditory information to the primary and secondary auditory cortex which simultaneously receives auditory input from the medial geniculate of the thalamus, the pulvinar, and (sparingly) the midbrain (Amaral et al., 1983; Pandya & Yeterian, 2015). As will be detailed below, it is within the primary and neocortical auditory association areas where linguistically complex auditory signals are analyzed and reorganized so as to give rise to complex, grammatically correct, human language.
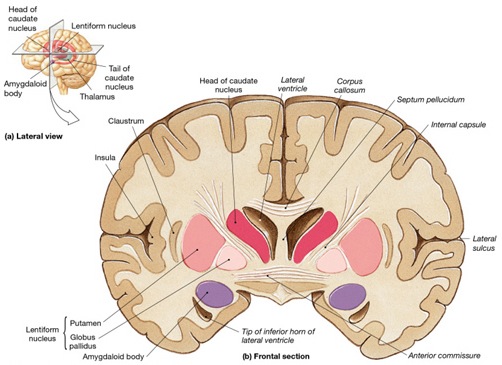
It is noteworthy that immediately beneath the insula and approaching the auditory neocortex is a thick band of amygdala-cortex, the claustrum. Over the course of evolution the claustrum apparently split off from the amygdala due to the expansion of the temporal lobe and the passage of additional axons coursing throughout the white matter (Gilles et al., 1983) including the arcuate fasciculus. Nevertheless, the claustrum maintains rich interconnections with the amygdala, the insula, and to some extent, the auditory cortex (Gilles et al., 1983). This is evident from dissection of the human brain which reveals that the fibers of the arcuate fasciculus do not merely pass through this structure (breaking it up in the process) but sends and receives fibers from it.
CORTICAL ORGANIZATION
The functional neural-architecture of the auditory cortex is quite similar to the somesthetic and visual cortex (Peters & Jones, 2015). That is, neurons in the same vertical columns have the same functional properties and are activated by the same type or frequency of auditory stimulus. The auditory cortex, therefore, is basically subdivided into discrete columns which extend from the white matter (layers VII/6b) to the pial surface (layer I).
However, although most neurons in a single column receive excitatory input from the contralateral ear, some receive input from the ipsilateral ear which may exert excitatory or inhibitory influences so as to suppressed, within the same column, input from itself or from the contralateral ear (Imig & Brugge, 1978). These interactions have been referred to as summation interaction (excitatory/excitatory) and suppression interaction (inhibitory/inhibitory, inhibitory/excitatory).
Moreover, some columns tend to engage in excitatory summation, whereas others tend to engage in inhibitory suppression (Imig & Brugge, 1978)--a process that would contribute to the focusing of auditory attention and the elimination of neural noise thereby promoting the ability to selectively attend to motivationally significant environmental and animal sounds including human vocalizations (Nelken et al., 2008).
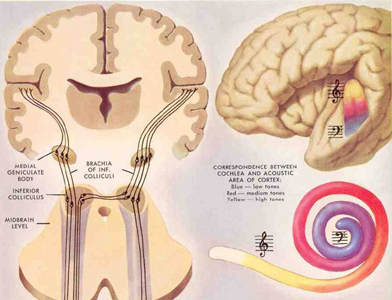
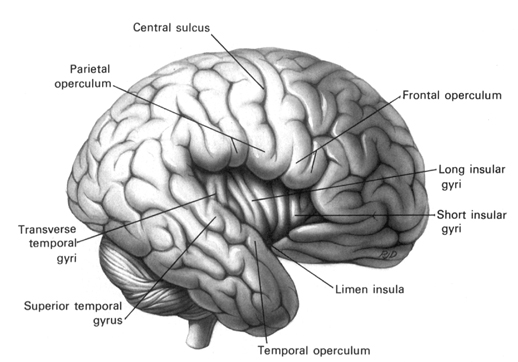
Among humans, the primary auditory neocortex is located on the two transverse gyri of Heschl, along the dorsal-medial surface of the superior temporal lobe. The center most portion of the anterior transverse gyri contains the primary auditory receiving area (Broadman's area 41) and neocortically resembles the primary visual (area 17) and somesthetic cortices (area 3). The posterior transverse gyri consists of both primary and association cortices (areas 41 and 42 respectively). The major source of auditory input is derived from the medial geniculate thalamic nucleus as well as the pulvinar (which also provides visual input).

Heschl's gyri is especially well developed and no other species has a Heschl's gyrus that as prominent as is the case with humans (Yousry et al., 1995). In fact, some individuals appear to posses multiple Heschl gyri--though the significance of this is not clear. However, it has been reported that this may be a reflection of genetic disorders, learning disabilities, and so on (see Leonard, 1996).
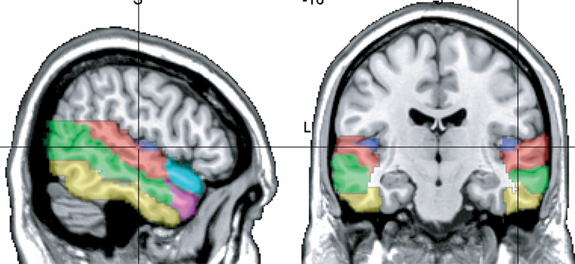
Heschl's gyrus is the blue area.
In over 80-90% of right handers and in over 50% to 80% of left handers, the left hemisphere is dominant for expressive and receptive speech (Frost et al., 2008; Pujol, et al., 2008). In humans, the auditory cortex including Wernicke's (i.e. the planum temporale) is generally larger on the left temporal lobe (Geschwind & Levitsky, 1968; Geschwind & Galaburda 2015; Habib et al. 1995; Wada et al., 1975; Witelson & Pallie, 1973). Specifically, as originally determined by Geschwind & Levitsky (1968), the planum temporal is larger in the left hemisphere in 65% of brains studied. By contrast, the planum temporal is larger on the right in 25%, whereas there is no difference in 10% of subjects studied. Geschwind, Galaburda and colleagues argue that the larger left planum temporale is a significant factor in the establishment of left hemisphere dominance for language.


AUDITORY TRANSMISSION FROM THE COCHLEA TO THE TEMPORAL LOBE
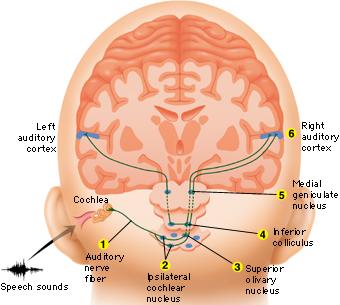
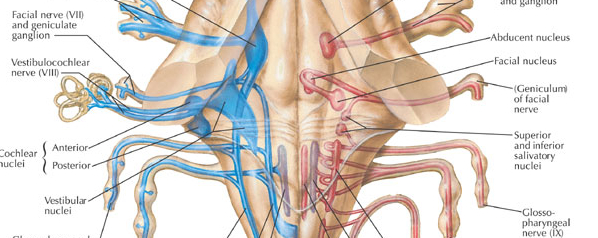
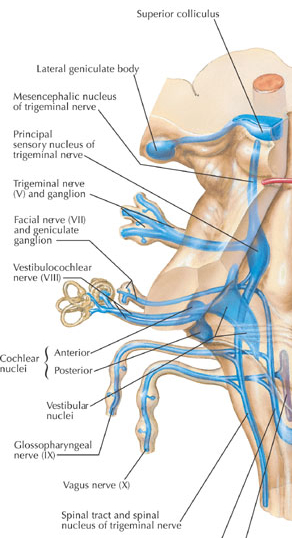
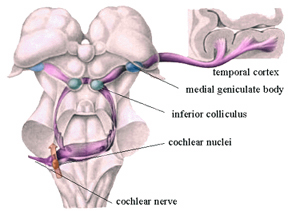
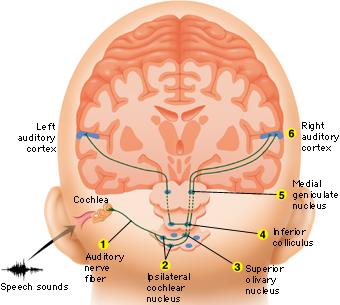
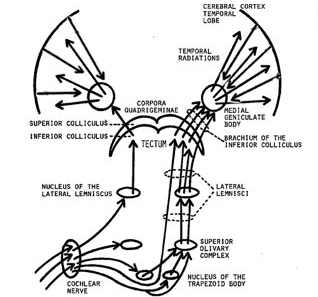
Within the cochlea of the inner ear are tiny hair cells which serve as sensory receptors. These cells give rise to axons which form the cochlear division of the 8th cranial nerve; i.e. the auditory nerve. This rope of fibers exits the inner ear and travels to and terminates in the cochlear nucleus which overlaps and is located immediately adjacent to the vestibular-nucleus from which it evolved within the brainstem (see chapter 5).
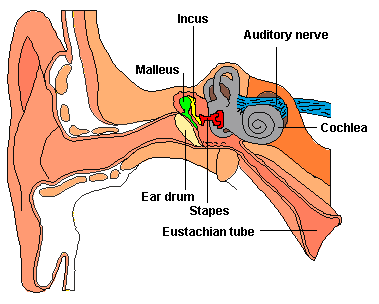
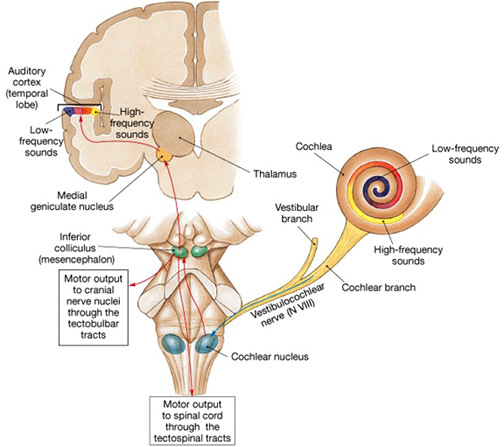
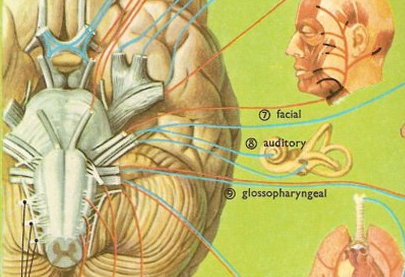
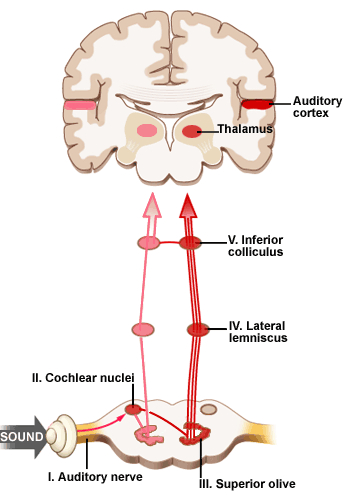
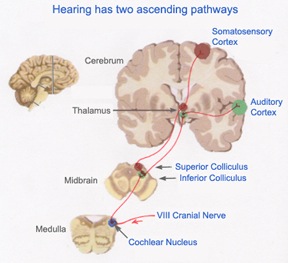
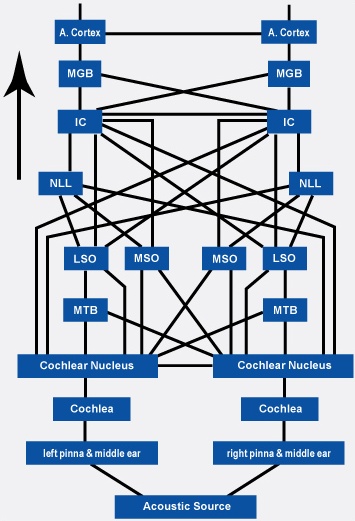
Among mammals once auditory stimuli are received in the cochlear nucleus there follows a series of transformations as this information is relayed to various nuclei, i.e., the superior olivary complex, the nucleus of the lateral lemniscus of the brainstem, the midbrain inferior colliculus and medial geniculate nucleus of the thalamus as well as the amygdala (which extracts those features which are emotionally or motivationally significant), and cingulate gyrus (Carpenter 2011; Devinksy et al. 1995; Edeline et al. 1990; Parent 1995). Auditory information is then relayed from the medial geniculate nucleus of the thalamus as well as via the amygdala (through the inferior fasciculus) to Heschl's gyrus, i.e. the primary auditory receiving area (Brodmann's areas 41 & 42) located within the superior temporal gyrus and buried within the depths of the Sylvian fissure.
Here auditory signals undergo extensive analysis and reanalysis and simple associations are established. However, by time they have reached the neocortex, auditory signals have undergone extensive analysis by the medial thalamus, amygdala, and the other ancient structures mentioned above.
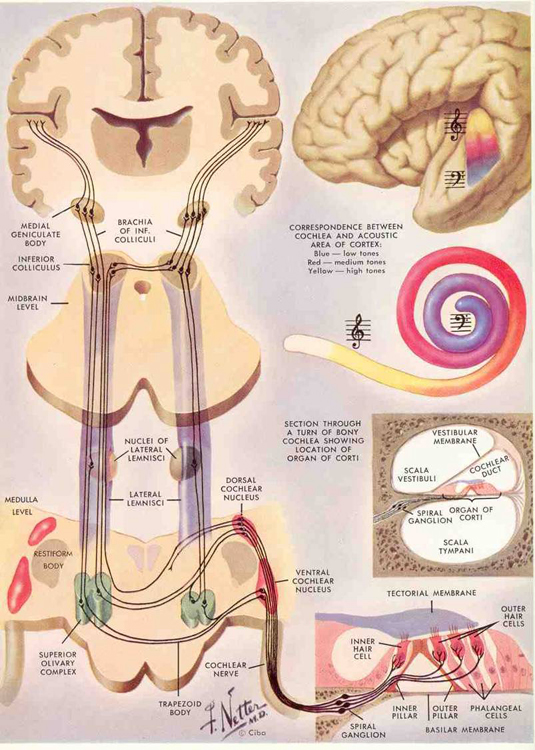
As noted, unlike the primary visual and somesthetic areas located within the neocortex of the occipital and parietal lobes which receive signals from only one half of the body or visual space, the primary auditory region receives some input from both ears, and from both halves of auditory space (Imig & Adrian, 1977). This is a consequence of the considerable interconnections and cross-talk which occurs between different subcortical nuclei as information is relayed to and from various regions prior to transfer to the neocortex. Predominantly, however, the right ear transmits to the left cerebral neocortex and vice versa.
Hallucinations
Although the majority of auditory neurons respond to auditory stimuli, approximately 25% also respond to visual stimuli, particularly those in motion. In fact, Penfield and Perot (1963) were able to trigger visual responses from stimulation in the superior temporal lobe. In addition, these neurons are involved in short-term memory, as lesions to the superior temporal lobe can induce short-term memory deficits (Heffner & Heffner, 1986).
Conversely, electrical stimulation not only induces visual responses, but complex auditory responses. Penfield and Perot (1963) report that electrical stimulation can produce the sound of voices, and the hearing of music. These neurons will also respond, electrophysiologically, to the sound of human voices, including the patient's own speech (Creutzfeldt, et al., 2008). Conversely, injury to the left and right superior temporal lobe can result in an inability to correctly perceive or hear complex sounds; a condition referred to as pure word deafness, and if limited to the right ear, agnosia for environmental and musical sounds (see below). In fact, right temporal injuries can disrupt the ability to remember musical tunes or to create musical imagery (Zatorre & Halpen, 1993).
AUDITORY RECEIVING AREA NEUROANATOMICAL-FUNCTIONAL ORGANIZATION.
The primary auditory area is tonotopically organized (Edeline et al. 1990; Merzenich & Brugge, 1973; Pantev et al. 2008; Woolsey & Fairman, 1946), such that different auditory frequencies are progressively anatomically represented. In addition, there is evidence to suggest that the auditory cortex is also "cochleotopically" organized (Swarz & Tomlinson 1990). Specifically, high frequencies are received and analyzed in the anterior-medial portions and low frequencies in the posterior-lateral regions of the superior temporal lobe (Merzenich & Brugge, 1973). In addition, the primary auditory cortex processes and perceives pitch and intensity, as well as modulations in frequency. Primary auditory neurons are especially responsive to the temporal sequencing of acoustic stimuli (Wolberg & Newman, 1972).
Moreover, the auditory cortex appears to be organized so as to detect tones in noise, i.e. the non-random structure of natural sounds, which enables it to selectively attend to environmental and animal sounds including human vocalizations (Nelken et al., 2008). In fact, these same neural fields can be activated by sign language, that is, among the congenitally deaf (Nishimura et al., 2008).
Some neurons are also specialized to perceive specific-specific calls (Hauser, 2011) or the human voice (Crutzfeldt et al., 2008). This has been determined by single unit recording. Some neurons will react to the patient's voice, whereas yet others will selectively respond to a particular (familiar) voice or call, but not to others--which in regard to voices, indicates that these cells (or neural assemblies) have engaged in learning.
Auditory neurons in fact, display neurplasticity in response to learning as well as injury. For example, not only due auditory synapses display learning-induced changes, but auditory neurons can be classically conditioned so as to form associations between stimuli. For example, if a neutral sound is paired with a noxious stimulus, the neurons response properties will be greatly altered (Weinberger, 1993).
These neurons also display neuroplasticity in response to injury and loss of hearing. For example, due to cochlear or peripheral hearing loss, neurons that no longer receive high frequency auditory input, begin to respond instead to middle frequencies (Robertson & Irvine, 2008). In fact, in response to complete loss of hearing, such as congenital deafness, these neurons may cease to respond to auditory input and may instead respond to body language, such as the signing used by the deaf (Nishimura et al., 2008).
FILTERING, FEEDBACK & TEMPORAL-SEQUENTIAL REORGANIZATION
The old cortical centers located in the midbrain and brain stem evolved long before the appearance of neocortex and became specialized for performing a considerable degree of auditory analysis long before mammals evolved (Buchwald et al. 1966). This is evident from observing the behavior of reptiles and amphibians where auditory cortex is either absent or minimally developed.
Auditory information would be projected to the brainstem via two pathways; from the vestibular-cochlear nerve, also known as the 8th cranial nerve, and from the medial geniculate nucleus which is part of the thalamus. This information would then be sent to the inferior colliculus of the midbrain, and in mammals it would be transmitted to the auditory neocortex.
Moreover, many of these old cortical nuclei also project back to each other such that each subcortical structure might hear and analyze the same sound repeatedly (Brodal 2011; Pandya & Yeterian, 2015). In this manner the brain is able heighten or diminish the amplitude of various sounds via feedback adjustment (Joseph, 1993; Luria, 1980). In fact, not only is feedback provided, but the actual order of the sound elements perceived can be rearranged when they are played back.

This same process continues at the level of the neocortex which has the advantage of being the recipient of signals that have already been highly processed and analyzed (Buchwald et al. 1966; Edeline et al. 1990; Scharz & Tomlinson 1990). Primary auditory neurons are especially responsive to the temporal sequencing of acoustic stimuli (Wolberg & Newman, 1972). It is in this manner, coupled with the capacity of auditory neurons to extract non-random sounds from noise, that language related sounds begin to be organized and recognized (e.g. Nelken et al., 2008; Scwazrz & Tomlinson 1990).
For example, neurons located in the primary auditory cortex can determine and recognize differences and similarities between harmonic complex tones and demonstrated auditory response patterns that vary in response to lower and higher frequency and to specific tones (Nelken et al. 2008; Scwarz & Tomlinson 1990). Some display "tuning bandwitdths" for pure tones, whereas others are able to identify up to seven components of harmonic complex tones. In this manner, pitch can also be discerned (e.g. Pantev et al. 2008).
Sustained Auditory Activity.
One of the main functions of the primary auditory neocortical receptive area appears to be the retention of sounds for brief time periods (up to a second) so that temporal and sequential features may be extracted and discrepancies in spatial location identified; i.e. so that we can determine from where a sound may have originated (see Mills & Rollman 1980). This prolonged activity, presumably also allows for additional processing and so that comparisons to be made with sounds that were just previously received and those which are just arriving. Hence, as based on functional imaging, the left temporal lobe becomes increasingly active as word length increases (Price, 2011), due presumably to the increased processing necessary.
Moreover, via their sustained activity, these neurons are able to prolong (perhaps via a perseverating feedback loop with the thalamus) the duration of certain sounds so that they are more amenable to analysis--which may explain why activity increases in response to unfamiliar words and as word length increases (Price, 2011). In this manner, even complex sounds can be broken down into components which are then separately analyzed. Hence, sounds can be perceived as sustained temporal sequences. It is perhaps due to this attribute that injuries to the superior temporal lobe result in short-term auditory memory deficits as well as disturbances in auditory discrimination (Hauser, 2011; Heffner & Heffner, 1986).
Although it is apparent that the auditory regions of both cerebral hemispheres are capable of discerning and extracting temporal-sequential rhythmic acoustics (Milner, 1962; Wolberg & Newman, 1972), the left temporal lobe contains a greater concentration of neurons specialized for this purpose as the left half of the brain is clearly superior in this capacity (Evers et al., 2008).
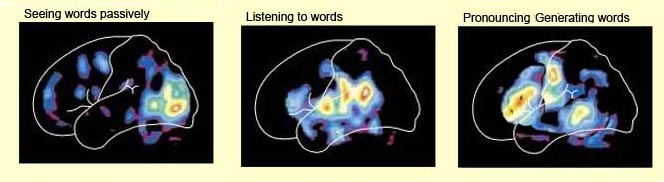
For example the left hemisphere has been repeatedly shown to be specialized for sorting, separating and extracting in a segmented fashion, the phonetic and temporal-sequential or linguistic-articulatory features of incoming auditory information so as to identify speech units. It is also more sensitive to rapidly changing acoustic cues be they verbal or non-verbal as compared to the right hemisphere (Shankweiler & Studdert-Kennedy, 1967; Studdert-Kennedy & Shankweiler, 1970). Moreover, via dichtoic listening tasks, the right ear (left temporal lobe) has been shown to be dominant for the perception of real words, word lists, numbers, backwards speech, morse code, consonants, consonant vowell syllables, nonsense syllables, the transitional elements of speech, single phonemes, and rhymes (Blumstein & Cooper, 1974; Bryden, 1967; Cutting, 1974; Kimura, 1961; Kimura & Folb, 1968; Levy, 1974; Mills & Rollman, 1979; Papcunm et al., 1974; Shankweiler & Studert-Kennedy, 1966, 1967; Studdert-Kennedy & Shankweiler, 1970). In addition, and as based on functional imaging, activity significantly increases in the left hemisphere during language tasks (Nelken et al., 2008; Nishimura et al., 2008), including reading (Binder et al., 2014; Price, 2011).
In part the association of the left hemisphere and left temporal lobe with performing complex temporal-sequential and linguistic analysis is due to its interconnections with the inferior parietal lobule (see chapters 6, 11)--a structure which also becomes highly active when reading and naming (Bookheimer, et al., 1995; Menard, et al., 1996; Price, 2011; Vandenberghe, et al., 1996) and which acts as a phonological storehouse that becomes activated during short-term memory and word retrieval (Demonet, et al., 2014; Paulesu, et al., 1993; Price, 2011).
As noted in chapters 6, 11, the inferior parietal lobule is in part an outgrowth of the superior temporal lobe but also consists of somesthetic and visual neocortical tissue. However, the inferior parietal lobule (the angular and supramarginal gyrus) also acts to impose temporal sequences on incoming auditory, as well as visual and somesthetic stimuli, and also serves to provide (via its extensive interconnections with surrounding brain tissue) and integrate related associations thus making complex and grammatically correct human language possible (chapters 5, 11).

However, the language capacities of the left temporal lobe are also made possible via feedback from "subcortical" auditory neurons, and via sustained (vs diminished) activity and analysis. That is, because of these "feedback" loops the importance and even order of the sounds perceived can be changed, filtered or heightened; an extremely important development in regard to the acquisition of human language (Joseph, 1993; Luria, 1980). In this manner sound elements composed of consonants, vowels, and phonemes and morphemes can be more readily identified, particularly within the auditory neocortex of the left half of the brain (Cutting 1974; Shakweiler & Studdert-Kennedy 1966, 1967; Studdert-Kennedy & Shankweiler, 1970).
For example, normally a single phoneme may be scattered over several neighboring units of sounds. A single sound segment may in turn carry several successive phonemes. Therefore a considerable amount of reanalysis, reordering, or filtering of these signals is required so that comprehension can occur (Joseph 1993). These processes, however, presumably occurs both at the neocortical and old cortical level. In this manner a phoneme can be identified, extracted and analyzed and placed in its proper category and temporal position (see edited volume by Mattingly & Studdert-Kennedy, 2011 for related discussion).
Take, for example, three sound units, "t-k-a," which are transmitted to the superior temporal auditory receiving area. Via a feedback loop the primary auditory area can send any one of these units back to the thalamus which again sends it back to the temporal lobe thus amplifying the signal and/or which allows for rearranging their order, "t-a-k," or "K-a-t." A multitude of such interactions are in fact possible so that whole strings of sounds can be arranged or rearranged in a certain order (Joseph 1993). Mutual feed back characterizes most other neocortical-thalamic interactions as well, be it touch, audition, or vision (Brodal 2011; Carpenter 2011; Parent 1995).
Via these interactions and feedback loops sounds can be repeated, their order can be rearranged, and the amplitude on specific auditory signals can be enhanced whereas others can be filtered out. It is in this manner, coupled with experience and learning (Edeline et al. 1990; Diamond & Weinberger 2008) that fine tuning of the nervous system occurs so that specific signals are attended to, perceived, processed, committed to memory and so on. Indeed, a significant degree of plasticity in response to experience as well as auditory filtering occurs throughout the brain not only in regard to sound, but visual and tactual information as well (Greenough et al. 2007; Hubel & Wiesel, 1970; Juliano et al. 2014). Moreover, the same process occurs when organizing words for expression.
This ability to perform so many operations on individual sound units has in turn greatly contributed to the development of human speech and language. For example, the ability to hear human speech requires that temporal resolutions occur at great speed so as to sort through the overlapping and intermixed signals being perceived. This requires that these sounds are processed in parallel, or stored briefly and then replayed in a different order so that discrepancies due to overlap in sounds can be adjusted for (see Mattingly & Studdert-Kennedy, 2011 for related discussion), and this is what occurs many times over via the interactions of the old cortex and the neocortex. Similarly, when speaking or thinking, sound units must also be arranged in a particular order so that what we say is comprehensible to others and so that we may understand our own verbal thoughts.
HEARING SOUNDS & LANGUAGE
Humans are capable of uttering thousands of different sounds all of which are easily detected by the human ear. And yet, although own vocabulary is immense, human speech actually consists of about 12-60 units of sound depending, for example, if one is speaking Hawaiian vs English. The English vocabulary consists of several hundred thousand words which are based on the combinations of just 45 different sounds.
Animals too, however, are capable of a vast number of utterances. In fact monkeys and apes employ between 20-25 units of sound, whereas a fox employs 36. However, these animals cannot string these sounds together so as to create a spoken language. Most animals tend to use only a few units of sound at one time which varies depending on their situation, e.g. lost, afraid, playing.
Humans combine these sounds to make a huge number of words. In fact, employing only 13 sound units, humans are able to combine them to form five million word sounds (see Fodor & Katz 1964; Slobin 1971)
PHONEMES
As noted, the auditory areas are specialized to perceive a variety of sounds including those involving temporal sequences and abrupt change sin acoustics--characteristics which also define phonemes, which are the smallest units of sound and which are considered the building blocks of human language. For example, p and b as in bet vs pet are phonemes. When phonemes are strung together as a unit they in turn comprise morphemes. Morphemes such as "cat" are composed of three phonemes, "k,a,t". Hence, phonemes must be arranged in a particular temporal order in order to form a morpheme, and phoneme perception is associated with the auditory area of the left temporal lobe (Ojemann, 2011).
Morphemes in turn make up the smallest unit of meaningful sounds such as those used to signal relationships such as "er" as in "he is older than she." All languages have rules that govern the number of phonemes, their relationships to morphemes and how morphemes may be combined so as to produce meaningful sounds (Fodor & Katz 1964; Slobin 1971).
Each phoneme is composed of multiple frequencies which are in turn processed in great detail once they are transmitted to the superior temporal lobe. As noted, the primary auditory area is tonotopically organized, such that related albeit differing auditory frequencies are analyzed by adjoining cell columns. As also noted, however, it is the left temporal lobe which has been shown to be dominant for the perception of real words, word lists, numbers, syllables, the transitional elements of speech, as well as single phonemes, consonants, and consonant-vowell syllables.
CONSONANTS AND VOWELS
In general, there are two large classes of speech sounds: consonants and vowels. Consonants by nature are brief and transitional and have identification boundaries which are sharply defined. These boundaries enable different consonants to be discerned accurately (Mattingly & Studdert-Kennedy, 2011). Vowels are more continuous in nature and in this regard, the right half of the brain plays an important role in their perception.
Consonants are more important in regard to left cerebral speech perception. This is because they are composed of segments of rapidly changing frequencies which includes the duration, direction and magnitude of sounds segments interspersed with periods of silence. These transitions occur in 50 msecs or less which in turn requires that the left half of the brain take responsibility for perceiving them. Moreover, many consonants are silent.
In contrast, vowels consist of a slowly changing or steady frequencies with transitions taking 350 or more msec. In this regard, vowels are more like natural environmental sounds which are more continuous in nature, even those which are brief such as a snap of a twig. They are also more likely to be processed and perceived by the right half of the brain, though the left cerebrum also plays a role in their perception (see chapter 11).
The differential involvement of the right and left hemisphere in processing consonants and vowels is a function of their neuroanatomical organization and the fact that the left cerebrum is specialized for dealing with sequential information. Moreover, the left temporal lobe is able to make fine temporal discriminations with intervals between sounds as small as 50 msec. However, the right hemisphere needs 8-10 times longer and has difficulty discriminating the order of sounds if they are separated by less than 350 msecs.
Hence, consonants are perceived in a wholly different manner from vowels. Vowels yield to nuclei involved in "continuous perception" and are processed by the right (as well as left) half of the brain. Consonants are more a function of "categorical perception" and are processed in the left half of cerebrum. In fact, the left temporal lobe acts on both vowels and consonants during the process of perception so as to sort these signals into distinct patterns of segments via which these sounds become classified and categorized (Joseph 1993).
Nevertheless, be they processed by the right or left brain, both vowels and consonants are significant in speech perception. Vowels are particularly important when we consider their significant role in communicating emotional status and intent.
GRAMMAR & AUDITORY CLOSURE
Despite claims regarding "universal grammars" and "deep structures," it is apparent that human beings speak in a decidedly non-grammatical manner with many pauses, repetitions, incomplete sentences, irrelevant words, and so on. However, this does not prevent comprehension since the structure of the nervous system enables H. sapiens sapiens to perceptually alter word orders so they make sense, and even fill in words which are left out.
For example, when subjects heard a taped sentence in which a single syllable (gis) from the word "legislatures," had been deleted and filled in with static (i.e. le...latures), the missing syllable was not noticed. Rather, all subjects heard "legislatures" (reviewed in Farb 1975)
Similarly, when the word "tress" was played on a loop of tape 120 times per minute (tresstresstress....") subjects reported hearing words such as dress, florists, purse, Joyce, and stress (Farb 1975). In other words they organized these into meaningful speech sounds which were then coded and perceived as words.
The ability to engage in gap filling, sequencing, and to impose temporal order on incoming (supposedly grammatical speech) is important because human speech is not always fluent as many words are spoken in fragmentary form and sentences are usually four words or less . Much of it also consists of pauses and hesitations, "uh" or "err" sounds, stutters, repetitions, and stereotyped utterances, "you know," "like".
Hence, a considerable amount of reorganization as well as filling in must occur prior to comprehension. Therefore, these signals must be rearranged in accordance with the temporal sequential rules imposed by the structure and interaction of Wernicke's area, the inferior parietal lobe and the nervous system -what Noam Chompsky (1957) referred to as "deep structure"- so that they may be understood.
Consciously, however, most people fail to realize that this filling in and reorganization has even occurred, unless directly confronted by someone who claims that she said something she believes she didn't. Nevertheless, this filling in and process of reorganization greatly enhances comprehension and communication.
UNIVERSAL GRAMMARS
Regardless of culture, race, environment, geographical location, parental verbal skills or attention, children the world over go through the same steps at the same age in learning language (Chompsky, 1972). Unlike reading and writing, the ability to talk and understand speech is innate and requires no formal training. One is born with the ability to talk, as well as the ability to see, hear, feel, and so on. However, one must receive training in reading, spelling and mathematics as these abilities are acquired only with some difficulty and much effort. On the other hand, just as one must be exposed to light or he will lose the ability to see complex forms, one must be exposed to language or he will lose the ability to talk or understand human speech.
In his book Syntactic Structures, Chompsky (1957) argues that all human beings are endowed with an innate ability to acquire language as they born able to speak in the same fashion, albeit according to the tongue of their culture, environment and parents. They possess all the rules which govern how language is spoken and they process and express language in accordance with these innate temporal-sequential motoric rules which we know as grammar.
Because they possess this structure, which in turn is imposed by the structure of our nervous system, children are able to learn language even when what they hear falls outside this structure and is filled with errors. That is, they tend to produce grammatically correct speech sequences, even when those around them fail to do so. They disregard errors because they are not processed by their nervous system which acts to either impose order even where there is none, or to alter or delete the message altogether. It is because humans possess these same Wernicke-inferior parietal lobe "deep structures" that speakers are also able to realize generally when a sentence is spoken in a grammatically incorrect fashion.
It is also because apes, monkeys, and dogs and cats do not possess these deep structures that they are unable to speak or produce grammatically complex sequences or gestures. Hence, their vocal production does not become punctuated by temporal-sequential gestures imposed upon auditory input or output by the Language Axis.
LANGUAGE ACQUISITION: FINE TUNING THE AUDITORY SYSTEM
The auditory cortex is organized so as to extract non-random sounds form noise, and in this manner in adaptive for perceiving and detecting animals sounds and human speech (Nelken et al., 2008). However, by time humans have reached adulthood, they have learned to attend to certain language-related sounds and to generally ignore those linguistic features that are not common to speakers native tongue. Some sounds are filtered and ignored as they are irrelevant or meaningless. Humans also lose the ability to hear various sounds because of actual physical changes, such as deterioration and deafness, which occur within the auditory system.
Initially, however, beginning at birth and continuing throughout life there is a much broader range of generalized auditory sensitivity. It is this generalized sensitivity that enables children to rapidly and more efficiently learn a foreign tongue, a capacity that decreases as they age (Janet et al. 2004). It is due, in part, to these same changes that generational conflicts regarding what constitutes "music" frequently arise.
Nevertheless, since much of what is heard is irrelevant and is not employed in the language the child is exposed to, the neurons involved in mediating their perception either drop out and die from disuse, which further restricts the range of sensitivity. This further aids the fine tuning process so that, for example, one's native tongue can be learned (Janet et al. 2004; Joseph 1993).

For example, no two languages have the same set of phonemes. It is because of this that to members of some cultures certain English words, such as pet and bet, sound exactly alike. Non-English speakers are unable to distinguish between or recognize these different sound units. Via this fine tuning process, only those phonemes essential to one's native tongue are attended to.
Language differs not only in regard to the number of phonemes, but the number which are devoted to vowels vs consonants and so on. Some Arabic dialects have 28 consonants and 6 vowels. By contrast, the English language consists of 45 phonemes which include 21 consonants, 9 vowels, 3 semivowels (y, w, r),4 stress, 4 pitches, 1 juncture (pauses between words) and 3 terminal contours which are used to end sentences (Fodor & Katz 1964; Slobin 1971).
It is from these 45 phonemes that all the sounds are derived which make up the infinity of utterances that comprise the English language. However, in learning to attend selectively to these 45 phonemes, as well as to specific consonants and vowels, required that the nervous system become fine tuned to perceiving them while ignoring others. In consequence, those cells which are unused, die (Joseph 1993).
Children are able to learn their own as well as foreign languages with much greater ease than adults because initially infants maintain a sensitivity to a universal set of phonetic categories (e.g. Janet et al. 2004). Because of this they are predisposed to hearing and perceiving speech and anything speech-like regardless of the language employed.
These sensitivities are either enhanced or diminished during the course of the first few years of life so that those speech sounds which the child most commonly hears becomes accentuated and more greatly attended to such that a sharpening of distinctions occurs (Janet et al. 2004). However, this generalized sensitivity in turn declines as a function of acquiring a particular language and presumably the loss of nerve cells not employed (see chapter 15 for related discussion). The nervous system becomes fine tuned so that familiar language-like sounds become processed and ordered in the manner dictated by the nervous system; i.e., the universal grammatical rules common to all languages.
Fine tuning is also a function of experience which in turn exerts tremendous influence on nervous system development and cell death. Hence, by the time most people reach adulthood they have long learned to categorize most of their new experiences into the categories and channels that have been relied upon for decades.
Nevertheless, in consequence of this filtering, sounds that arise naturally within one's environment can be altered, rearranged, suppressed, and thus erased. By fine tuning the auditory system so as to learn culturally significant sounds and so that language can be acquired occurs at a sacrifice. It occurs at the expense of one's natural awareness of their environment and its orchestra of symphonic sounds. In other words (at least from the perspective of the left hemisphere), the fundamental characteristics of reality are subject to language based alterations (Sapir 1966; Whorf 1956).

According the Edward Sapir (1966) "Human beings are very much at the mercy of the particular language which has become the medium of their society...the real world is to a large extent built up on the language habits of the group. No two language are ever sufficiently similar to be considered as representing the same social reality."
According to Benjamin Whorf (1956) "language.... is not merely a reproducing instrument for voicing ideas but rather is itself the shaper of ideas... We dissect nature along lines laid down by language." However, Whorf believed that it was not just the words we used but grammar which has acted to shape human perceptions and thought. A grammatically imposed structure forces perceptions to conform to the mold which gives them not only shape, but direction and order.
Hence, distinctions imposed by temporal order and the employment of linguistically based categories and the labels and verbal associations which enable them to be described can therefore enrich as well as alter thoughts and perceptual reality. This is because language is intimately entwined with conscious experience.
For example, Eskimos possess an extensive and detailed vocabulary which enables them to make fine verbal distinctions between different types of snow, ice, and prey, such as seals. To a man born and raised in Kentucky, snow is snow and all seals may look the same. But if that same man from Kentucky had been raised around horses all his life he may in turn employ a rich and detailed vocabulary so as to describe them, e.g. appaloosa, paint, pony, stallion, and so on. However, to the Eskimo a horse may be just a horse and these other names may mean nothing to him. All horses look the same.


Moreover, both the Eskimo and the Kentuckian may be completely bewildered by the thousands of Arabic words associated with camels, the twenty or more terms for rice used by different Asiatic communities, or the seventeen words the Masai of Africa use to describe cattle.
Nevertheless, these are not just words and names, for each cultural group are also able to see, feel, taste, or smell these distinctions as well. An Eskimo sees a horse but a breeder may see a living work of art whose hair, coloring, markings, stature, tone, height, and so on speak volumes as to its character, future, and genetic endowment.
Through language one can teach others to attend to and to make the same distinctions and to create the same categories. In this way, those who share the same language and cultural group, learn to see and talk about the world in the same way, whereas those speaking a different dialect may in fact perceive a different reality. Thus, the linguistic foundations of conscious experience, and thus linguistic-perceptual consciousness can be shaped by language.
For example, when A.F. Chamberlain (1903), visited the Kootenay and Mohawk Indians of Brittish Columbia during the late 1800s, he noted that they even heard animal and bird sounds differently from him. For example, when listening to some owls hooting, he noted that to him it sounded like "tu-whit-tu-whit-tu-whit," whereas the Indians heard "Katskakitl." However, once he became accustomed to their language and began to speak it, he soon developed the ability to hear sounds differently once he began to listen with his "Indian ears." When listening to a whip poor will, he noted that instead of saying whip-poor-will, it was saying "kwa-kor-yeuh."
Observations such as these thus strongly suggest that if one were to change languages they might change their perceptions and even the expression of their conscious thoughts and attitudes. Consider for example, the results from an experiment reported by Farb (1975). Bilingual Japanese born women married to American Serviceman were asked to answer the same question in English and in Japanese. The following responses were typical.
"When my wishes conflict with my family's..."
"....it is a time of great unhappiness (Japanese)
....I do what I want. (English)
"Real friends should...."
"...help each other." (Japanese)
"...be very frank." (English)
Obviously, language does not shape all attitudes and perceptions. Moreover, language is often a consequence of these differential perceptions, which in turn requires the invention of new linguistic labels so as to describe these new experiences. Speech of course is also filtered through the personality of the speaker, whereas the listener is also influenced by their own attitudes, feelings, beliefs, prejudices and so on, all of which can affect what is said, how it is said, and how it is perceived and interpreted (Joseph 1993).
In fact, regardless of the nature of a particular experience, be it visual, olfactory or sexual, language not only influences perceptual and conscious experiences but even the ability to derive enjoyment from them, for example, by labeling them cool, hip, sexy, bad, or sinful, and by instilling guilt or pride. In this manner, language serves not only to label and filter reality, but affects one's ability to enjoy it.
SPATIAL LOCALIZATION, ATTENTION & ENVIRONMENTAL SOUNDS
In conjunction with the inferior colliculus and the frontal lobe (Graziano et al., 2008), and due to bilateral auditory input, the primary auditory area plays a significant role in orienting to and localizing the source of various sounds (Sanchez-Longo & Forster, 1958); for example, by comparing time and intensity differences in the neural input from each ear. A sound arising from one's right will reach and sound louder to the right ear as compared to the left.
Indeed, among mammals, a considerable number of auditory neurons respond or become highly excited only in response to sounds from a particular location (Evans & Whitfield, 1968). Moreover, some of these neurons become excited only when the subject looks at the source of the sound (Hubel et al., 1959). Hence, these neurons act so that location may be identified and fixated upon. In addition to the frontal lobe (Graziano et al., 2008) these complex interactions probably involve the parietal area (7), as well as the midbrain colliculi and limbic system. As based on lesions studies in humans, the right temporal lobe is more involved than the left in discerning location (Nunn et al., 2008; Penfield & Evans, 1934; Ploner et al., 199; Shankweiler, 1961).
There is also some indication that certain cells in the auditory area are highly specialized and will respond only to certain meaningful vocalizations (Wollberg & Newman, 1972). In this regard they seemed to be tuned to respond only to specific auditory parameters so as to identify and extract certain meaningful features, i.e. feature detector cells. For example, some cells will respond to cries of alarm and sounds suggestive of fear or indicating danger, whereas others will react only to specific sounds and vocalizations.
Nevertheless, although the left temporal lobe appears to be more involved in extracting certain linguistic features and differentiating between semantically related sounds (Schnider et al. 2014), the right temporal region is more adept at identifying and recognizing acoustically related sounds and non-verbal environmental acoustics (e.g. wind, rain, animal noises), prosodic-melodic nuances, sounds which convey emotional meaning, as well as most aspects of music including temp and meter (Heilman et al. 1975, 2004; Joseph, 1988a; Kester et al. 2011; Schnider et al. 2014; Spinnler & Vignolo 1966).
Indeed, the right temporal lobes spatial-localization sensitivity coupled with its ability to perceive and recognize environmental sounds no doubt provided great survival value to early primitive man and woman. That is, in response to a specific sound (e.g. a creeping predator), one is able to immediately identify, localize, locate, and fixate upon the source and thus take appropriate action. Of course, even modern humans relie upon the same mechanisms to avoid cars when walking across streets or riding bicycles, or to ascertain and identify approaching individuals, etc.
HALLUCINATIONS
Electrical stimulation of Heschyl's gyrus produces elementary hallucinations (Penfield & Jasper, 1954; Penfield & Perot, 1963). These include buzzing, clicking, ticking, humming, whispering, and ringing, most of which are localized as coming from opposite side of the room. Tumors involving this area also give rise to similar, albeit transient hallucinations, including tinnitus (Brodal, 2011). Patients may complain that sounds seem louder and/or softer than normal, closer and/or more distant, strange or even upleasent (Hecaen & Albert, 1978). There is often a repetitive quality which makes the experience even more disagreeable.
In some instances the hallucination may become meaningful. These include the sound of footsteps, clapping hands, or music, most of which seem (to the patient) to have an actual external source.
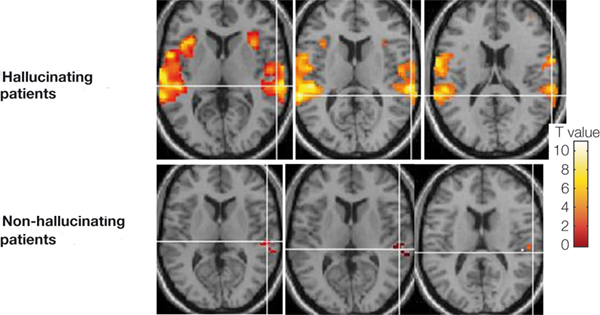
Penfield and Perot (1963) report that electrical stimulation of the superior temporal gyrus, the right temporal lobe in particular results in musical hallucinations. Patients with tumors and seizure disorders, particularly those involving the right temporal region, may also experience musical hallucinations. Frequently the same melody is heard over and over. In some instances patients have reported the sound of singing voices and individual instruments may be heard (Hecaen & Albert, 1978).
Conversely, it has been frequently reported that lesions or complete surgical destruction of the right temporal lobe significantly impaires the ability to name or recognize melodies and musical passages. It also disrupts time sense, the perception of timbre, loudness, and meter (Chase, 1967; Joseph, 1988a; Kester et al. 2011; Milner, 1962; Shankweiler, 1966).
Auditory verbal hallucinations seem to occur with right or left temporal destruction or stimulation (Hecaen & Albert, 1978; Penfield & Perot, 1963; Tarachow, 1941) --although left temporal involvement is predominant. The hallucination may involve single words, sentences, commands, advice, or distant conversations which can't quite be made out. According to Hecaen and Albert (1978), verbal hallucinations may precede the onset of an aphasic disorder, such as due to a developing tumor or other destructive process. Patients may complain of hearing "distorted sentences", "incromprehensible words" etc.
CORTICAL DEAFNESS
In some instances, such as due to middle cerebral artery stroke, the primary auditory receiving areas of the right or left cerebral hemisphere and/or the unerlying "white matter" fiber tracts may be destroyed. This results in a disconnection syndrome such that sounds relayed from the thalamus cannot be received or anlayzed by the temporal lobe. In some cases, however, the strokes may be bilateral. When both primary auditory receiving areas and their subcortical axonal connections have been destroyed the patient is said to be suffering from cortical deafness (Goodglass & Kaplan, 2008; Tanaka et al. 2011).
However, sounds continue to be processed subcortically --much like cortical blindness. Hence the ability to hear sounds per se, is retained. Nevertheless, since the sounds which are heard are not received neocortically and thus cannot be transmitted to the adjacent association areas, sounds become stripped of meaning. That is, meaning cannot be extracted or assigned and the patient becomes agnosic for auditory stimuli (Albert et al. 1972; Schnider et al. 2014; Spreen et al. 1965). Rather, only differences in intensity can be discerned --much like cortical blindness.



When lesions are bilateral, patients cannot respond to questions, do not show startle responses to loud sounds, lose the ability to discern the melody for music, cannot recognize speech or enviornmental sounds, and tend to experience the sounds they do hear as distorted and disagreeable, e.g. like the baning of tin cans, buzzing and roaring, etc. (Albert et al. 1972; Auerbach et al., 1982; Earnest et al. 1977; Kazui et al. 1990; Mendez & Geehan, 1988; Reinhold, 1950; Tanaka et al. 2011).
Commonly such patients also experience difficulty discriminating sequences of sound, detecting difference in temporal patterning, and determining sound duration wheareas intensity discriminations are better preserved. For example, when a 66 year old right handed male suffering from bitemporal subcortical lesions, "woke up in the morning and turned on the television, he found himself unable to hear anything but buzzing noises. He then tried to talk to himself, saying "This TV is broken." However, his voices sounded only as noise to him. Although the patient could hear his wife's voice, he could not interpret the meaning of her speech. He was also unable to identify many environmental sounds" (Kazui, et al. 1990; p. 477).
Nevertheless, individuals who are cortically deaf are not aphasic (e.g. Kazui et al. 1990. They can read, write, speak, comprehend pantomime, and are fully aware of their deficit. However, afflicted individuals may also display auditory inattention (Hecaen & Albert, 1978) and a failure to respond to loud sounds. Nevertheless, although not aphasic, per se, speech is sometimes noted to be hypophonic and contaminated by occasional literal paraphasias.
In some instances, rather than bilateral, a destructive lesion may be limited to the primary receving area of just the right or left cerebral hemisphere. These patient's are not considered cortically deaf. However, when the auditory receiving area of the left temporal lobe is destroyed the patient suffers from a condition referred to as pure word deafness. If the lesion is in the right temporal receiving area, the patient is more likely to suffer a non-verbal auditory agnosia (Schnider et al. 2014).
PURE WORD DEAFNESS
With a destructive lesion involving the left auditory receiving area, Wernicke's area becomes disconnected from almost all sources of acoustic input and patients are unable to recognize or perceive the sounds of language, be it sentences, single words, or even single letters (Hecaen & Albert, 1978). All other aspects of comprehension are preserved, including reading, writing, and expressive speech.
Moreover, if the right temporal region is spared, the ability to recognize musical and environmental sounds is preserved. However, the ability to name or verbally describe them is impaired --due to disconnection and the inability of the right hemisphere to talk.
Pure word deafness is most common with bilateral lesions in which case environmental sound recognition is also effected (e.g. Kazui et al. 1990). In these instances the patient is considered cortically deaf. Pure word deafness is more common with bilateral lesions simply because this prevents the any remaining intact auditory areas in the left hemisphere from receiving auditory input from the right temporal lobe via the corpus callosum.
Pure word deafness, when due to a unilateral lesion of the left temporal lobe, is partly a consequence of an inability to extract temporal-sequential features from incoming sounds. Hence, linguistic messages cannot be recognized. However, pure word deafness can sometimes be partly overcome if the patient is spoken to in an extremely slowed manner (Albert & Bear, 1974). The same is true of those with Wernicke's aphasia.
AUDITORY AGNOSIA
An individual with cortical deafness, due to bilateral lesions suffers from a generalized auditory agnosia involving words and non-linguistic sounds. However, in many instances an auditory agnosia, with preserved perception of language may occur with lesions restricted to the right temporal lobe (Fujii et al., 1990). In these instances, an individual loses the capability to correctly discern environmental (e.g. birds singing, doors closing, keys jangling) and acoustically related sounds, emotional-prosodic speech, as well as music (Nielsen, 1946; Ross, 1993; Samson & Zattore, 1988; Schnider et al. 2014; Spreen et al. 1965; Zatorre & Hapern, 1993).
These problems are less likely to come to the attention of a physician unless accompanied by secondary emotional difficulties. That is, most individuals with this disorder, being agnosic, would not know that they have a problem and thus would not complain. If they are their families notice (for example, if a patient does not respond to a knock on the door) the likelihood is that the problem will be attributed to faulty hearing or even forgetfulness.
However, because such individuals may also have difficulty discerning emotional- melodic nuances, it is likely that they will misperceive and fail to comprehend a variety of paralinguistic social-emotional messages; a condition referred to as social-emotional agnosia (Joseph, 1988a) and phonagnosia (van Lancker, et al., 1988). This includes difficulty correctly identifying the voices of loved ones or friends, or discerning what others may be implying, or in appreciating emotional and contextual cues, including variables such as sincerity or mirthful intonation. Hence, a host of behavioral difficulties may arise (see chapter 10).

For example, a patient may complain that his wife no longer loves him, and that he knows this from the sound of her voice. In fact, a patient may notice that the voices of friends and family sound in some manner different, which, when coupled with difficulty discerning nuances such as humor and friendliness may lead to the development of paranoia and what appears to be delusional thinking. Indeed, unless appropriately diagnoses it is likely that the patients problem will feed upon and reinforce itself and grow more severe.
It is important to note that rather than completely agnosic or word deaf, patients may suffer from only partial deficits. In these instances they may seem to be hard of hearing, frequently misinterpret what is said to them, and/or slowly develop related emotional difficulties.

THE AUDITORY ASSOCIATION AREAS
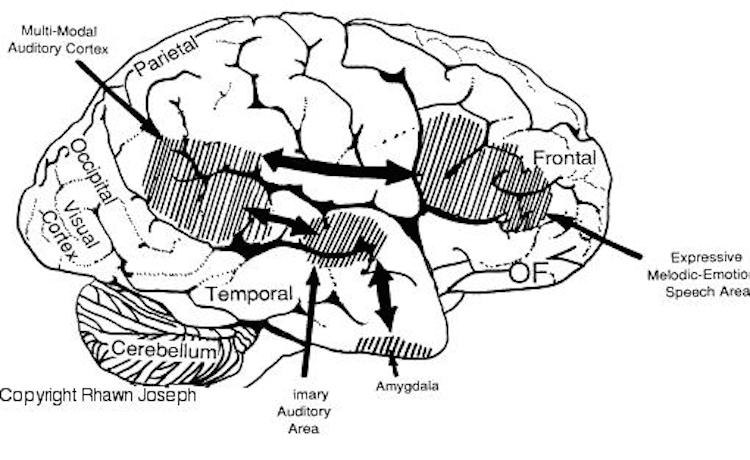
The auditory area although originating in the depths of the superior temporal lobe extends in a continuous belt-like fashion posteriorly from primary and association (e.g. Wernicke's) area toward the inferior parietal lobule, and via the arcuate fasciculus onward toward Broca's area. Indeed, in the left hemisphere, this massive rope of interconnections forms an Axis such that Wernickes Area, the inferior parietal lobule, and Broca's area. These areas often become activated in parallel or simultaneously during language tasks, and together, are able to mediate the perception and expression of most forms of language and speech (Joseph, 1982, Goodglass & Kaplan, 2000; Ojemann, 2011).
The rope-like arcuate and inferior and superior fasciculi are bidirectional fiber pathways, however, that run not only from Wernickes through to Broca's area but extends inferiorly deep into the temporal lobe where contact is established with the amygdala. Hence, via these connections auditory input comes to be assigned emotional-motivational significance, whereas verbal output becomes emotionally-melodically colored . Within the right hemisphere, these interconnections which include the amygdala appear to be more extensively developed.

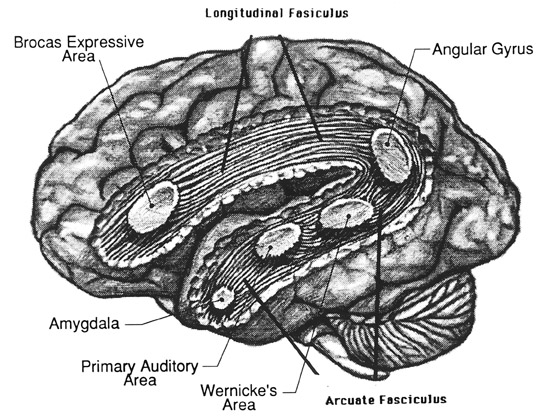
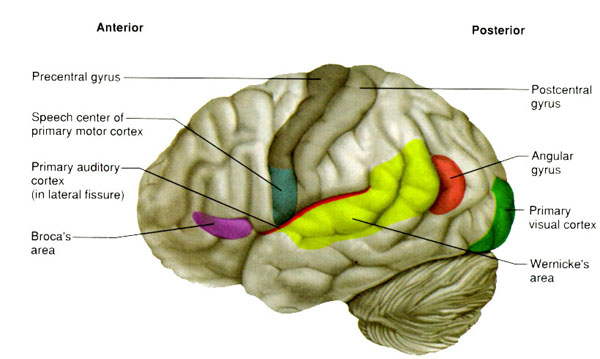
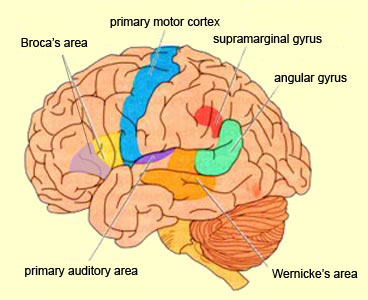
WERNICKE'S AREA
Following the analysis performed in the primary auditory receiving area, auditory information is transmitted to the immediately adjacent association cortex (Brodmanns area 22), where more complex forms of processing take place. In both hemispheres this region partly surrounds the primary area and then extends posteriorly merging with the inferior parietal lobule with which it maintains extensive interconnections. Within the left hemisphere the more posterior portion of area 22, and part of area 42 corresponds to Wernicke's area.
Wernickes area, and the corresponding region in the right hemisphere are not merely auditory association areas as they also receive a convergence of fibers from the somesthetic and visual association cortices (Jones & Powell, 1970; Zeki, 1978b). These interlinking pathways include the inferior and middle temporal lobes which are also multi-modal areas concerned with language, somesthesis, and complex visual functioning (Buchel et al., 1998; Luders et al., 1986; Nobre et al., 2014; Price, 2011), and the immediately adjacent inferior parietal lobule which becomes active when viewing words (Bookheimer, et al., 1995; Vandenberghe, et al., 1996; Menard, et al., 1996; Price, 2011) when performing syllable judgements (Price, 2011), and when reading ( Bookheimer, et al., 1995; Menard, et al., 1996; Price, et al., 1996).

The auditory association area (area 22) also receives fibers from the contralateral auditory association area via the corpus callosum and anterior commissure and projects to the frontal convexity and orbital region, the frontal eye fields, and cingulate gyrus (Jones & Powell, 1970). Hence, Wernicke's area receives input from a variety of convergence zones, and is involved in multimodal as well as auditory associational analysis and becomes highly active during a variety of language tasks (Buchel et al., 1998; Price, 2011).
Broadly considered, Wernicke's area encompasses a large expanse of tissue that extends from the posterior superior and middle temporal lobe to the IPL and up a over the parietal operculum, around the sylvian fissure, extending just beyond the supramarginal gyrus. Electrical stimulation, the surgical destruction, or lesions of this region results in significant language impairments (Kertesz, 1979; Penfield & Roberts, 1959; Ojemann, 2011). However, this vast expanse of cortex is actually made up of a mosaic of speech area, each concerned with different aspects of language, including comprehension, word retrieval, naming, verbal memory, and even expressive speech.
For example, only 36% of stimulation sites in the classically defined Wernicke's area (anterior to the IPL), interfere with naming, whereas phoneme identification is significantly impaired (Ojemann, 2011). By contrast, and as noted above, the IPL (which is an extension of Wernicke's area) becomes highly active when retrieving words or engaged in naming. In this regard, the IPL and Wernicke's area interact so as to comprehend and express the sounds and words of language--all of which is then transmitted via the arcuate fasiculus to Broca's area.
RECEPTIVE APHASIA
When the posterior portion of the left auditory association area is damaged there results severe receptive aphasia i.e. Wernicke's aphasia. Individuals with Wernicke's aphasia, in addition to severe comprehension deficits usually suffer abnormalities involving expressive speech, reading, writing, repeating, word finding, etc. Spontaneous speech, however, is often fluent, and sometimes hyperfluent such that they speak at an increased rate and seem unable to bring sentences to an end --as if the temporal-sequential separations between words have been extremely shortened or in some instances abolished. In addition, many of the words spoken are contaminated by neologistic and paraphasic distortions. As such, what they say is often incomprehensible (Chirstman 2014; Goodglass & Kaplan 2008; Hecaen & Albert, 1978). Hence, this disorder has also been referred to as fluent aphasia.
Individuals with Wernicke's aphasia, however, are still able to perceive the temporal-sequential pattern of incoming auditory stimuli to some degree (due to perseveration of the primary region). Nevertheless, they are unable to perceive spoken words in their correct order, cannot identify the pattern of presentation (e.g. two short and three long taps) and become easily overwhelmed (Hecaen & Albert, 1978; Luria, 1980).
Patients with Wernicke's aphasia, in addition to other impairments, are necessarily "word deaf". As these individuals recover from their aphasia, word deafness is often the last symptom to disappear (Hecaen & Albert, 1978).
THE MELODIC-INTONATIONAL AXIS
It has been consistently demonstrated among normals (such as in dichotic listening studies), that the right temporal lobe (left ear) predominates in the perception of timbre, chords, tone, pitch, loudness, melody, and intensity --the major components (in conjunction with harmony) of a musical stimulus (chapter 10). For example, when listening to Bach (the third movement of Bach's Italian concerto), the right temporal lobe becomes highly active, whereas when performing scales activity increases in the left temporal lobe (Parsons & Fox, 2011). Likewise, Evers and colleagues (2008) in evaluating cerebral blood velocity, found that a right hemisphere increase in blood flow when listening to harmony (but not rhythm), among non-musicians in general, and especially among females. Rhythm increased left hemisphere activity (Evers et al., 2008). Hence, the left hemisphere is clearly dominant in regard to the rhythmical and temporal sequential aspects of a musical stimulus (Evers et al., 2008; Parsons & Fox, 2011).
In part, it is due to its dominance for perceiving melodic information that the right temporal lobe becomes activated when engaged in a variety of language tasks. For example, the right temporal and parietal areas are activated when reading (Bottini et al., 2014; Price et al., 1996), and the right temporal lobe becomes highly active when engage in interpreting the figurative aspects of language (Bottini et al., 2014).
When the right temporal lobe is damaged (e.g. right temporal lobectomy, right amygdalectomy) there results a disrupts in time sense, rhythm, the ability to sing, carry a tune, perceive, recognize or recall tones, loudness, timbre, and melody. Similarly, the ability to recognize even familiar melodies and the capacity to obtain pleasure while listening to music is abolished or disrupted or significantly reduced; a condition referred to as amusia.
For example, a woman described by Takeda and colleagues (1990) and who was described as an expert singer of traditional Japanese songs, and who would accompany them on an instrument referred to as a samisen, lost this ability following a hemorrhagic stroke of the of the right superior gyrus including Heschl's gyrus. Although she was able to accurately produce the rhythmic aspects of music, melody and pitch were abolished.
In addition, lesions involving the right temporal-parietal area, have been reported to significantly impair the ability to perceive and identify environmental sounds, comprehend or produce appropriate verbal prosody, emotional speech, or to repeat emotional statements (Joseph, 1988a; Ross, 1993; van Lancker et al., 1988). Indeed, when presented with neutral sentences spoken in an emotional manner, right temporal-parietal damage has been reported to disrupt the perception and comprehension of emotional prosody regardless of its being positive or negative in content (see chapter 10).
Hence, the right temporal-parietal area is involved in the perception, identification, and comprehension of environmental and musical sounds and various forms of melodic and emotional auditory stimuli, and probably acts to prepare this information for expression via transfer to the right frontal convexity which is dominant regarding the expression of emotional-melodic and even environmental sounds. Indeed, it appears that an emotional-melodic-intonational Axis, somewhat similar to the Language Axis in anatomical design is maintained within the right hemisphere (Joseph 1982, 1988a; Gorelick & Ross, 2007; Ross, 1993).
When the posterior portion of the Melodic-Emotional Axis is damaged, the ability to comprehend or repeat melodic-emotional vocalizations in disrupted. Such patients are thus agnosic for non-linguistic sounds. With right frontal convexity damage speech becomes bland, atonal, and monotone.
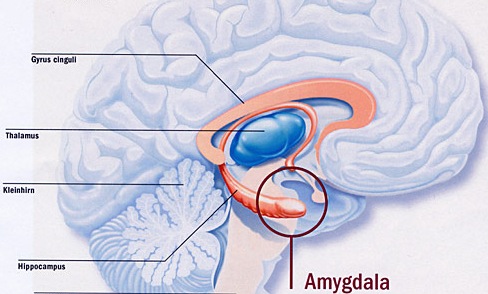
THE AMYGDALA.
As detailed in chapters 13 and 15, the right cerebral hemisphere appears to maintain more extensive as well as bilateral interconnections with the limbic system. Indeed, the limbic system also appears to be lateralized in regard to certain aspects of emotional functioning such that the right amygdala, hippocampus and hypothalamus seem to exert dominant influences.
As noted, the arcuate fasciculus extends from the amygdala (which is buried within the anterior-inferior temporal lobe) through the auditory association area and inferior parietal region and into the frontal convexity. It is possibly through these interconnections that emotional colorization is added to neocortical acoustic perceptions as well as sounds being prepared for expression. In fact, the human amygdala can produce as well as perceive emotional vocalizations (Halgren, 2002; Heit, Smith, & Halgren, 1988). Moreover, when the right amygdala has been destroyed or surgically removed, the ability to sing as well as to properly intonate is altered (see Chapter 13). In this regard, the amygdala should be considered part of the melodic-intonational axis of the right hemisphere as it not only subcortically responds to and analyzes environmental sounds and emotional vocalizations but imparts emotional significance to auditory input and output processed and expressed at the level of the neocortex.
Nevertheless, it is possible that emotional-prosodic intonation is imparted directly to speech variables as they are being organized for expression within the left hemisphere. That is, although the right hemisphere is dominant in regard to melodic-emotional vocalizations, the two amygdalas are in direct communication via the anterior commissure. Hence, although originating predominantly within the right amygdala/hemisphere, these influences can be directly transmitted to the left half of the brain via the left amygdala and through anterior commissure and via the arcuate fasciculus which transmits this data into the linguistic stream of the Language Axis.
THE INFERIOR & MIDDLE TEMPORAL LOBE
The basal temporal region can be subdivided into a medial, inferior, middle, and anterior and posterior zones, harbors the amygdala and hippocampus in its depths. These tissues are extensively interconnected, and project to the auditory association area, frontal convexity, and orbital region (Jones & Powell, 1970; Pandya & Kuypers, 1969). The inferior arcuate fasciculus in it's journey from the amygdala to Wernickes areas also passes through this neocortical auditory territory. Hence, when considered a whole, the inferior and middle temporal lobe is a complex multifunctional tissue which is functionally lateralized and has extensive visual and auditory and linguistic capabilities.
In general, the anterior basal temporal lobe is more involved in auditory functioning, whereas the posterior basal temporal lobe is more intimately concerned with the processing of higher level visual information including form recognition and (in the left temporal lobe) the reading of the written language. Indeed, this generalized dichotomy, first proposed in the 1990 edition of this text, has since been born out by a variety of functional imaging studies and is supported by lesions and neurosurgical stimulation studies.
Specifically, the left inferior-middle posterior temporal lobe (Brodmann's area 37), is located between the visual cortex and the anterior temporal cortex and becomes activated during a variety of language tasks, including reading and object and letter naming (Price, 2011). This has been demonstrated not only by functional imaging (Buchel et al., 1998; Price, 2011), but direct cortical recoding (Nobre et al., 2014), and electrical stimulation (Luders et al., 1986). In fact, both normal, cognitally blind, and late-blind subject display activity in this area (Buchel et al., 1998). For example, reading of concrete and abstract words activates the left posterior basal temporal lobe and the left frontal operculum (Buchel et al., 1998).
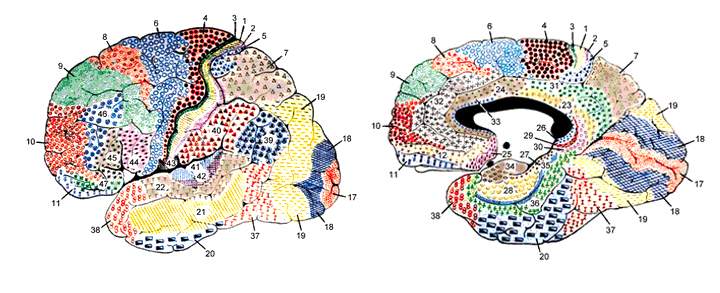
Hence, because of its role in language, when the middle temporal lobe is stimulated there can result aphasic abnormalities (Penfield & Rasmussen, 1950). Lesions involving this area are also associated with subtle disturbances involving language, including word finding difficulty, confrontive naming deficits, abnormalities in the maintenance of temporal order and sequence, as well as verbal memory impairments (Luria, 1980). Patients may suffer from reading and naming deficits (Rapcsak, et al., 2007); a condition referred to as phonological alexia. Moreover, patients with developmental dyslexia have been found to have abnormalities in this area (Rumsey et al., 2011).
As noted, left temporal lobe activity increases as word length increases. Hence, with injuries in this area, including the basal temporal lobe, patients may have difficulty recalling word lists or order of word presentation, such that the longer the list, the greater the difficulty. Hence, if a list of four words were read, the patient cannot repeat the correct order even after repeated presentations, although individuals words may be recalled correctly (Luria, 1980).
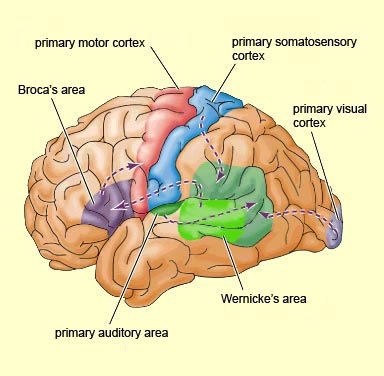
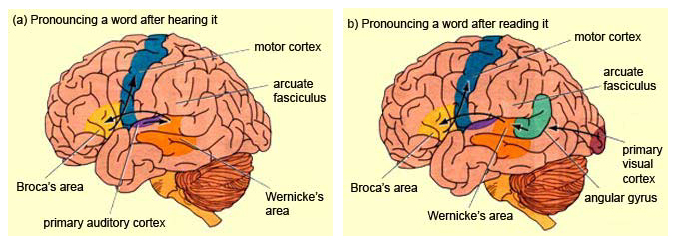
However, the nature of the disturbances depends on the exact location of the lesion (Coltheart, 1998; Miozzo & Caramazza, 1998), for as noted, the anterior and posterior and inferior and middle temporal lobes perform interrelated but also divergent functions. In general, the more posterior aspect is concerned with the visual aspects of perception and language, including reading and object naming. Indeed, the left posterior basal temporal lobe is a convergence zone for visual, auditory, and tactile information. Hence, patients may suffer from alexia as well as visual and tactile anomia if this area is injured (Rapcsak, et al., 2007). In addition, these structures are lateralized, and although both the right and left temporal lobe become activated by various language and reading tasks (reviewed in Price, 2011), the right basal temporal lobe is much more concerned with non-verbal visual functioning.
THE 'VISUAL" MIDDLE INFERIOR TEMPORAL LOBE
The middle temporal lobe, (MTL) like other structures throughout the brain, appears to be functionaly lateralized such that the right temporal region is more involved in visual and non-verbal auditory functioning, whereas the left MTL is more concerned with auditory-linguistic capabilities. Hence, whereas the auditory-linguistic zones appear to be more extensive within the left temporal lobe (and include portions of the posterior temporal region), the right MTL seems to be more associated with visual responsiveness. In general, however, the middle temporal gyrus of both hemispheres appear to contain visually responsive neurons (Beckers & Zeki 1995; Felleman & Kaas, 1974; Heit et al. 1990; Maunsell & Van Essen 2004; Selemon et al. 2014). Nevertheless, since there have been relatively few published studies of the effects of lesions or the functional capabilities of the middle temporal lobes in humans, notions regarding the extensiveness of auditory vs. visual lateralized representation are admittedly speculative.
MIDDLE AND INFERIOR TEMPORAL LOBE VISUAL FUNCTIONING
Visual cells in the MTL receive direct projections from the striate cortex, area 17 and the association area 18 (Wall et al. 1982). This area in turns projects to the MTL of the opposite hemisphere via the corpus callosum, ipsilaterally to areas 17, 18, 19, the inferior temporal and the parietal-occipital cortex (Tigges et al., 2011; Wall et al., 1982), and maintains interconnections with the pulvinar, superior colliculus and pontine nuclei of the brainstem (Walls et al., 1982). Hence, this regions has connections with areas concerned with visual and visual attentional capabilities.



MTL neurons are sensitive to speed and direction of stimulus movement, motion, orientation, width, and disparity (Felleman & Kaas, 2004; Maunsell & Van Essen, 1983). However, most MTG neurons are not particular sensitive to the form of a stimulus--most preferring narrow stimuli (Albright et al. 2004; Maunsell & Van Essen, 1983). A large number of cells have binocular capabilities and respond to stimuli from either or both eyes. Hence, these cells are significantly involved in stereoscopic vision as well as determining distance.
Although not specialized for perception of form per se, via the combined analysis of width, orientation, speed and direction of movement these cells can process visual stimuli in a three-dimensional fashion, particularly in regard to trajectory, depth and position, i.e. if an object is near or far, approaching or withdrawing (Maunsell & Van essen, 1983).

Via its interconnections with the parietal-occipital lobe (an area involved in depth perception, visual fixation, and the visual-somesthetic guidance of movement) the MTL appears to provide visual guidance in regard to interactional object and body movement. That is, via the analysis of the flow of movement, local direction and speed, as well as the relative distances of objects, these neurons (in conjunction with area 7 cells) are important in guiding the movements of the body as well as the individuals limbs toward the object (Manunsell & Van Essen,1983).
The MTL maintains extensive interconnections with the inferior region and thus with limbic nuclei. It is probably via these connections that objects or conspecies of motivational significance can be discerned and attended to. That is, not only is the speed, movement, distance, etc. of a stimulus determined, but its emotional attributes (e.g. should it be feared, chased, eaten). Moreover, not just written words, but object recognition occurs in this area (in conjunction with the inferior temporal lobe), such that if injured, patients may suffer from agnosia, i.e. an inability to recognize visual images (Giannokapoulos et al., 2008).

HALLUCINATIONS.
Tumors involving the middle temporal lobe have been associated with the development of auditory and visual hallucinations, dreamy states, and alterations in emotional functioning--particularly as the lesion encroaches on the inferior regions (Luria, 1980). Electrical stimulation also initiates the development of complex hallucinations and alterations in consciousness (Penfield & Roberts, 1959).
INFERIOR TEMPORAL LOBE
As noted, it is possible to very loosely define the anterior-inferior temporal lobes as auditory cortex, although neurons in this vicinity receive visual as well as somesthetic input.. Hence, the inferior temporal lobes can respond to auditory as well as emotionally and visually significant stimuli (Gloor, 2011; Nakamura et al., 2014; Tovee et al., 2014).
That the inferior temporal lobe (ITL) processes complex auditory, visual and emotional stimuli is probably a function of it being a derivative of the amygdala (see chapter 14) as well as visual cortex (Diamond 1973; Gloor, 2011). Visual and auditory functions also reflect its reception of extensive (upper visual field) projections from the optic radiations, highly processed input from the visual association areas in the occipital lobes of both cerebral hemispheres (via the corpus callosum), and fibers from the superior colliculus by way of the pulvinar of the thalamus (Chow, 1950; Kaas & Krubitzer 2011; Kuypers et al., 1965; Previc 1990; Rocha-Miranda et al. 1975).
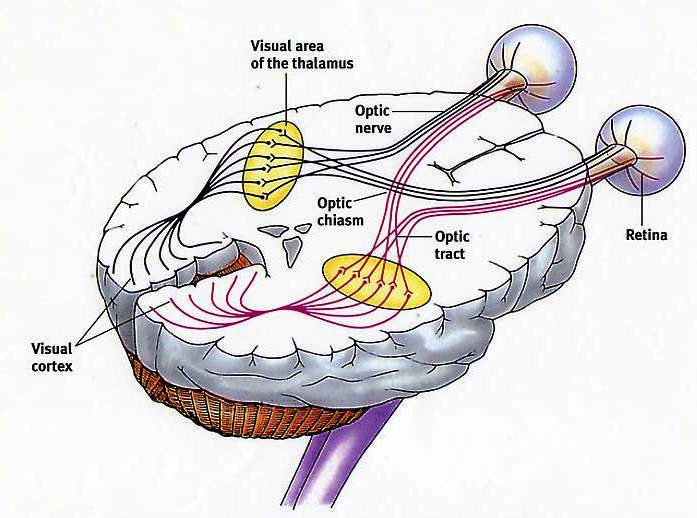
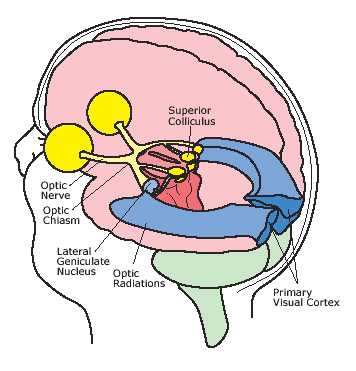
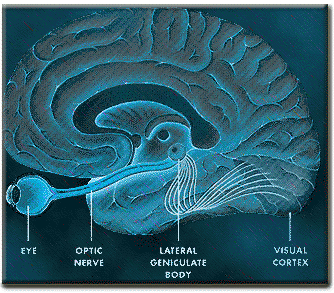
VISUAL CAPABILITIES & FORM RECOGNITION
Indeed, the neocortex of the ITL is specialized for receiving, analyzing, discriminating, recognizing and recalling complex visual information and is involved in attention and visually guided behavior (Gross & Graziano 1995; Gross et al. 1972; Tovee et al. 2014), including the recollection and learning of visual discriminations (Gross, 1972) and memory for objects and spatial locations (Nunn et al., 2008; Ploner et al., 2008). If the temporal lobe is injured, these functions become compromised.
For example, Kimura (1963) found that patients with right vs left temporal lobe injury were impaired when presented with overlapping nonsense shapes and then immediately tested for recognition. Likewise, Meier and French (1965), found that those with right vs left temporal lobe injuries were impaired when asked to make visual discriminations when presented with fragmented concentric circle patterns--skills which are also related to visual closure and gestalt formation. More recently, Nunn et al., (2008) found that right vs left temporal lobectomy patients were more impaired when required to remember and recognize toys and recall their location.
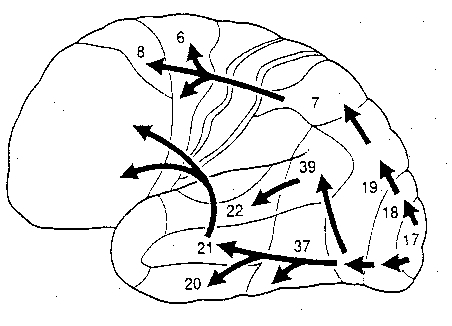
Cells in the ITL have very large, bilateral visual receptive fields which include the fovea, and many are sensitive to direction of stimulus movement, color, contrast, size, shape, orientation and are involved in the perception of three dimensional objects and the supramodal analysis of information already processed in the association areas (Eskander, et al. 2002; Gross & Graziano 1995; Gross, et al. 1972; Nakamura et al. 2014; Rolls, 2002; Sergent, et al.1990). Thus inferior temporal (IT) neurons appear to take part in the last stages of visual analysis for form recognition and receive terminal fibers from the primary and association visual areas (Gloor, 2011; Gross, et al. 1972; Ungerleider & Mishkin, 1982); that is, via the ventral visual stream. Indeed, a single neuron can respond to a combination of these features and many will fire selectively in response to particular shapes and faces (Eskander, et al. 2002; Gross, et al. 1972; Nakamura et al. 2014; Richmond, et al. 1983; Rolls, 2002; Sergent, et al.1990).
FORM & FACIAL RECOGNITION
Overall, the ITL appears to be involved in the highest level of visual integration containing highly developed neurons which seem to be the end station of a hierarchical system which mediates the perception and recognition of specific and particular shapes and forms (Desimone & Schein, 2007; Gross & Graziano 1995; Nakamura et al. 2014; Richmond, et al. 1983; Rolls, 2002.) Indeed, in addition to the optic radiations, there is a pathway (the ventral stream) beginning in the primary visual cortex which passes through areas 18 and 19 and terminates in the ITL (Kuypers et al., 1965; Previc 1990). As information is passed from the primary to these association areas various features important in the identification of specific objects become progressively and hiarchically analyzed and increasingly complex associations are formed.
In fact, based on single cell recordings, some of these ITL neurons have been found to become particularly excited when presented with two dimensional patterns or three dimensional objects such as hands, brushes, and in particular, faces (Deismone & Gross, 1979; Gross et al., 1972; Nakamura et al. 2014; Richmond et al., 1983; Richmond et al.2007). A variety of different feature detectors are found and the majority probably act collectively so as code and assemble a particular shape, including the formation of gestalts and thus the performance of visual closure, particularly the right temporal region (see chapter 10). Via closure an individual can detect a partial stimulus and recognize that it is a face vs a rabbit.
Some cells are responsive to particular facial orientations, such as a profile, some respond to only parts of the face, such as eyes or a mouth, whereas others respond only to the entire face; i.e. a correctly organized facial gestalt. Interestingly, the ITL also contains neurons which will fire even to a scrambled face, so long as all the features are present (Gross et al., 2004). Moreover, some cells respond preferentially to familiar faces, to the faces of certain individuals, to specific emotions conveyed via the face, and the direction of the face including the direction of gaze--especially cells within the amygdala. Specifically, the left amygdala acts to discriminate the direction of another person's gaze, whereas the right amygdala becomes activated while making eye-to-eye contact (Kawashima, et al., 2008). Hence, these cells can determine if someone is looking at them, or looking at someone else, if the facial expression conveys threat, and if there is a hand moving toward or away from the subject or another person.
Hence, these cells are especially equipped for determining social signals and to read the emotions conveyed facially between two people. Interestingly, these same capacities were originally mediated by the olfactory system which in turn gave rise to the amygdala and promoted its differentiation and thus the development of the temporal lobe. In this regard it is also noteworthy that with the bilateral removal of the anterior inferior temporal lobe (thus destroying the amygdala as well as parts of the hippocampus), patients (and non-human primates) develop so called "psychic blindness," and will pick up and smell and taste whatever object captures their attention. In other words, being deprived of an amygdala that can process, visually, social and emotional signals, the amygdalectomized patient/primate reverts back to those behaviors typical of creatures that rely on smell.


AGNOSIA AND TEMPORAL LOBE FUNCTIONAL LATERALITY
As noted, abnormalities affecting the middle temporal lobe (area 37) can result in agnosic disturbances (Giannokapoulos et al., 2008). There are however, to major forms of agnosia, and different subtypes as well, which can be produced by damage to tissues mediating complex visual perception or lesions disconnecting the IPL, for example, from visual, somesthetic, or auditory input.
For example, one type of agnosia, "apperceptive visual agnosia" refers to a disturbance in perceptual and visual-motor integration, such that patients have difficulty copying or matching various objects. This latter form of agnosia has been associated with lesions to the parietal occipital cortex (Mizuno, et al., 1996), as well as to bilateral damage to the inferior-occipital cortex (Shelton, et al., 2014).
By contrast, associative visual agnosia, which is a deficit in naming, such that auditory equivalents cannot be matched to a visual perception, is associated with left inferior and middle temporal (area 37) occipital abnormalities (Giannokapoulos et al., 2008), which may be accompanied with alexia (Feinberg et al., 2014), as well as to lesions to and atrophy of the parietal occipital cortex (Mizuno, et al., 1996).
It is noteworthy that depending on the laterality and location of the injury, e.g., right vs left inferior/medial vs superior temporal lobe, patients may display category specific agnosias. For example, a 27-year old man I examined who had sustained a massive right inferior-posterior temporal lobe injury that also involved surgical removal, was able to recognize pictures of tools (he had been a carpenter) but could not recognize or correctly name pictures of animals and he could not correctly remember facial stimuli that he had been shown five minutes earlier and could not differentiate them from faces he had not seen. By contrast, a 43 year old woman who had been a waitress, and had developed a left inferior temporal lobe glioma that required surgical removal (coupled with chemotherapy) was able to recognize and name pictures of animals and could remember different pictures of faces, but had considerable difficulty recognizing and naming common household objects.
In this regard a 47-year old woman I examined who was subsequently found to have a calcium cyst growing from the skull into the right superior temporal lobe, was able to name pictures of animals, tools and household objects. However, she was almost completely unable to recognize and correctly name animal and humans sounds (e.g. a baby crying, a crowd cheering, a lion roaring) which had been briefly presented, but was better able to recognize non-living sounds such as a creaking door, or a hammer hammering--though these abilities were also compromised.
These findings, which require independent confirmation, raises the possibility that the temporal lobes are not only able to distinguish between living and non-living things including tools and faces, but that the right temporal lobe is specialized for perceiving living creatures (and the sounds they make) whereas the left is specialized for perceiving and naming non-living things, such as tools and household objects.
VISUAL ATTENTION
Attention and visual fixation involves the activation of neurons in the superior parietal and frontal eye fields, the midbrain colliculi, and thalamic nuclei--regions with which the ITL maintains interconnections (Gloor, 2011; Gross & Graziano 1995; Previc 1990). Attention, however, generally requires that visual fixation be focused. Since many ITL neurons maintain large bilateral visual fields, when engaged in visual fixation ITL these cells appear to become partly suppressed. That is, the area of the visual field they respond to becomes restricted to the fovea (Pevic 1990; Richmond et al., 1983). In this manner, ITL neurons become less responsive to other objects such as those in the periphery as the receptive field contracts around the object attended to.
Hence, ITL neurons seems to scan the entire visual field so as to alert the organism to objects of interest or motivational importance (via interconnections with limbic nuclei). When detected, the frontal eye fields, the superior parietal lobe, and subcortical neurons are activated triggering visual fixation. Simultaneously, ITL visual form recognition neurons are activated whereas those with wide non-specific visual fields are inhibited. In this manner, objects of interest are detected and fixated upon (e.g. Previc 1990).
Of adjunctive importance is the middle temporal lobe which in turn can analyze the velocity and direction of the objects movement so that the individual may approach, and, via interaction with cells in area 7, grasp and manipulate the object.
As based on functional imaging (Sergent, et al., 2002), the medial temporal lobe, the right parahippocampal gyrus (in conjunction with areas 19, 37, 36), and the inferior and middle temporal lobe becomes highly active when viewing and categorizing faces and other complex stimuli. Likwise, electrical stimulation of these areas can produce hallucinations and memories of faces of complex visual stimuli (Gloor, 2011; Halgren, 2002).
Damage or removal of the inferior temporal lobe results in loss of the ability to recognize faces, as well as creating severe disturbances involving visual discrimination learning and retention (Braun et al. 2014; Gross & Mishkin, 1977; Mishkin, 1972), and difficulty performing visual closure and recognizing incomplete figural stimuli (Kimura, 1963; Lansdell, 1968,1970). For example, primates with lesions in this vicinity, have severe difficulty learning to discriminate between different shapes and patterns and objects which differ in regard to size or color, although visual acuity is normal.
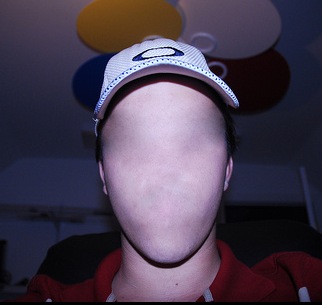
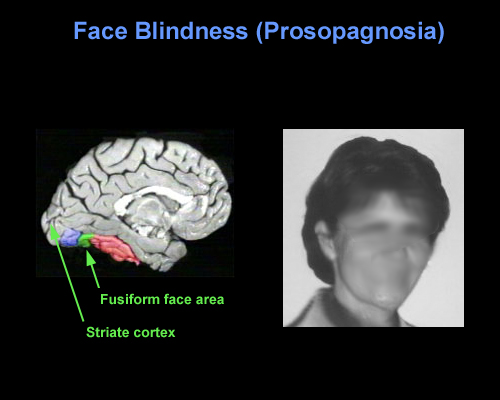
With damage to the right temporal-occipital region, there can result a severe disturbance in the ability to recognize the faces of friends, loved ones, or pets (Braun et al. 2014; DeRenzi, 1986; DeRenzi et. al., 1968; DeRenzi & Spinnler, 1966; Hanley et al. 1990; Hecaen & Angelergues, 1962; Landis et al., 1986; Levine, 1978; Whitely & Warrington, 1977l ) or to discriminate and identify even facial affect (Braun et al. 2014); a condition referred to as prosopagnosia. In fact, with gradual deterioration and degeneration of the right inferior temporal lobe, patients may suffer a progressive prosopagnosia (Evans et al. 1995). Some patients may in fact be unable to recognize their own face in the mirror. For example, one patient was unable to even discriminate between people on the basis of sex but instead had to relie on the presence of details, such as lipstick, rouge, hair length, a moustache, so as to make discriminations (Levine, 1978).
Presumably, the inability to recognize faces is due to the destruction of facial recognition neurons, including those within the amygdala (Young et al. 1995) and hippocampus (which becomes activated when memorizing faces, Kapur et al. 1995). However, because, at a neocortical level, global facial recognition cells appear to be in a minority whereas those specialized for analyzing facial parts are more numerous, the ability to recognize facial details is therefore more likely to be preserved following inferior temporal destruction -particularly if the left temporal lobe is spared.
Nevertheless, the association with the amygdala is particularly important, as facial stimuli are also social emotional stimuli, and facial recognition, for example, that of a friend or loved one, is generally associated with emotional feelings. Moreover it is via the face that emotional intent and affective states, including threat or fear, is conveyed--information that is processed by the amygdala (e.g. Morris et al., 1996). Hence, damage to the overlying temporal lobe may result in prosopagnosia, including an inability to recognize the face of friends or relatives, due to also to disconnection and an inability to attribute and associate the emotion of recognition to these facial stimuli. It is perhaps for this reason, that some patients with prosopagnosia are also unable to recognize their pets or their homes--the emotional significance of these stimuli can no longer be evoked. These stimuli are no longer personalized and thus cannot be recognized.
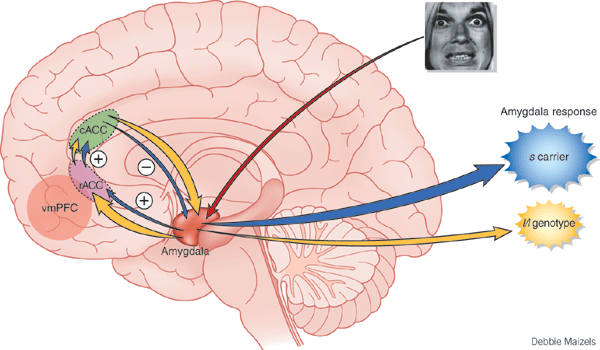
Lateralization & Facial Recognition.
Although patients with prosopagnosia often suffer from bilateral injuries, in many cases the lesions are restricted to the right hemisphere (DeRenzi, 1986; DeRenzi et. al., 1968; DeRenzi & Spinnler, 1966; Evans et al. 1995; Hanley et al. 1990; Hecaen & Angelergues, 1962; Landis et al., 1986; Levine, 1978; Whitely & Warrington, 197l). Disturbances involving facial recognition do not usually occur with isolated left cerebral lesions. Indeed, the right hemisphere appears to be dominant in regard to the recognition of both familiar and unfamiliar faces as has been well demonstrated in numerous studies of brain injured as well as normal, intact individuals (Bradshaw et al. 1980; DeRenzi, 1982; DeRenzi et al.1968; DeRenzi & Spinnler, 1966; Evans et al. 1995; Geffen et al. 1971; Levy et al. 1972; Ley & Bryden, 1977; Hecaen & Angelergues, 1962). The left hemisphere, however, is involved in the recognition of famous faces (Marzi & Berlucchi, 1977; Rizzolatti, et al. 1971; Hanley et al. 1990).
Among neurosurgical patients, it has also been reported that electrical stimulation of the posterior right temporal gyrus disrupts visual-spatial memory for faces in general (Fried et al., 1982). Electrical stimulation of the posterior portion of the right middle temporal gyrus also results in an inability to correctly lable emotion faces.
Agnosias.
When the ITL is damaged, in addition to prosopagnosia there may occur difficulty identifying various familiar stimuli and objects, e.g. utensils, cars, as well as differentiating among similar visual stimuli. Many patients also have difficulty with color recognition (Green & Lessell, 1977; Meadows, 1974b). Frequently these types of agnosic disturbances are related to left cerebral or bilateral dysfunction.
MEMORY
The IT is able to code for and learn new stimuli, can recall the code of the previous stimuli, and can make comparisons between what was perceived on one occasion versus another (Eskandar et al. 2002). This enables IT neurons to make judgments regarding temporal order; if the stimulus was first or second or last (Baylis, & Rolls, 2007; Eskander, et al. 2002). Thus, IT neurons can perform sequential analysis and can compare initial with previous visual representations (Eskandar et al. 2002). Hence, neurons in the IT are involved in form and facial recognition, discrimination, temporal-sequencing, and thus learning and memory for a variety of stimuli.

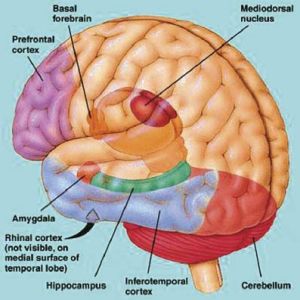
In addition, IT neurons are directly connected with the entorhinal cortex (Jones & Powel, 1970; Van Hoesen & Pandya, 1975b) -the "gateway to the hippocampus"- as well as to the amygdala (Amaral et al. 2007). Thus neurons located in the inferior temporal lobe are also involved in the maintenance of short term emotional, visual and cognitive memory, and many display heightened activity during delay periods while learning (Fuster & Jevey, 2011; Miyashita & Chang, 1988). Moreover, IT neurons are are also highly concerned with the behavioral context in which learning occurs (Baylis & Rolls, 2007; Gross, et al. 1979; Riches, et al. 2011).
For example, Eskandar, et al. (2002) found in their studies of electrophysiological activity that neurons in the inferior temporal gyrus convey information concerning current versus previous stimulus patterns and their behavioral context. IT neurons are therefore involved in remembering this information, matching it with previously learned information, and are capable of simultaneously transmitting these impressions (including contextual details) to other brain areas. However, IT neurons are also capable of discriminating between stimuli independent of context (Eskandar, et al., 2002).
Hence, IT neurons are involved in both encoding, storage, and recall and interact with the amygdala and hippocampus in regard to learning, memory and recognition. It is in this manner and through these interconnects that IT neurons are involved in emotional as well as non-emotional cognitive processing and memory storage. Conversely, with injuries to the inferior temporal lobe, visual and verbal memory functions suffer (see chapter 14).
MEMORY, THE HIPPOCAMPUS & INFERIOR MEDIAL TEMPORAL LOBE
The entorhinal, perirhinal, and parahippocampal neocortex are located in the inferior and medial temporal lobe and are adjacent to and richly interconnected with the hippocampus which is buried within its depths. Like the hippocampus and amygdala, the inferior and medial temporal lobes are involved in memory functioning (Gloor, 1990, 2011; Murray, 2002; Nunn et al., 2008; Ploner et al., 2008; Squire, 2002) and may in fact serve as neuronal memory depots that becomes activated during recognition (e.g. Brewer et al., 1998, Wagner et al., 1998).
As argued by Heit and colleagues (1990), "MTL firing patterns may contribute to the reactivation of neocortical circuits encoding a particular stimulus-context gestalt" which in turn makes recognition and retrieval possible. For example, the medial temporal lobe (MTL) becomes activated during recognition memory tasks including those involving words and faces (Heit et al. 1990). Indeed, the greater the activation, the greater is the likelihood that material will be remembered (e.g. Brewer et al., 1998; Wagner et al., 1998).
When the inferior and medial temporal lobe is electrically stimulated exceedingly vivid personal memories may be triggered and recalled (Gloor, 1990, 2002, 2011; Halgren 2002; Halgren, et al. 1978; Penfield, 1954; Penfield & Perot, 1963). Patients may report seeing a complex scene from their past or early childhood, including hearing conversations, seeing faces, experiencing somesthetic sensations, and related events. Curiously, however, these scenes do not move forward in time and are otherwise quite static (See Gloor, 1990, 2011; Halgren 2002), like a wide angle, multi-sensory snapshot.
Conversely, left MTL lesions results in recognition deficits for words (Milner & Teuber 1968), whereas those involving the right MTL disrupt memory for visual forms and faces (e.g. Hanley et al. 1990). However, the entorhinal cortex and hippocampus appear to be the crucial structures, whereas the neocortex may be the site where memories are stored. Consider, for example, patients who undergo "hippocampal removals" whereas the overlying neocortex is spared (Milner, 1990) and patients such as the famous H.M., who underwent bilateral mesial temporal removals: amygdala, hippocampus, entorhinal cortex (Milner, 1968). These patients (particularly those with right sided destruction) perform exceedingly poorly on visual recognition memory tests, including those involving recurring nonsense figures (Kimura, 1963) and human faces (Milner, 1990).
In that the inferior and inferior medial temporal lobes also contain neurons which are selectively sensitive to particular sensory features, and will fire in response to faces, hands, and geometric patterns and objects (Gross et al. 1979; Gross et al. 1972; Sergent et al. 1990), it is possible that not only are auditory and visual memories stored within the inferior temporal lobe, but that it may act as an associational warehouse (so to speak) from which particular auditory-visual images can be activated, retrieved and so that comparisons can be made. Through the neocortex the hippocampus and amygdala can gain access to particular perceptual images as well as store associated information via the aid and guidance of the neural circuits maintained in the entorhinal cortex and through which information is transmitted to both nuclei.
For example, the entorhinal cortex acts as a gateway via which information from the immediately adjacent perirhinal cortex (which sits above and is richly interconnected with the amygdala) and the parahippocampal gyrus (which receives visual, auditory and tactual neocortical information) is analyzed and is then transmitted into the hippocampus (see Horel et al. 2007; Issausti et al. 2007; Squire, 2002). It is also via the entorhinal area that the hippocampus receives amygdaloid projections (Carlsen et al., 1982; Gloor, 1955, 2011; Krettek & Price, 1976; Steward, 1977) and fibers from the orbital frontal and temporal lobes (Van Hoesen, et al., 1972). These neocortical regions are thus highly important in memory.
Squire (2002 p. 202) in fact argues that "the cortical structures adjacent to the hippocampus (entorhinal, perirhinal, and parahippocampal cortex) appear to participate with the hippocampus in a common memory function." He bases this argument on his review of numerous studies (many of which were conducted by Squire and Zola Morgan and colleagues) which indicate that severe memory impairments can result from lesions restricted to these cortical areas.

TEMPORAL LOBE INJURIES & MEMORY LOSS
Although a variety of neurochemical and neuroanatomical regions are involved in the formulation of memory, it has long been known that damage or the neurosurgical removal of the temporal lobes can produce profound disturbances in the learning and recollection of verbal and visual stimuli (Milner, 1958; Kimura, 1963; Nunn et al., 2008; Ploner et al., 2008; Squire, 2007, 2002). For example, left temporal lobectomy, siezures or lesions involving the inferior temporal areas can moderately disrupt immediate and severely impair delayed memory for verbal passages, and the recall of verbal paried-associates, consonant trigrams, word lists and number sequences (Delaney et al. 1980; Meyer, 1959; Meyer & Yates,1955; Milner, 1958, 1968; Milner & Teuber, 1968; Weingartner, 1968). Similarly, severe anterograde and retrograde memory loss for verbal material has been noted when the anterior and posterior temporal regions (respectively) are electrically stimulated (Ojeman et al., 1968, 1971).
In contrast, right temporal lesions or lobectomy significantly impair recognition memory for tactile and recurring visual stimuli such as faces and meaningless designs, as well as memory for object position and orientation, and visual-pictorial stimuli (Corkin, 1965; Delaney et al., 1980; Evans et al. 1995; Kimura, 1963; Milner, 1968; Nunn et al., 2008; Ploner et al., 2008; Taylor, 1969). Electrical stimulation of the right anterior and posterior temporal region also causes respectively, severe anterograde and retrograde memory loss for designs and geometric stimuli, and impairs memory for faces Fried et al., 1982; Ojeman et al., 1968).
With bilateral removal of the inferior temporal region there results a condition which has been variably referred to as "psychic blindness" and the "Kluver-Bucy syndrome". However, as explained in chapter 13, this is due to destruction of the amygdala. If the mesial regions are removed, severe memory disturbances involving visual and auditory stimuli result such that the patient suffers a permanent anterograde amnesia, including facial processing impairments (Young et al. 1995).
Based on lesion, temporal lobectomy and electrical stimulation studies it thus appears that the anterior temporal region is more involved in initial consolidation storage phase of memory, whereas the posterior region is more involved in memory retrieval and recall. In addition, as based on event-related functional magnetic imaging (Brewer et al., 1998; Wagner et al., 2008), it appears that the temporal lobes directly interact with the frontal lobes in memorization and remembering. Indeed, the greater the activation of the frontal and temporal lobes (and associated tissues), the greater is the likelihood that subjects will remember whereas reduced activity is associated with forgetting. Hence, these areas interact to promote memory and retention. In consequence, if the frontal lobe is injured, and even if the temporal lobes are spared, patients may demonstrate significant memory loss --due to an inability to correctly search for and find the memory.

Bilateral destruction of the anterior hippocampus results in striking and profound disturbances involving memory and new learning (i.e. anterograde amnesia). For example, one such individual who underwent bilateral destruction of this nuclei (H.M.), was subsequently found to have almost completely lost the ability to recall anything experienced after surgery. If you introduced yourself to him, left the room, and then returned a few minutes later he would have no recall of having met or spoken to you. Dr. Brenda Milner has worked with H.M. for almost 20 years and yet she is an utter stranger to him.
H.M. is in fact so amnesic for everything that has occurred since his surgery (although memory for events prior to his surgery is comparatively exceedingly well preserved), that every time he rediscovers that his favorite uncle died (actually a few years before his surgery) he suffers the same grief as if he had just been informed for the first time.
H.M., although without memory for new (non-motor) information, has adequate intelligence, is painfully aware of his deficit and constantly apologizes for his problem. "Right now, I'm wondering" he once said, "Have I done or said anything amiss?" You see, at this moment everything looks clear to me, but what happened just before? That's what worries me. It's like waking from a dream. I just don't remember...Every day is alone in itself, whatever enjoyment I've had, and whatever sorrow I've had...I just don't remember" (Blakemore, 1977, p.96).
Presumably the hippocampus acts to protect memory and the encoding of new information during the storage and consolidation phase via the gating of afferent streams of information and the filtering/exclusion (or dampening) of irrelevant and interfering stimuli. When the hippocampus is damaged there results input overload, the neuroaxis is overwhelmed by neural noise, and the consolidation phase of memory is disrupted such that relevant information is not properly stored or even attended to. Consequently, the ability to form associations (e.g. between stimulus and response) or to alter preexisting schemas (such as occurs during learning) is attenuated (Douglas, 1967).
HALLUCINATIONS
The functional integrity of the temporal lobes, the inferior regions in particular, are highly important in regard to the memorization and recollection of various auditory, visual, olfactory, and emotional experiences. When destroyed, disconnected from sources of input, or compromised in some fashion, the ability to store information and to draw visual-verbal mnemonic imagery from memory is severely attenuated.
Conversely, when the temporal lobes and/or the limbic nuclei buried within its depths (i.e. the amygdala and hippocampus) are artificially or abnormally activated it sometimes occurs that visual-auditory imagery as well as a variety of emotional reactions are evoked involuntarily. These may take the form of complex hallucinations, dream-like states, confusional episodes, or may involve the abnormal attribution of emotional significance to otherwise neutral thoughts and external experiences.
HALLUCINATIONS & THE INTERPRETATION OF NEURAL "NOISE".
Hallucinations may occur secondary to tumors or seizures involving the occipital, parietal, frontal, and temporal lobe (Gloor, 2011; Halgren, 2002; Penfield & Perot, 1963), or arise secondary to toxic exposure, high fevers, general infections, exhaustion, starvation, extreme thirst, partial or complete hearing loss including otosclerosis, and with partial or complete blindness such as due to glacoma (Bartlet, 1951; Flournoy 1923; Lindal et al. 2014; Pesme, 1939; Rhein, 1913; Ross et al., 1975; Rozanski & Rosen, 1952; Semrad, 1938; Tarachow, 1941). Interestingly, when secondary to peripheral hearing loss, frequently individuals report hearing certain songs and melodies from their childhood --melodies which they had usually long forgotten. In addition, individuals suffering from cortical blindness, i.e. Anton's syndrome (Redlich & Dorsey, 1945) and deafness (Brown, 1972), as well as those recovering from Wernicke's aphasia, frequently experience hallucinations.
In general, hallucinations secondary to loss of visual or auditory input appears to be secondary to the interpretation of neural noise. That is, with loss of input various brain regions begin to extract or assign meaningful significance to random neural events, or to whatever input may be received. Thus we find that subjects will hallucinate when placed in sensory reduced environments or even when movement is restricted (Lilly, 1956, 1972; Lindsley, 1961; Shurley, 1962; Zuckerman & Cohen, 1964).
Conversely, hallucinations can occur due to increased levels of neural noise as well. For example, if an area of the neocortex is abnormally activated that area in turn may act to interpret its own neural activity, and/or as a function of the activation of "feature detectors." For example, those neurons that subserve facial recognition, and word recognition, and object recognition, may become simultaneously activated--as well as all associated memories--and in consequence, the brain attempts to interpret what it experiences.
However, the degree of interpretative activity depends on the type of processing performed in the region involved. In this regard we find that hallucinations become increasingly complex as the disturbance expands from primary to association areas and as involvement moves from the occipital to anterior temporal regions (Critchley, 1939; Penfield & Perot, 1963; Tarachow, 1941) --which is one of the major interpretive regions of the neocortex (Gibbs, 1951; Gloor 1990, 2002; Halgren 2002; Penfield & Perot, 1963). That is, in the primary regions, neural noise is given a simple interpretation (simple hallucinations), whereas in the association and multi-associational areas, the individual begins to hallucination secondary to "feature detector" activation, such that they may see faces, chairs, trees, hear voices, music, and so on, all of which is experienced as a mosaic of something real.
For example, tumors or electrical stimulation of the occipital lobe produce simple hallucinations such as colors, stars, spots, balls of fire, flashes of light, whereas with superior temporal involvement the patient may experience crude noises, such as buzzing, roaring sounds, bells, and an occasional voice or sounds of music. However, these same neurons become activated when presented with colors, spots, flashes of light, bells, and so on.
However, with anterior and inferior temporal abnormalities, the hallucinations become increasing complex consisting of both auditory and visual features, including faces, people, objects, animals, etc. (Critchley, 1939; Penfield & Perot, 1963; Tarachow, 1941). This is because there is a ventral stream of visual experience which becomes increasingly complex as information is transmitted from the primary to secondary to association, to multi-modal areas in the temporal lobes where neurons fire in response to complex objects, including faces. The anterior-inferior temporal regions, therefore, give rise to the most complex forms of imagery because cells in this area are specialized for the perception and recognition of specific forms. Moreover, structures such as the amygdala and hippocampus become activated and in consequence, memories and emotions may also be evoked, such that the experience may also become personally meaningful and include real individuals and real events that are produced from memory (Gloor, 2011; Penfield & Perfot, 1963).
It has frequently been reported that as compared to other cortical areas, the most complex and most forms of hallucination occur secondary to temporal lobe involvement (Critchley, 1939; Malh et al., 1964; Horowitz et al., 1968; Penfield & Perot, 1963; Tarachow, 1941) and that the hippocampus and amygdala (in conjunction with the temporal lobe) appear to be the responsible agents (Gloor 1990, 2002, 2011; Gloor et al., 1982; Horowitz et al., 1968; Halgren et al., 1978). For example, Bancaud et al. (2014), Halgren et al., (1978), and Horowitz and colleagues (1968) note that hippocampal stimulation was predominatly associated with either fully formed and/or memory-like hallucinations including feelings of familiarity, and secondarily dream-like hallucinations. However, stimulation limited to the neocortex generally failed to produce complex hallucinations unless the amygdala became activated (Gloor et al., 1982). In this regard, it appears that limbic activation, and activation of the amygdala in particular (Gloor, 2011) is necessary in order to bring to a conscious level percepts which are being processed in the temporal lobes.
LSD. This does not mean, however, that neocortical involvement is not necessary for frequently it is the interpretive interaction of the temporal lobe which gives rise to certain types of hallucinations; i.e. bringing them to a conscious level. That is, although the amygdala (perhaps acting on the hippocampus) may be responsible for the hallucination, the hallucination requires an interactions between the amygdala, hippocampus, and the neocortex, for it is only when the "hallucination" envelops the neocortex of the temporal lobe that the individual becomes "conscious of them."
For example, it is well known that the ingestion of LSD will trigger the formation of vivid and complex auditory and visual hallucinations. Following LSD administration electrophysiological abnormalities are noted in the amygdala and hippocampus (Chapman et al., 1963). However, if the temporal lobes are surgically removed there is a significant decrease (with unilateral removal, Serafetinides, 1965) or complete abolishon (with bilateral temporal lobe removal) of LSD induced hallucinatory activity (Baldwin et al., 1955)--even when the amygdala and hippocampus are spared. That is, if the overlying neocortex is destroyed the individual will fail to hallucination--or at least to become conscious of the hallucination. Of course, if the overlying temporal lobe is destroyed the amygdala and hippocampus also become disconnected, and cannot help but be injured.
Nevertheless, studies with LSD and temporal lobe neocortical resection suggest that the neocortex is associated with consciousness and the limbic system with the unconscious, and if the pathways between them are destroyed, hallucinations cease to be experienced consciously.
Interestingly, the hallucinatory effect of LSD appears to be greatest in the right temporal lobe (Serafetinides, 1965). That is, destruction of the right temporal lobe abolishes LSD-induced hallucinations, where with left temporal destruction, the individual continues to hallucination. Likewise, Penfield and Perot (1963) report that the most vivid hallucination tend to be triggered from the right not the left temporal lobe.


DREAMING
Vivid, visual-auditory and sometimes intensely emotional hypnogic dream imagery is clearly associated with REM sleep (Foulkes, 1962; Goodenough, Shapiro, Holden, & Steinschriber,1959; Monroe, Rechtschaffen, Foulkes, & Jensen, 1965). As discussed in detail in Chapter 10, electrophysiological studies or measures of cerebral blood flow have indicated that the right hemisphere becomes highly active during REM, whereas conversely the left brain becomes more active during N-REM (Goldstein et al., 1972; Hodoba, 1986; Meyer et al., 2007).
It has also been reported that abnormal and seizure-like activity in the right temporal and temporal-occipital area acts to increase dreaming and REM sleep for an atypically long time period. Similarly, REM sleep increases activity in this same region much more than in the left hemisphere (Hodoba, 1986) indicating that there is a specific complementary relationship between REM sleep, dreaming, and right temporal-occipital electrophysiological activity.
In this regard, although not conclusive, there seems to be a convergence of evidence which suggests that the right temporal lobe may be more involved than the left in the production of visual-auditory hallucinations and dream-like mental states be they produced secondary to LSD, electrical stimulation, abnormal seizure activity, or occurring naturally during sleep.
As pertaining to right vs. left cerebral involvement in the production and recollection of hallucinations and dreams it is interesting to note that Horowitz et al., (1968), reported that electrically induced hallucinated events were usually forgotten by patients within 10 to 15 minutes after the experience, and when questioned the next day memory was not improved. Similar forgetting patterns are characteristic of memory for normal sleep induced dreams as well (Joseph, 1988a). That is, it becomes progressively more difficult to recall one's dreams as one spends time in or awakens during NonREM (Wolpert & Trosman, 1958) -which is associated with high left temporal lobe and low right lobe activitity.
TEMPORAL LOBE (PARTIAL COMPLEX) SEIZURES & EPILEPSY
Temporal lobe epilepsy is often associated with atrophy and sclerosis of the hippocampus in about 50% of such patients (Mathern, 2008; Mitchell et al., 2008), with yet others demonstrating sclerosis in the amygdala (Wolf & Blumcke 2008). In fact, according to Gloor (2011, p. 692), "studies on temporal lobe epilepsy provide evidence that the amygdala is involved inmany of the common symptoms and signs that occur in the course of temporal lobe seizures." Gloor (2011, p. 693), also argues that "hippocampal sclerosis is most likely a consequence of prolonged seizures or status epilepticus, particularly when occurring in early childhood and can therefore be regarded as a lesion induced by seizures rather than a causative agent." Gloor (2011) further suggests that the seizure is either produced by the amygdala, which then envelops the hippocampus, and/or that both must give rise to the seizure simultaneously. In support of this position are the findings of Feindel and Rasmussen (2011) who report 65 of 100 neurosurgery epilepsy patients in whom the amygdala and the hippocampus was removed showed a favorable outcome, whereas an equal number showed a favorable outcome if just the amygdala was removed and the hippocampus was spared.
Because the hippocampus and amygdala have the lowest seizure thresholds of all other brain structures, when injured they are thus highly likely to develop seizure activity and thus give rise to temporal lobe epilepsy.
There are a number of factors which can independently give rise to temporal lobe epilepsy (Kotagal & Luders, 2008; Mathern, 2008; Mitchell et al., 2008; Trimble, 2011; Wolf & Blumcke 2008). For example, incisural hernation at the time of brith is a major cause of amygdala injury and hippocampal sclerosis. Head injuries also cause injuries to the temporal region due to contusion from the bony structures in which it is encased. That is, if the head is struck, the inferior and anterior temporal regions may strike against the skull and may even be sheared due to rotational forces. Also middle ear infections are associated with disturbances involving the temporal lobe which result in TLE (Kotagal & Luders, 2008).
In addition, as detailed in chapter 30, severe and repeated early emotional trauma can in fact injure the immature hippocampus and temporal lobe, giving rise to a propensity to develop kindling as well as abnormal neural networks. This would make these individuals are more likely to develop psychotic and severe emotional and dissociative disorders.

Usually, during the course of a temporal lobe seizure, there are no abrupt and drastic alterations in motor activity such as tonic-clonic spasms (Kotagal & Luders, 2008; Trimble, 2011, 1996). However, some patients may simply cease to respond and stare blankly straight ahead, make licking or smacking movements of the lips, and/or fiddle with their clothing, as if picking up pieces of lint. Although conscious-awareness is lost, these patients are not unconscious. Rather, their mental state is one of absence. In fact, they may appear awake and conscious, although unable to speak or respond to questions, and behaviorally their actions seem semi-purposeful. Nevertheless, sometimes it is extremely obvious that they are experiencing a seizure, whereas in some cases an inexperienced observor may only have the impression that the person is acting somewhat odd.
Nevertheless, unless the patient has several different seizure foci, the same characteristic behavioral manifestations are elicited every time a patient has a seizure. The patient does not simply stare on one occasion and on the next begin rolling his eyes and cry out.
AURAS & AUTOMATISMS
Some patients also note an aura immediately prior to seizure onset (Kotagal & Luders, 2008; Trimble, 2011, 1996). This may involve feelings of fear or anxiety, alterations in gastric motility, or unpleasant tastes or in particular, odors (e.g. burning rubber or feces), i.e. an olfactory aura (Gloor, 2011). Some patients may lick and repeatedly smack their lips.
Presumably, the experience of olfactory hallucinations is due to abnormal activation of the rhinencephalon (the "nose brain") and thus limbic nuclei such as the amygdala (Gloor, 2011). Possibly the licking and smacking movements are also due to activation of the amygdala and other limbic structures associated with food consumption. Changes in gastrict motility may be secondary to insular activation and/or limbic participation.
Automatisms associated with TLE occur in up to 75-95% of patients which is twice what occurs with lesions outside the temporal lobe (Kotagal & Luders, 2008; Trimble, 2011, 1996). These includes staring, searching, groping, lip smacking, spitting, groping, staring, salivation. Also laughing, cryng, hissing, gritting or gnashing of teeth, clenching the fist, confused talking, screaming, shouting, standing, running, walking, kissing, and so on.
Common visceral reactions associated with TLE auras include feelings of a racing or flutering heart, incuding pounding or throbbing (Kotagal & Luders, 2008; Trimble, 2011, 1996) also there may be feelings of smothering or chocking. Body sensation include numbness, tenseness, pressure and heaviness. Ther may also be visual sensations such as macro or micro or of things be very hear or far (Gloor, 2011).
Feelings of strangeness or familiarity are also common (Gloor, 2011; Kotagal & Luders, 2008; Trimble, 2011, 1996). deja vue occurs in up to 20% of such patients, usually with right cerebral seizures within the temporal lobe. Some claim to have feelings like a desire to be alone, or of wanting something but not knowing what.
Olfactory hallucinations are not uncommon and are usually quite disagreeable and include smells like burning meat, fish, lime, acid fumes, burning shit. There may also be gustatory sesatniosn which are also usually disagreeable coupled with very bad and bitter or metalic and sour tastes.
Fear is one of the most common feelings, which include feelings of panic, terror, or of something horrible about to happen (Daly, 1958; Gibbs, 1958; Penfield & Jasper, 1954; Williams, 1956). Some patients have experienced fear coupled with sexual sensations, including fear associated with a feeling of sexual climax, or feelings of being sexually penetrated following by orgasm then a loss of consciousness. Although one patient stated it was like "a red hot pocker being inserted into her vagina."
Hence, there are a vast range of aura and experimental phenomenon associated with temporal lobe epilepsy, many of which reflect the possible activation of those structures innervated by the amygdala. Not uncommonly these experiential phenomenon begin just before the seizure. In summary, these include fear, coupled with the remembrance of a fearful or traumatic memories, rising epigastric sensations, chest sensations, nausea, heart palpations, feelings of cold or warmth, shivering, pallor or flushing of the face, respiratory changes inlcuding apnea, salavation, belching, farting, sweating, and vaginal secretions in women which may be accompanied by sexual feelings or behaviors (Daly, 1958; Gloor, 2011; Penfield & Jasper, 1954; Remillard et al., 1983). Moreover, many of these same exact feelings and behaviors can be triggered by direct stimulation of the amygdala (Chapman, 1960; Chapman et al., 1954; Gloor, 2011; Halgren, 2002; Kaada, 1951, 1972), including deja vu--the feeling of familiarity/reminiscence.
In addition, patients may act out on these emotions and auras. There have been reported instances of patient's suddenly lashing out and even attempting to attack those close by, while in the midst of a seizure (Saint-Hilaire et al., 1980), and/or attacking, kicking, and destroying furniture and other objects (Ashford et al., 1980). Patient's have also behaved violently with direct stimulation of the amygdala (Mark et al., 1972), as have a variety of animal subjects (see chapter 13).
Others have responded with behaviors indicative of extreme fear, or conversely, extreme pleasure, including laughter and mirth. Moreover, some patients have acted out sexually during a seizure, such as by displaying their penis, or lying on the floor with legs spread and thrusting, even moaning with pleasure and experiencing libidinous feelings in the vagina and thighs, coupled with an orgasm (Currier, Little, Suess and Andy, 1971; Gloor, 2011; Remillard et al., 1983).
to develop psychotic and severe emotional and dissociative disorders.
SEXUAL SEIZURES
The primate amygdala is sexually differentiated (Nishizuka & Arai, 2011) and in conjunction with the overlying temporal lobe, is capable of detecting sexually significant stimuli, and can determine and detect gender differences such as male and female faces and the emotions they convey (Hasselmo, Rolls, & Baylis, 2008, Heit et al., 1988; Rolls, 2004). Because the amygdala is involved in sexuality and is sexually differentiated, activation of the amygdala can produce penile erection (Kling and Brothers, 2002; MacLean, 1990; Robinson and Mishkin, 1968; Stoffels et al., 1980) sexual feelings (Bancaud et al., 1970; Remillard et al., 1983), sensations of extreme pleasure (Olds and Forbes, 2011), memories of sexual intercourse (Gloor, 1986), as well as ovulation, uterine contractions, lactogenetic responses, and orgasm (Backman and Rossel, 2004; Currier et al., 1971; Freemon and Nevis,1969; Warneke, 1976; Remillard et al., 1983; Shealy and Peel, 1957).
By contrast, injuries to and/or seizure activity within the amygdala/temporal lobe may result in bizarre sexual changes, such as continuous masturbation and indiscriminate, often hypersexual hetero- and homosexual behaviors including attempts at sex with inanimate objects (Kling and Brothers, 2002; Kluver and Bucy, 1939; Pribram and Bagshaw 1953; Terzian and Ore, 1955). Hypersexuality following amygdala injury has been documented among numerous species, including cats and dogs (Blumer 1970; Kling and Brothers, 2002).
Humans with an abnormally activated or severely injured amygdala/temporal lobe may expose and manipulate their genitals (Leutmezer et al., 2008), masturbate in public, and attempt to have sex with family members or individuals of the same sex (Blumer, 1970; Kolarsky, Freund, Macheck, and Polak, 1967; Terzian and Ore, 1955). Moreover, abnormal activity involving the amygdala and overlying temporal lobe has been associated with the the development of hyposexuality (Taylor, 1971; Heirons and Saunders, 1966; Toon, Edem, Nanjee, and Wheeler, 2008), hypersexuality (Blumer, 1970) as well as homosexuality, transvestism, and thus confusion over sexual orientation (Davies and Morgenstern, 1960; Kolarsky et al., 1967). In fact, abnormal- or seizure activity within the amygdala or overlying temporal lobe may induce an individual to engage in "sexual intercourse" even in the absence of a partner. For example, Currier and colleagues (1971, p. 260) described a female temporal lobe seizure patient who was "sitting at the kitchen table with her daughter making out a shopping list" when she suffered a seizure. "She appeared dazed, slumped to the floor on her back, lifted her skirt, spread her knees and elevated her pelvis rhythmically. She made appropriate vocalizations for sexual intercourse such as: It feels so good...further, further."
RUNNING, LAUGHING & CRYING SEIZURES
In some instances patients with temporal lobe seizures (triggered perhaps by tumor) may display extremely odd and bizzare behaviors during the course of the seizure (Chen & Forster, 1973; Trimble 2011) For example, a number of authors have presented case reports of individuals who developed laughing (gelastic epilepsy), crying (dacrystic epilepsy), and/or running seizures (cursive epilepsy) -running apparently being triggered via amygdala-striatal activation (chapter 13, 16).
One patient's seizure consisted of suddenly bursting into laughter, rubbing his upper abdomen and running wildly about the room with an expression of fear on his face (Sethi & Rao, 1976). Another individual displayed paroxysmal attacks of weeping, sobbing, and mournful moaning (Offen et al., 1976). These behaviors were involuntary, however, and part of the seizure. Once consciousness returned, the patients would have no recollection of their actions.
EMOTION
The most common emotional reactions and sensations which occur secondary to or during the course of a temporal lobe seizure include feelings of fear, anxiety, depression, depersonalization, pleasure, unpleasure, and familiarity (Bear, 1979; Cendes, et al. 2014; Herman & Chhabria, 1980; Gloor, 2011; Gloor et al., 1982; Perez & Trimble, 1980; Trimble 2011; Slater & Beard 1963; Strauss et al., 1982; Weil, 1956; Williams, 1956), fear being the most frequently experienced. Many of these same feelings are also triggered by electrical stimulation of the temporal lobes, amygdala and hippocampus (Gloor, 1972, 2011; Gloor et al., 1982; Heath, 1964; Mullan & Penfield, 1959).
Depression (lasting from hours to weeks) may occur as an immediate sequela to the seizure, many patients also experiencing confusion. A depressive aura may also precede and thus hearald the coming of a seizure by hours or even days (Trimble 2011, 1996; Williams, 1956). This may be due in part to the role of the temporal lobe (and amygdala) is storing emotional and personal memories, including those with are negative, depressing and traumatic (Abrams & Taylor, 1979; Cimino, et al. 2011 Cohen, Penick & Tarter, 1974; Deglin & Nikolaenko, 1975; Shagass et al.,1979; Wexler, 1973) such that when activated, depressive moods and memories may be provoked. For example, Abrams and Taylor (1979) and Shagass et al. (1979) have found that depressed patients demonstrated more right vs left temporal lobe electrophysiological activity and EEG abnormalities. Moreover, others have argued that ECT administered to the right vs left temporal lobe is more likely to alleviate depressive symptoms (Cohen, et al, 1974; Deglin & Nikolaenko, 1975).
Rather than increased emotionality, some patients complain of emotional blocking, and feelings of emptiness: "feelings don't reach me anymore" (Weil, 1956). Presumably this is a consequence of limbic disconnection. That is, the seizure foci (or lesion) acts to deconnect the limbic areas from the temporal (or orbital frontal) lobes. In consequence, percepts and thoughts no longer come to be assigned emotional or motivational significance.
TEMPORAL LOBE EPILEPSY & RELIGIOUS EXPERIENCE
It has been reported by a number of neuroscientists that some patients who experience temporal lobe epilepsy, seizures, or related abnormalities sometimes experience very sexual as well as bizarre, unusual and fearful mental phenomenon including dissociative states, feelings of depersonalization, and hallucinogenic and dream-like recollections involving threatening men, naked women, sexual intercourse, religion, the experience of god, as well as demons and ghosts and pigs walking upright dressed as people (Bear 1979; Daly 1958; Gloor 1986, 2002; Halgren 2002; Horowitz et al. 1968; Penfield & Perot 1963; Slater & Beard 1963; Taylor 1972 1975; Trimble 2011; Weingarten, et al. 1977; Williams 1956). Moreover, some individuals report communing with spirits or receiving profound knowledge from the Hereafter, following temporal lobe activation (Daly 1958; MacLean 1990; Penfield & Perot 1963; Williams 1956).

One young many had repeated conversations with God. One day God informed this young man that he was Jesus Christ, could perform miracles and could fly. Believing that he was in fact The Christ, since god had told him so, he climbed upon a roof and leaped into the air, with disasterious results (Sommer 1880).
Intense activation of the temporal lobe, hippocampus, and amygdala has been repeatedly reported to give rise to a host of sexual, religious and spiritual experiences; and chronic hyperstimulation can induce an individual to become hyper-religious or visualize and experience ghosts, demons, angels, and even God, as well as claim demonic and angelic possession or the sensation of having left their body (Bear 1979; Daly 1958; Gloor 1986, 2002; Horowitz et al. 1968; MacLean 1990; Mesulam 2011; Penfield & Perot 1963; Schenk, & Bear 2011; Slater & Beard 1963; Subirana & Oller-Daurelia, 1953; Trimble 2011; Weingarten, et al. 1977; Williams 1956). Indeed, as detailed in chapter 9, the temporal lobe, amygdala and hippocampus enables humans to have religious, spiritual and mystical experiences (Bear 1979; Daly 1958; d'Aquili & Newberg 1993; Mesulam 2011; Trimble 2011).

In one case, a 37 year-old manager of a car dealership had been struck by a car while walking across a street, and was slammed against the pavement, striking his head. According to this fellow (GM), once he gained "consciousness" he realized he was standing next to his body, which was still lying in the street. He could see that his head was bloody, and he could see that he was unconscious and he could see other people rushing over to where he lay. However, this dissociative hallucination only lasted a few seconds, and then he was back in his body, regaining consciousness. GM states that his experience was a "revelation" and although he had never been religious, that he became very religious, and began reading the Bible, volunteering at a local church, and writing a religious column for a small local newspaper. He also began having repeated episodes where he would float beside his body. As his wife complained that he was repeatedly "blanking out" and as he was also having memory problems, he sought medical help, was referred for neuropsychological testing, which revealed significant disturbances of memory, and evidence of temporal lobe epilepsy. Subsequent EEG demonstrated bilateral spiking and theta activity over the temporal lobes.
OUT OF BODY, HEAVENLY & "OTHERWORLDLY EXPERIENCES"
Penfield and Perot (1963) describe several patients who during a seizure claimed they could see themselves in different situations. One woman stated that "it was though I were two persons, one watching, and the other having this happen to me," and that it was she who was doing the watching as if she was completely separated from her body. According to Penfield, "It was as though the patient were attending a play and was both actor and audience.
Other patients claim to have quite pleasant auras and describe feelings such as elation, security, eternal harmony, immense joy, paradisiacal happiness, euphoria, completeness. Between .5 and 20% of such patients claim such feelings (Williams, 1956; Daly, 1958). One patient of Williams (1956) claimed that his attacks began with a "sudden feeling of extreme well being involving all my senses. I see a curtain of beautiful colors before my eyes and experience a pleasant but indescribable taste in my mouth. Objects feeling pleasurably warm. the room assumes vast proportions, and I feel as if in another world."
A patient described by Daly said his seizure felt like "a sunny day when your friends are all around you." He then felt dissociated from his body, as if he were looking down upon himself and watching his actions.
Williams (1956) describes a patient who claimed that during an aura she experienced a feeling that she was being lifted up out of her body, coupled with a very pleasant sensation of elation and the sensation that she was "just about to find out knowlegde no one else shares, something to do with the line between life and death."
Subirana and Oller-Daurelia (1953) described two patients who experienced ecstatic feelings of either "extraordinary beatitude" or of paradise as if they had gone to heaven and noted that his fantastic feelings lasted for hours.
Other patients have noted that feelings and things suddenly become "crystal clear" or that they have a feeling of clairvoyance, or of having the truth revealed to them, or of having achieved a sense of greater awareness and of a new awareness such that sounds, smells and visual objects seemed to have a greater meaning and sensibility.
PERSONALITY & PSYCHIATRIC DISTURBANCES
Personality and psychiatric disorders have been frequently reported as a common associated complication among a small percentage of individuals (between 4.5 to 7%) with seizures-disorders localized to the temporal lobe (Harrison, 2008; Trimble 2011, 1996). Such patients may appear depressed, paranoid, hysterical, or suffering from schizophrenic-like symptomology (However, see Gold et al. 2014).
Some authors have argued that, like hallucinations, the nearer the seizure foci to the anterior temporal lobe, the greater the probability of significant psychiatric abnormality (Gibbs, 1951; Gloor 2002; Trimble 2011, 1996). Presumably this is a function of hyper-activation of underlying limbic structures and thus abnormal attribution of emotional significance to different afferent streams of perceptual experience (Bear, 1979).
SCHIZOPHRENIA & THE TEMPORAL LOBE
It has been repeatedly reported that a significant minority of individuals with temporal lobe epilepsy suffer from prolonged and/or repeated instances of major psychopathology (Bruton et al. 2014; Buchsbaum, 1990; Falconer, 1973; Flor-Henry, 1969, 1983; Gibbs, 1951; Jensen & Larsen, 1979; Perez & Trimble 1980; Sherwin, 1977, 2011; Slater & beard, 1963; Taylor, 1972; Trimble 2011, 1996). Conversely, those with affective disturbances, e.g. mania, have been found to suffer from seizures involving the right temporal lobe (Bear et al., 1982; Falconer & Taylor, 1970; Flor-Henry, 1969; Trimble 2011, or right frontal lobe (chapter 19), although depression may be more likely with left vs right hemisphere injuries (see chapter 11).
Estimates of psychosis and affective disorders among individuals suffering from temporal lobe epilepsy, be it a single episode or chronic psychiatric condition have varied from 2-81%. Probably a more realistic figure is 3% to 7% (see Trimble, 2011, 2002, 1996).
There are several distinct neurological explanations for the association between temporal lobe damage and psychotic and affective disorders. As discussed in detail in previous chapter 10, the right hemisphere is dominant for most aspects of emotional functioning. Hence, when damaged or abnormally activated, such as from a seizure or chronic subclinical seizure activity, emotional functioning becomes altered and disturbed. Moreover, since the amygdala and hippocampus are often secondarily aroused (or hyperaroused), the possibility of abnormal emotionality is enhanced even further.
In this regard, Gloor et al., (1982) in a depth electrode stimulation study of patients suffering from epilepsy presented evidence indicating that all experiential and emotional alterations were encountered following right amygdala and hippocampal activation, with the majority of phenomenon elicited from the amygdala. As discussed in chapter 13, the limbic system seem to be lateralized and the right amygdala and hippocampus appear to be more involved in emotional functioning . Hence, abnormal activation of these nuclei (such as during the course of a temporal lobe seizure) are more likely to give rise to abnormal emotional reactions, with the exception of depression which is common with left frontal and left temporal lobe injuries.
In addition, some individuals with temporal lobe seizures may complain of emotional blunting. Hence, rather than a limbic hyperconnection, these individuals suffer from a disconnection; similar to what occurs with a left frontal injury.
It has also been repeatedly demonstrated in histological studies that the temporal lobe of schizophrenics are characterized by an excessive focal temporal lobe neuronal damage including gliosis (Akbarian et al. 1993; Bruder, et al., 2008; Bruton 1988; Bruton et al. 1990; Harrison, 2008; Kwon et al., 2008; Stevens 1982; Taylor 1972). Hence, these patients may experience auditory hallucinations and are more likely to demonstrate formal thought disorders and aphasic thinking including the production of neologisms and syntactical speech errors (Kwon et al., 2008), as well as emotional (or thought) blocking or blunting due to limbic system-temporal lobe disconnection (Perez & Trimble, 1980; Slater & Beard, 1963). As often occurs with receptive aphasia and left temporal lobe dysfunction, individuals suffering from schizphrenia may be unaware of their illness (Amador et al. 2014; Lysaker, et al., 2008; Wilson et al. 1986).
Hence, certain subgroups of schizophrenics display significant abnormalities involving speech processing, such that the semantic, temporal-sequential, and lexical aspects of speech organization and comprehension are disturbed and deviantly constructed (Chaika, 1982; Clark et al. 2014; Flor-Henry, 1983; Hoffman, 1986; Hoffman et al. 1986; Rutter, 1979). Indeed, significant similarities between schizophrenic discourse and aphasic abnormalties have been reported (Faber, Abrams, Taylor, Kasprisin et al. 1983). It is often difficult to determine what a schizophrenic individual may be talking about, particularly in that some of these individuals complain that what they say often differs from what they intended to say (Chapman, 1966). Temporal-sequentual (i.e. syntactical) abnormalities have also been noted in their ability to reason. The classic example being: "I am a virgin; the Virgin Mary was a virgin; therefore I am the Virgin Mary."
Hence, there is considerable evidence indicating that a significant relationship exists between abnormal left hemisphere and left temporal lobe functioning and schizophrenic language, thought and behavior. As detailed in chapter 19, the left frontal region and medial frontal cortices are also implicated in the genesis of schiophrenic-like abnormalities including catatonia (chapter 19).
However, in contrast to those with frontal lobe schizophrenia who are more likely to present with "negative" symptoms and emotional blunting, temporal lobe schziophrenics are likely to present with "positive" symptoms, including retention of the capacity to experience and express "warmth" (Crow, 1980; Crowe & Kuttner 2011). Emotional expression, however, tends to be abnormal, inappropriate or incongruent, and/or exaggerated (Bear & Fedio, 1977; Crow, 1980; Crowe & Kuttner 2011).
It has also been repeatedly reported that irritative lesions more greatly involves the lateral convexity of the left temporal lobe are he most likely to result in schizophrenic-like disturbances (DeLisi et al. 2011; Dauphinais et al. 1990; Flor-Henry 1983; Harrison, 2008; Perez et al. 2015; Rossi et al. 1990, 2011; Sherwin 2011). Given the role of the temporal lobe in language (and the fact that the superior temporal lobe is derived from amygdala and hippocampal tissue) it is also not uncommon for these individuals to demonstrate aphasic abnormalities in their thought and speech (Chaika, 1982; Clark et al. 2014; Flor-Henry, 1983; Hoffman, 1986; Hoffman et al. 1986; Perez & Trimble, 1980; Rutter, 1979; Slater & Beard, 1963).
However, the type and form of temporal lobe schizophrenia may differ dependent on which regions have been compromised. In some instances the amygdala and hippocampus are involved, (Bogerts et al. 2015; Brown et al. 1986; Falkai & Bogerts 1986) in which case memory and emotional functioning may be most severely effected, and patients may experience visual hallucinations. Temporal lobe schizophrenics with limbic involvement may behave violently and aggressively (Crow & Kuttner 2011; Lewis et al. 1982; Taylor 1969). They may also experience extreme feelings of religiousness (Bear, 1979, Trimble, 2011, 2002), fear, paranoia, and depression (Crow & Kuttner 2011; Perez & Trimble, 1980; Trimble, 2011, 2002), as well as hallucinations.
APHASIA & PSYCHOSIS
Since the left temporal lobe is intimately involved in all aspects of language comprehension as well as the organization of linguistic expression, altered neocortical activity involving this region is likely to result in significant disruptions in language functioning and the formulation of linguistic thought. As described in chapter 11, individuals with left temporal lobe damage and Wernicke's aphasia are likely to be diagnosed as suffering from a formal thought disorder due to their fluent aphasic language disturbances their failure to comprehend.
Conversely, several authors have noted significant similarities between schizophrenic discourse and aphasia (Faber et al. 1983; Hillbom. 1951), such that the semantic, temporal-sequential, and lexical aspects of speech organization and comprehension are disturbed and deviantly constructed (Chaika, 1982; Flor-Henry, 1983; Hoffman, 1986; Hoffman, Stopek & Andreasen, 1986; Rutter, 1979). Similar findings have been reported for individuals developing post-traumatic schizophrenic-like symptoms following head injury and missile wounds (Hillbom, 1951). Moreover, just as some aphasic individuals may be unaware of their disorder, similar findings have been noted with schizophrenics and those with schizoaffective disorders (Amador et al. 2014).
TEMPORAL LOBE SEIZURES, PSYCHOSIS & APHASIA
Patients with left temporal seizures commonly become globally aphasic during the course of their seizure. When spoken to or if their name is repeatedly called they may make only fleeting eye contact, grunt or utter partial words such as "huh?" Some individuals remain aphasic for seconds to minutes after seizure termination as well.
Language impairments, verbal memory disorders and associated linguistic deficiencies are usually apparent even between seizures if adequately assessed. Moreover, individuals with left sided foci tend to have lower (WAIS-R) Verbal IQ's as compared to their Performance IQ's and as compared to those with right sided foci. This is not surprising as language is represented in the left hemisphere. However, in some cases, excessive activity within the left (or right) temporal (or frontal) lobe can actually result in what appears to be enhanced functional activity.
It is possible that abnormal activity in the left temporal lobe, as well as the presence of an active lesion, can result in significant alterations in not only language, but the organization of linguistic thought such that the patient appears psychotic. In this regard, abnormal thought formation may become a characteristic pattern. In fact, even the neuroanatomical representation of language can be drastically altered due to left cerebral damage (Joseph, 1986a; Novelly & Joseph, 1983). Moreover, if the left temporal region is periodically disconnected from centers mediating emotion, patients may demonstrate what appears to be emotional blunting as well as a formal thought disorder.
BETWEEN SEIZURE PSYCHOSES
The range of disturbances among those with temporal lobe epilepsy includes a strikingly high rate of sexual abberation as well as hyposexuality, aggressiveness, paranoia, depression, deepening of emotion, intensification of religious concerns, disorders of thought, depersonalization, hypergraphia, complex visual and auditory halluciantions and schizophrenia (Bear, 1982; Bear et al., 1982; Flor-Henry, 1969, 1983; Sherwin, 1977, 2011; Schiff et al., 1982; Stevens et al. 1979; Taylor, 1975). However, this association has also been disputed (see Gold et al. 2014).
Frequently the seizure related disturbances in emotional functioning waxe and wane, such that some patients experience islands of normality followed by islands of psychosis. Possibly these changes are a function in variations in subclinical seizure activity and kindling.
In some instances, the psychiatric disturbance may develop over days as a prelude to the actual onset of a seizure, due presumably to increasing levels of abnormal activity until the seizure is triggered. In other cases, particularly in regard to depression, the alterations in personality and emotionality may occur following the seizure and may persist for weeks or even months (Trimble 2011, 1996).
In yet other instances, patients may act increasingly bizzare for weeks, experience a seizure, and then behave in a normal fashion for some time ("forced normalization", Flor-Henry 1983), only to again begin acting increasingly bizzarre. This has been referred to as an inter-ictal (between seizure) psychosis and is possibly secondary to a build up of abnormal activity in the temporal lobes and limbic system (Bear, 1979; Flor-Henry 1969, 1983) Hence, once the seizure occurs, the level of abnormal activity is decreased and the psychosis goes away only to gradually return as abnormal activity again builds up.
Caveats
Some authors have argued that there is no significant relationship between these psychotic disorders and temporal lobe epilepsy (Gold et al. 2014; Stevens & Hermann, 2011), whereas others have drawn attention to possible social-developmental contributions (Matthews et al., 1982). That is, growing up with a seizure disorder, feeling victimized, not knowing when a seizure may strike, loss of control over ones life, etc. can independently create significant emotional aberrations.
It is also important to emphasize that it is probably only a subpopulation of individuals with temporal lobe epilepsy who come to the attention of most researchers; e.g. those with the most serious or intractable problems. Thus one should not immediately view an individual with temporal lobe (or any type of epilepsy) as immediately suspect for emotional-psychotic abnormalities. Nevertheless, it is noteworthy that not only is there an association between the temporal lobe and certain forms of schizophrenia, but that temporal lobe abnormalities have been noted across generations of individuals and families afflicted with psychotic disturbances (Honer et al. 2014).
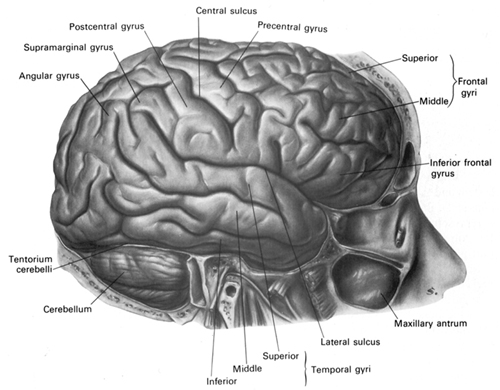
TEMPORAL LOBE INJURY SUSCEPTIBILITY
As detailed in chapters 30, 32, the temporal lobes are highly susceptible to injury from a variety of causes, including head injury, stroke, tumor, and epilepsy. In part, this susceptibility is due to the position of the temporal lobe within the skull. With whiplash injuries, or if the skull is struck from the back or the front, the temporal lobes will slam into the inside of the skull and may be ripped, torn, and sheared. These are called coup and contra coupe injuries.


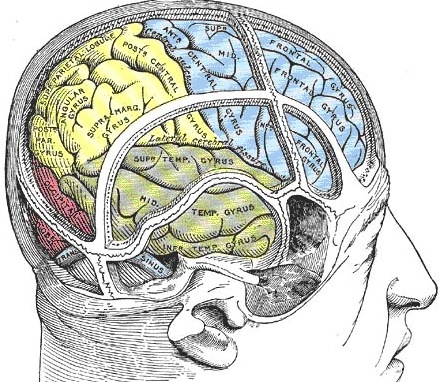
The inferior temporal lobes are also slow to mature which in turn increases the likelihood that abnormal neural networks may be formed in response to adverse early experience (see chapters, 2, 28, 30). Hence, not surprisingly, abnormal early environmental influences, including profound traumatic stress, can induce language, emotional, and memory disorders including repression for childhood experiences, as well as severe psychiatric abnormalities including schizophrenia and dissociative phenomenon; disturbances which implicate the temporal lobes as well as the amygdala and hippocampus.











